Abstract
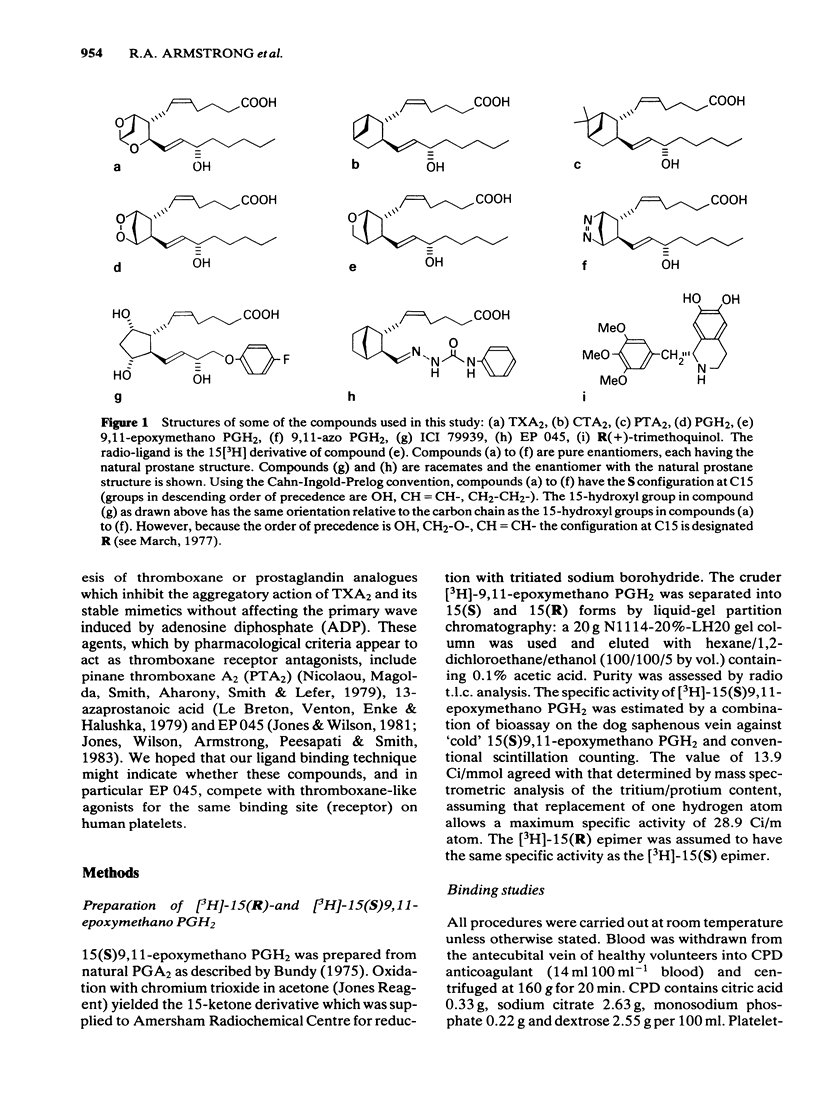
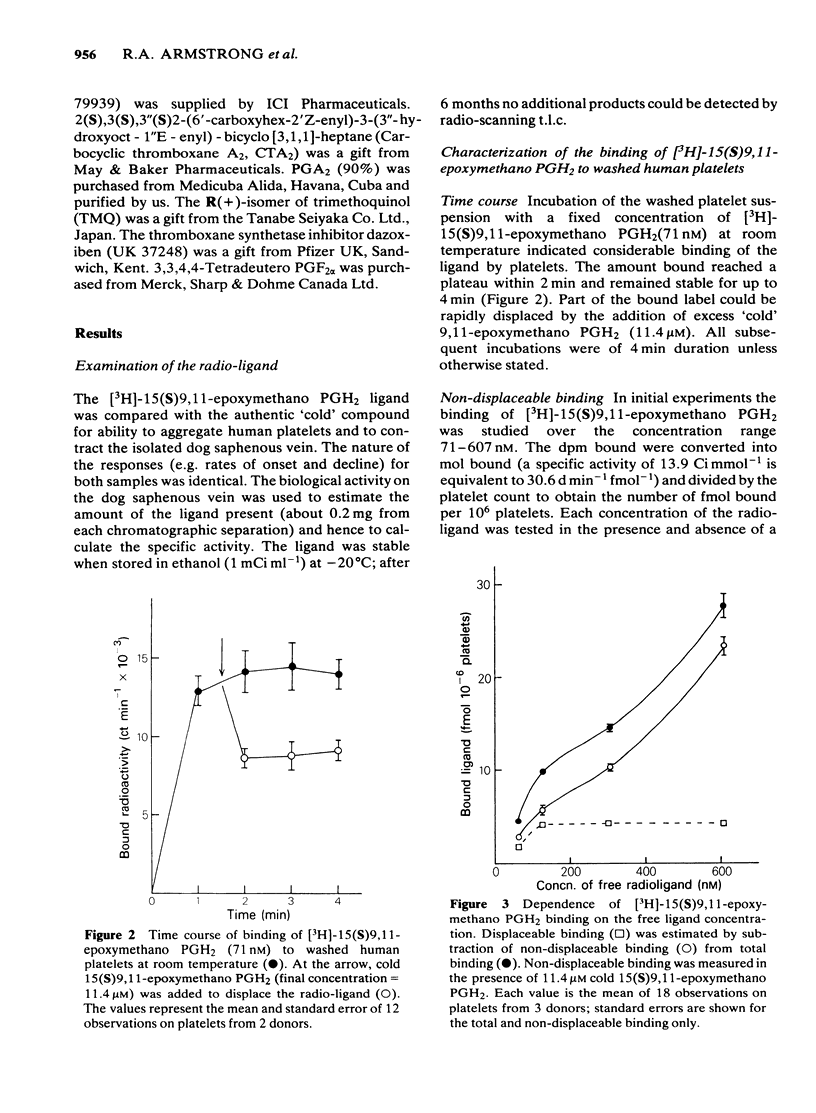
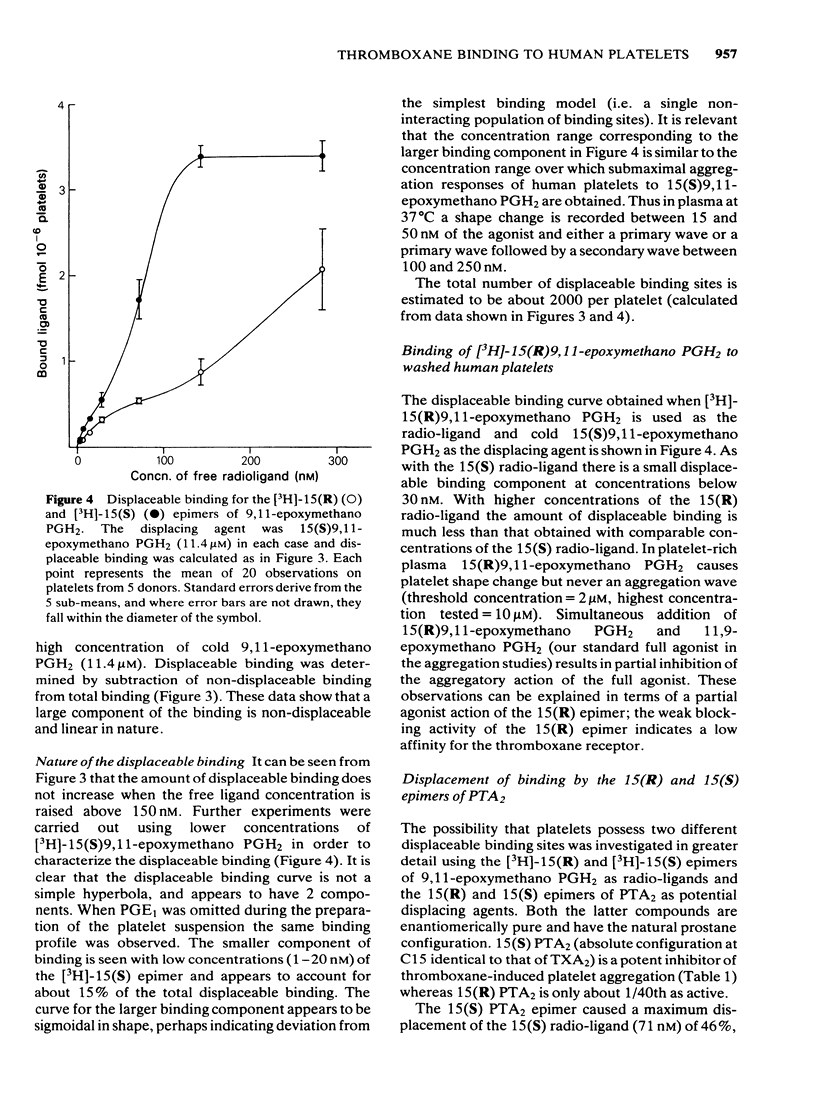
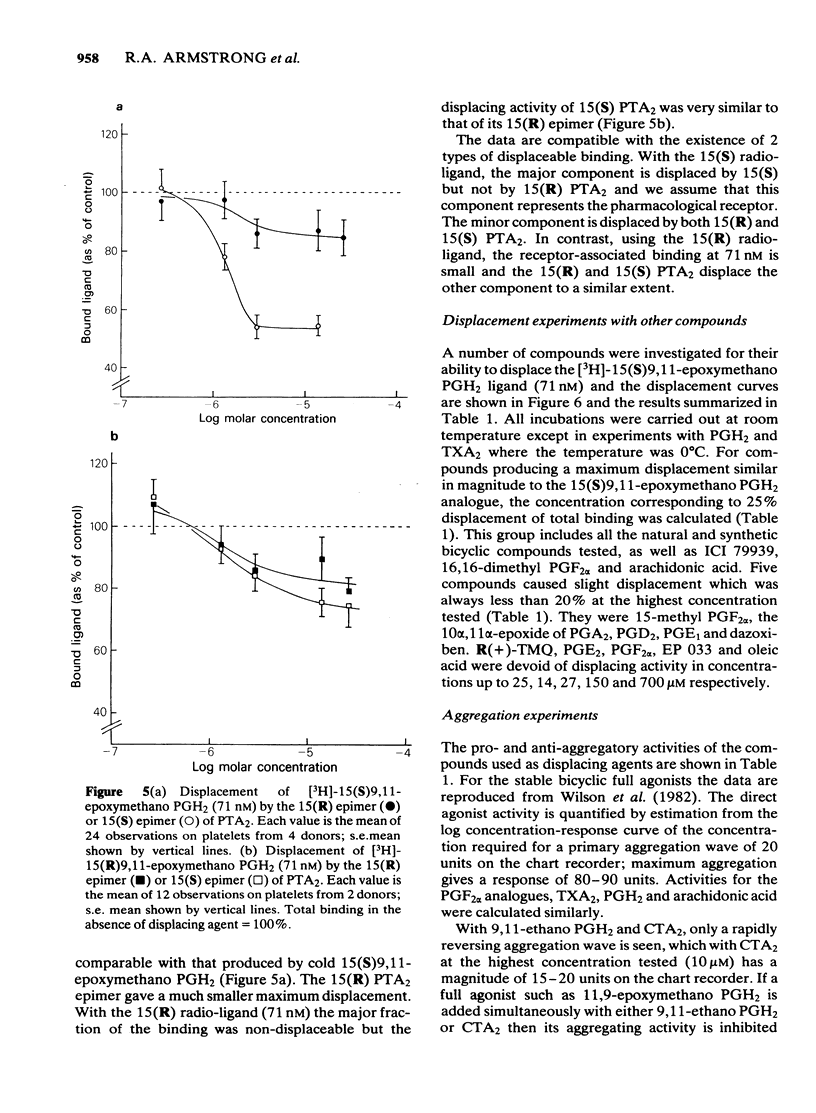
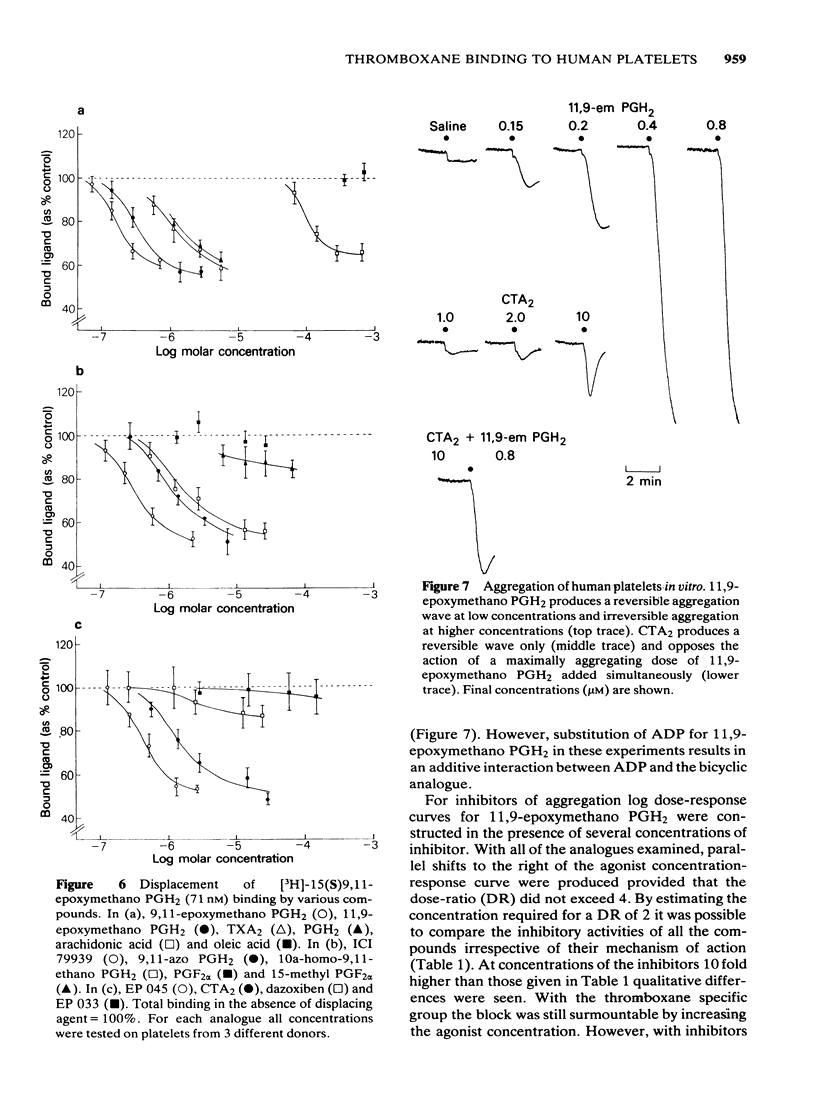
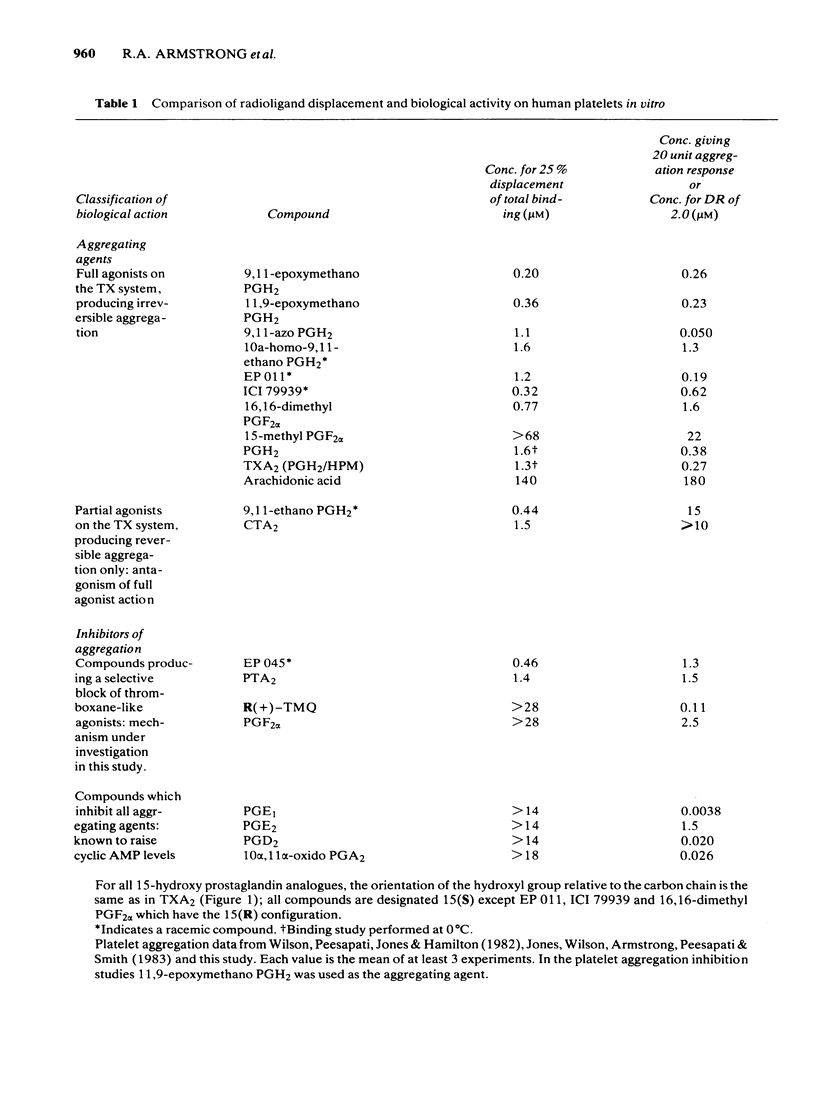
The preparation of enantiomerically pure [3H]-15 (S) 9, 11-epoxymethano PGH2 (a thromboxane A2-like agonist) has enabled the binding of ligands to the thromboxane receptor of the human platelet to be studied. The binding of the radio-ligand to washed human platelets has 3 components. One component is not displaceable by 'cold' 9, 11-epoxymethano PGH2 and its concentration-binding plot is roughly linear. The other 2 components are displaceable and saturable, and the larger of the two, which is sensitive to the stereochemistry of the C15 secondary alcohol, appears to represent the thromboxane receptor. About 1700 15(S)9, 11-epoxymethano PGH2 molecules are specifically bound to a single platelet and 50% of this binding is achieved with a concentration of 75 nM. Displacement of [3H]-15(S)9, 11-epoxymethano PGH2 is effected by (a) TXA2 and PGH2 and a number of bicyclic stable analogues (e.g. 9,11-azo PGH2), all of which produce irreversible aggregation of human