Abstract
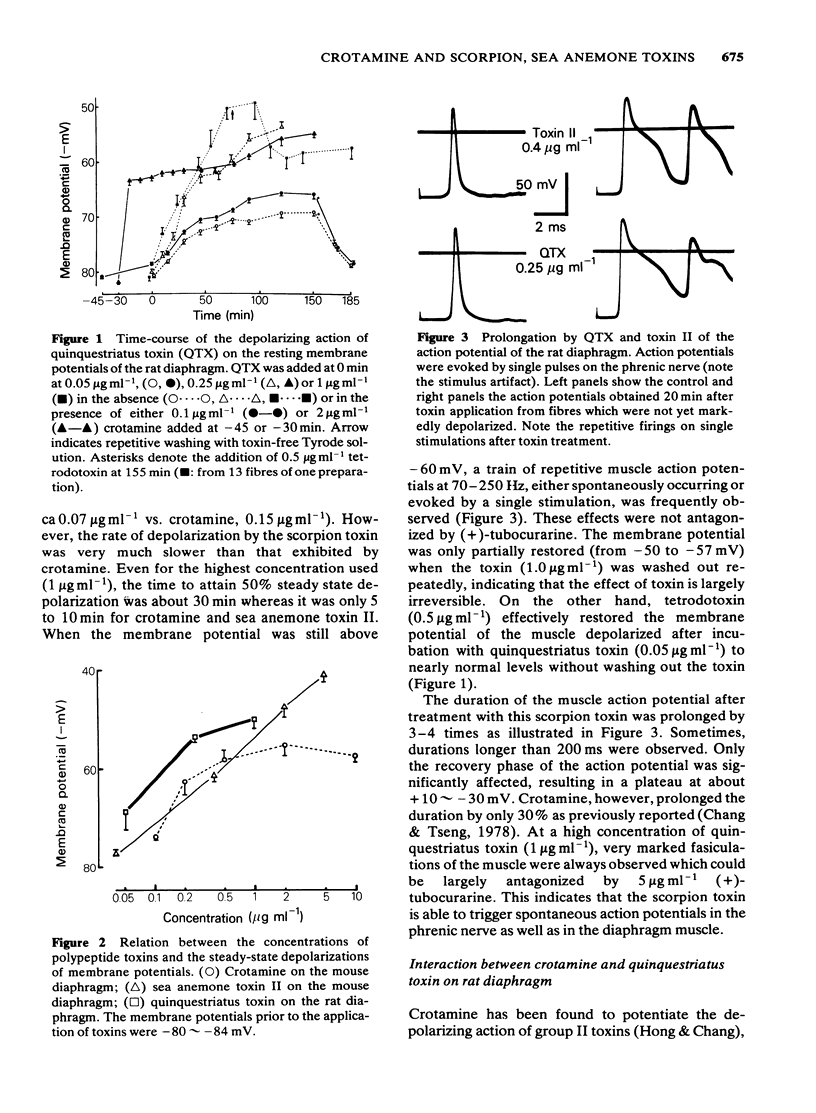
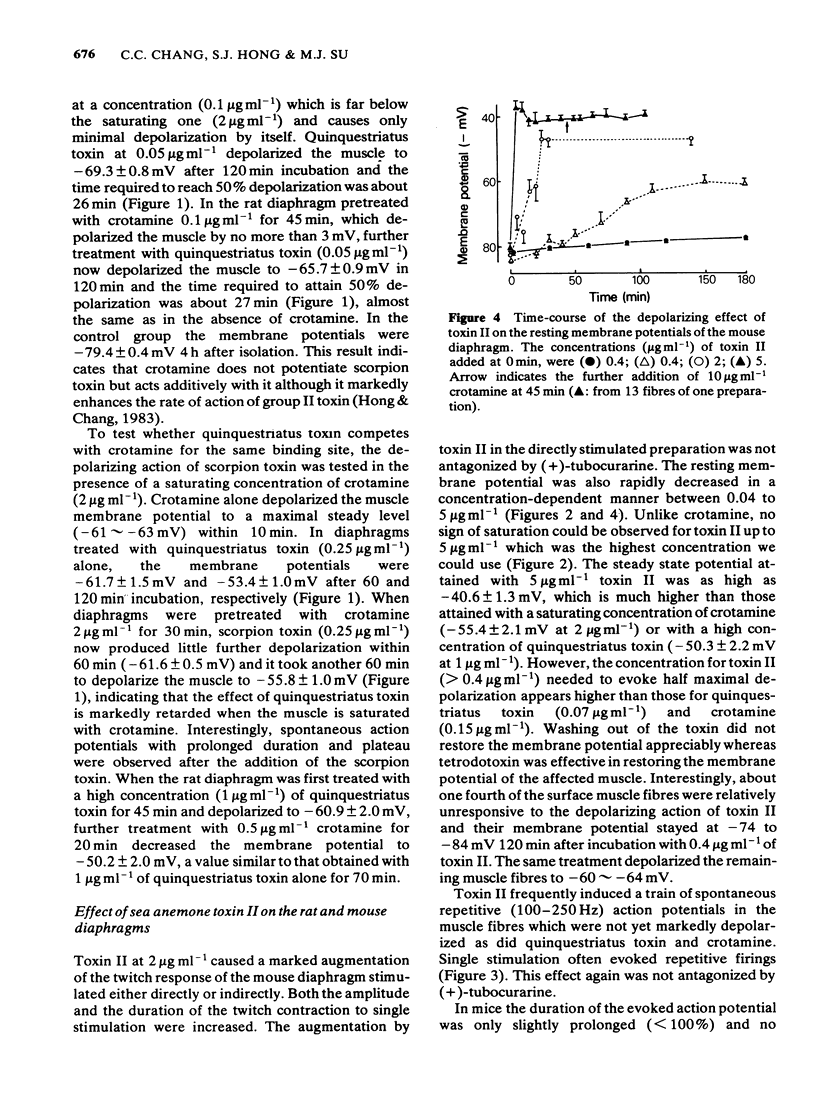
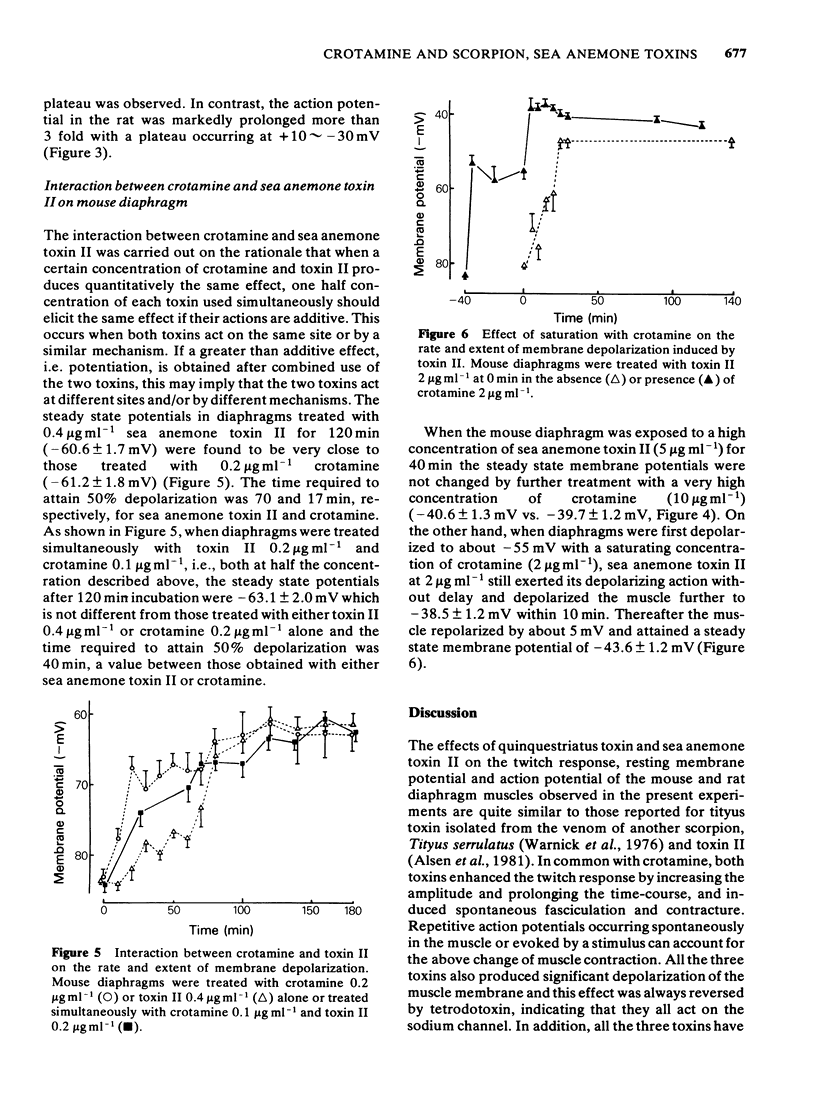
Quinquestriatus toxin (QTX) isolated from the venom of a scorpion (Leiurus quinquestriatus) and sea anemone (Anemonia sulcata) toxin II enhanced the twitch response of the rat and mouse diaphragms and like crotamine (isolated from the venom of Crotalus durissus terrificus) caused spontaneous fasciculation of the muscle. Trains of action potentials in muscles at 70-250 Hz, which could not be antagonized by (+)-tubocurarine, were triggered by single stimulation or occurred spontaneously after treatment with these toxins. QTX and toxin II prolonged the rat muscle action potential 3 to 4 fold whereas crotamine prolonged the action potential by only 30%. The membrane potential was depolarized from about -82 mV to -55 mV by crotamine 2 micrograms ml-1, -41 mV by toxin II 5 micrograms ml-1 and to -50 mV by QTX 1 microgram ml-1. The concentrations to induce 50% maximal depolarization (K0.5) were 0.07, 0.15 and greater than 0.4 microgram ml-1, respectively, for QTX, crotamine and toxin II, whereas the rates of depolarization were in the order toxin II greater than or equal to crotamine greater than QTX. The depolarizing effects of crotamine and QTX, but not of toxin II, were saturable. The depolarizing effects of all three toxins were irreversible whereas the membrane potential could be restored by tetrodotoxin non-competitively. Simultaneous treatment with crotamine and QTX or crotamine and toxin II at concentrations below K0.5 caused only additive effects on depolarization. When the muscle was depolarized by pretreating with a saturating concentration of crotamine, the onset of depolarization by QTX was greatly retarded whereas that by toxin II was unaffected.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAM K. R., WEISS C. Actions of scorpion venom on skeletal muscle. Br J Pharmacol Chemother. 1959 Sep;14:334–339. doi: 10.1111/j.1476-5381.1959.tb00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsen C., Harris J. B., Tesseraux I. Mechanical and electrophysiological effects of sea anemone (Anemonia sulcata) toxins on rat innervated and denervated skeletal muscle. Br J Pharmacol. 