platelets; (b) analogues of PGF2 alpha with potent thromboxane-like activity (e.g. ICI 79939); (c) compounds with partial agonist activity on the platelet thromboxane system (e.g. CTA2); (d) Thromboxane/endoperoxide analogues which specifically antagonize thromboxane-like actions on the human platelet (e.g. PTA2 and EP 045). Displacement is not achieved with the natural prostaglandins PGE2, PGD2 and PGF2 alpha. Neither the thromboxane-synthetase inhibitor dazoxiben nor R(+)-trimethoquinol have high displacing activity. The correlation of radio-ligand displacement with the biological activity of the competing ligands is discussed in relation to the nature of the thromboxane receptor on the human platelet.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar H., Navran S. S., Miller D. D., Feller D. R. Antiplatelet actions of trimetoquinol isomers: evidence for inhibition of a prostaglandin-independent pathway of platelet aggregation. Biochem Pharmacol. 1982 Mar 1;31(5):886–889. doi: 10.1016/0006-2952(82)90482-8. [DOI] [PubMed] [Google Scholar]
- Corey E. J., Narasaka K., Shibasaki M. A direct,stereocontrolled total synthesis of the 9,11-azo analogue of the prostaglandin endoperoxide, PGH2. J Am Chem Soc. 1976 Sep 29;98(20):6417–6418. doi: 10.1021/ja00436a074. [DOI] [PubMed] [Google Scholar]