1981 Sep;74(1):61–71. doi: 10.1111/j.1476-5381.1981.tb09955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman C., Dubois J. M., Rojas E., Rathmayer W. Decreased rate of sodium conductance inactivation in the node of Ranvier induced by a polypeptide toxin from sea anemone. Biochim Biophys Acta. 1976 Nov 11;455(1):173–184. doi: 10.1016/0005-2736(76)90162-0. [DOI] [PubMed] [Google Scholar]
- Bernard P., Couraud F., Lissitzky S. Effects of a scorpion toxin from Androctonus australis venom on action potential of neuroblastoma cells in culture. Biochem Biophys Res Commun. 1977 Jul 25;77(2):782–788. doi: 10.1016/s0006-291x(77)80046-6. [DOI] [PubMed] [Google Scholar]
- Bülbring E. Observations on the isolated phrenic nerve diaphragm preparation of the rat. 1946. Br J Pharmacol. 1997 Feb;120(4 Suppl):3–2. doi: 10.1111/j.1476-5381.1997.tb06771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Activation of the action potential Na+ ionophore by neurotoxins. An allosteric model. J Biol Chem. 1977 Dec 10;252(23):8669–8676. [PubMed] [Google Scholar]
- Catterall W. A., Beress L. Sea anemone toxin and scorpion toxin share a common receptor site associated with the action potential sodium ionophore. J Biol Chem. 1978 Oct 25;253(20):7393–7396. [PubMed] [Google Scholar]
- Catterall W. A. Binding of scorpion toxin to receptor sites associated with sodium channels in frog muscle. Correlation of voltage-dependent binding with activation. J Gen Physiol. 1979 Sep;74(3):375–391. doi: 10.1085/jgp.74.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Coppersmith J. Pharmacological properties of sodium channels in cultured rat heart cells. Mol Pharmacol. 1981 Nov;20(3):533–542. [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Risk M. Toxin T4(6) from Ptychodiscus brevis (formerly Gymnodinium breve) enhances activation of voltage-sensitive sodium channels by veratridine. Mol Pharmacol. 1981 Mar;19(2):345–348. [PubMed] [Google Scholar]