- Dukes M., Russell W., Walpole A. L. Potent luteolytic agents related to prostaglandin F2alpha. Nature. 1974 Jul 26;250(464):330–331. doi: 10.1038/250330a0. [DOI] [PubMed] [Google Scholar]
- Hamanaka N., Ohuchida S., Hayashi M. Syntheses of thromboxane A2 analogs: Thia- and dithiathromboxane A2. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:319–322. [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3824–3828. doi: 10.1073/pnas.71.10.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung S. C., Ghali N. I., Venton D. L., Le Breton G. C. Prostaglandin F2 alpha antagonizes thromboxane A2-induced human platelet aggregation. Prostaglandins. 1982 Aug;24(2):195–206. doi: 10.1016/0090-6980(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Marr C. G. Actions of 16-aryloxy analogues of prostaglandin F2alpha on preparations responsive to prostaglandin endoperoxides. Br J Pharmacol. 1977 Dec;61(4):694–696. doi: 10.1111/j.1476-5381.1977.tb07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Wilson N. H., Armstrong R. A., Peesapati V., Smith G. M. Effects of thromboxane antagonist EP 045 on platelet aggregation. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:345–350. [PubMed] [Google Scholar]
- Le Breton G. C., Venton D. L., Enke S. E., Halushka P. V. 13-Azaprostanoic acid: a specific antagonist of the human blood platelet thromboxane/endoperoxide receptor. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4097–4101. doi: 10.1073/pnas.76.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer A. M., Smith E. F., 3rd, Araki H., Smith J. B., Aharony D., Claremon D. A., Magolda R. L., Nicolaou K. C. Dissociation of vasoconstrictor and platelet aggregatory activities of thromboxane by carbocyclic thromboxane A2, a stable analog of thromboxane A2. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1706–1710. doi: 10.1073/pnas.77.3.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre D. E., Gordon J. L. Discrimination between platelet prostaglandin receptors with a specific antagonist of bisenoic prostaglandins. Thromb Res. 1977 Dec;11(6):705–713. doi: 10.1016/0049-3848(77)90099-8. [DOI] [PubMed] [Google Scholar]
- Mayo J. R., Navran S. S., Huzoor-Akbar, Miller D. D., Feller D. R. Stereo-dependent inhibition of human platelet function by the optical isomers of trimetoquinol. Biochem Pharmacol. 1981 Aug 15;30(16):2237–2241. doi: 10.1016/0006-2952(81)90093-9. [DOI] [PubMed] [Google Scholar]