- Chang C. C., Tseng K. H. Effect of crotamine, a toxin of South American rattlesnake venom, on the sodium channel of murine skeletal muscle. Br J Pharmacol. 1978 Jul;63(3):551–559. doi: 10.1111/j.1476-5381.1978.tb07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheymol J., Bourillet F., Roch-Arveiller M., Heckle J. Action neuromusculaire de trois venins de scorpions Nord-Africains (Leiurus quinquestriatus, Buthus occitanus et Androctonus australis) et de deux toxines extraites de l'un d"entre eux. Toxicon. 1973 Apr;11(3):277–282. doi: 10.1016/0041-0101(73)90055-x. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E., Deroubaix E., Tazieff-Depierre T. Effect of toxin II isolated from scorpion venom on action potential and contraction of mammalian heart. J Mol Cell Cardiol. 1975 Sep;7(9):643–653. doi: 10.1016/0022-2828(75)90141-8. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The effect of scorpion venoms on the sodium currents of the squid giant axon. J Physiol. 1980 Nov;308:479–499. doi: 10.1113/jphysiol.1980.sp013484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E., Beress L. Iodine labelling of sea anemone toxin II, and binding to normal and denervated diaphragm. Naunyn Schmiedebergs Arch Pharmacol. 1979 Nov;309(2):165–170. doi: 10.1007/BF00501225. [DOI] [PubMed] [Google Scholar]
- Jacques Y., Fosset M., Lazdunski M. Molecular properties of the action potential Na+ ionophore in neuroblastoma cells. Interactions with neurotoxins. J Biol Chem. 1978 Oct 25;253(20):7383–7392. [PubMed] [Google Scholar]
- Khodorov B. I., Revenko S. V. Further analysis of the mechanisms of action of batrachotoxin on the membrane of myelinated nerve. Neuroscience. 1979;4(9):1315–1330. doi: 10.1016/0306-4522(79)90159-3. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer E., Schmidt H. Die Wirkung von Skorpiongift auf die Ionenströme des Ranvierschen Schnürrings. II. Unvollständiage Natrium-Inaktivierung. Pflugers Arch. 1968;303(2):150–161. doi: 10.1007/BF00592632. [DOI] [PubMed] [Google Scholar]
- Laure C. J. Die Primärstruktur des Crotamins. Hoppe Seylers Z Physiol Chem. 1975 Feb;356(2):213–215. [PubMed] [Google Scholar]
- Lawrence J. C., Catterall W. A. Tetrodotoxin-insensitive sodium channels. Binding of polypeptide neurotoxins in primary cultures of rat muscle cells. J Biol Chem. 1981 Jun 25;256(12):6223–6229. [PubMed] [Google Scholar]