- Moncada S., Needleman P., Bunting S., Vane J. R. Prostaglandin endoperoxide and thromboxane generating systems and their selective inhibition. Prostaglandins. 1976 Sep;12(3):323–335. doi: 10.1016/0090-6980(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Needleman P., Minkes M., Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976 Jul 9;193(4248):163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- Needleman P., Moncada S., Bunting S., Vane J. R., Hamberg M., Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature. 1976 Jun 17;261(5561):558–560. doi: 10.1038/261558a0. [DOI] [PubMed] [Google Scholar]
- Nichol L. W., Jackson W. J., Winzor D. J. A theoretical study of the binding of small molecules to a polymerizing protein system. A model for allosteric effects. Biochemistry. 1967 Aug;6(8):2449–2456. doi: 10.1021/bi00860a022. [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C., Magolda R. L., Smith J. B., Aharony D., Smith E. F., Lefer A. M. Synthesis and biological properties of pinane-thromboxane A2, a selective inhibitor of coronary artery constriction, platelet aggregation, and thromboxane formation. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2566–2570. doi: 10.1073/pnas.76.6.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shio H., Ramwell P. Effect of prostaglandin E 2 and aspirin on the secondary aggregation of human platelets. Nat New Biol. 1972 Mar 15;236(63):45–46. doi: 10.1038/newbio236045a0. [DOI] [PubMed] [Google Scholar]
- Svensson J., Hamberg M., Samuelsson B. Prostaglandin endoperoxides IX. Characterization of rabbit aorta contracting substance (RCS) from guinea pig lung and human platelets. Acta Physiol Scand. 1975 Jun;94(2):222–228. doi: 10.1111/j.1748-1716.1975.tb05881.x. [DOI] [PubMed] [Google Scholar]
- Willis A. L., Vane F. M., Kuhn D. C., Scott C. G., Petrin M. An endoperoxide aggregator (Lass), formed in platelets in response to thrombotic stimuli: purification, identification and unique biological significance. Prostaglandins. 1974 Dec 25;8(6):453–507. doi: 10.1016/0090-6980(74)90062-8. [DOI] [PubMed] [Google Scholar]
- Wilson N. H., Peesapati V., Jones R. L., Hamilton K. Synthesis of prostanoids with bicyclo[2.2.1]heptane, bicyclo[3.1.1]heptane, and bicyclo[2.2.2]octane ring systems. Activities of 15-hydroxy epimers on human platelets. J Med Chem. 1982 May;25(5):495–500. doi: 10.1021/jm00347a004. [DOI] [PubMed] [Google Scholar]