- Lawrence J. C., Catterall W. A. Tetrodotoxin-insensitive sodium channels. Ion flux studies of neurotoxin action in a clonal rat muscle cell line. J Biol Chem. 1981 Jun 25;256(12):6213–6222. [PubMed] [Google Scholar]
- Ravens U. Electromechanical studies of an Anemonia sulcata toxin in mammalian cardiac muscle. Naunyn Schmiedebergs Arch Pharmacol. 1976 Dec;296(1):73–78. doi: 10.1007/BF00498842. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Romey G., Abita J. P., Schweitz H., Wunderer G., Lazdunski Sea anemone toxin:a tool to study molecular mechanisms of nerve conduction and excitation-secretion coupling. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4055–4059. doi: 10.1073/pnas.73.11.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romey G., Renaud J. F., Fosset M., Lazdunski M. Pharmacological properties of the interaction of a sea anemone polypeptide toxin with cardiac cells in culture. J Pharmacol Exp Ther. 1980 Jun;213(3):607–615. [PubMed] [Google Scholar]
- Schmidt H., Schmitt O. Effect of aconitine on the sodium permeability of the node of Ranvier. Pflugers Arch. 1974 Jun 11;349(2):133–148. doi: 10.1007/BF00586624. [DOI] [PubMed] [Google Scholar]
- Seyama I., Narahashi T. Modulation of sodium channels of squid nerve membranes by grayanotoxin I. J Pharmacol Exp Ther. 1981 Dec;219(3):614–624. [PubMed] [Google Scholar]
- Tamkun M. M., Catterall W. A. Ion flux studies of voltage-sensitive sodium channels in synaptic nerve-ending particles. Mol Pharmacol. 1981 Jan;19(1):78–86. [PubMed] [Google Scholar]
- Ulbricht W. The effect of veratridine on excitable membranes of nerve and muscle. Ergeb Physiol. 1969;61:18–71. doi: 10.1007/BFb0111446. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Balerna M., Barhanin J., Fosset M., Lazdunski M. Binding of sea anemone toxin to receptor sites associated with gating system of sodium channel in synaptic nerve endings in vitro. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1646–1650. doi: 10.1073/pnas.77.3.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnick J. E., Albuquerque E. X., Diniz C. R. Electrophysiological observations on the action of the purified scorpion venom, tityustoxin, on nerve and skeletal muscle of the rat. J Pharmacol Exp Ther. 1976 Jul;198(1):155–167. [PubMed] [Google Scholar]


