Abstract
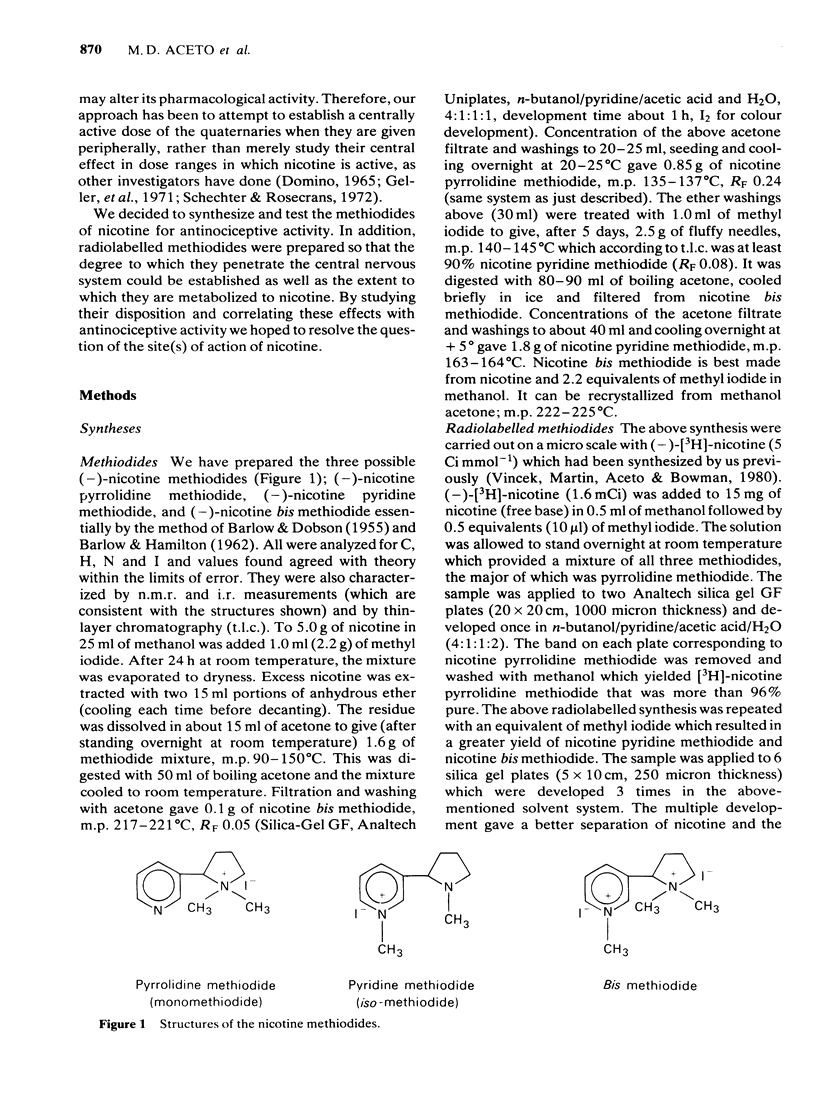
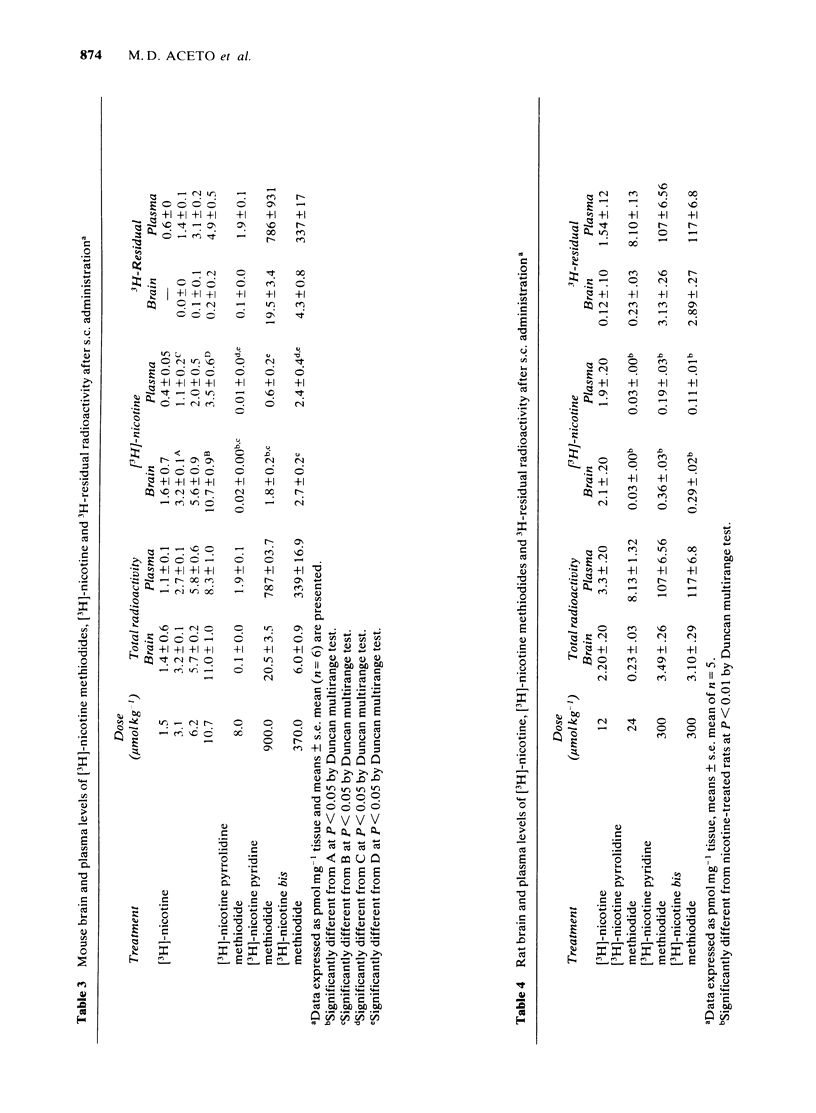
Three quaternary methiodides of nicotine were prepared and tested for antinociceptive activity in the mouse tail-flick, mouse phenylquinone and rat tail-flick tests. Following peripheral administration, all three methiodides were inactive in the mouse and rat tail-flick procedures, whereas nicotine was active in both tests, which suggested that nicotine was acting centrally. Quaternization of nicotine did not eliminate antinociceptive activity as demonstrated by the intraventricular injection of the methiodides in mice. Nicotine pyrrolidine and bis methiodides were somewhat more potent than nicotine, whereas nicotine pyridine methiodide was considerably less potent than nicotine in the tail-flick procedure. Systemically administered nicotine pyrrolidine methiodine was approximately one-third as active as nicotine in the mouse phenylquinone test; nicotine pyridine methiodide and nicotine bis methiodide were 100 and 300 times less active, respectively. Hexamethonium partially blocked nicotine and nicotine pyrrolidine methiodide, whereas mecamylamine blocked nicotine completely but nicotine pyrrolidine methiodide partially. Nicotine may have both central and peripheral actions in the mouse phenylquinone test, whereas nicotine pyrrolidine methiodide may have both nicotine and non-nicotine like antinociceptive activity. The radiolabelled methiodides were synthesized and their disposition in body tissues studied. The methiodides were found to penetrate brain poorly (plasma-to-brain ratios greater than 20). The methiodides were metabolized to nicotine to a small extent. This metabolism occurred to a greater extent in mice than in rats.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW R. B., DOBSON N. A. Nicotine monomethiodide. J Pharm Pharmacol. 1955 Jan;7(1):27–34. doi: 10.1111/j.2042-7158.1955.tb12000.x. [DOI] [PubMed] [Google Scholar]
- Dewey W. L., Harris L. S., Howes J. F., Nuite J. A. The effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and phenylquinone tests. J Pharmacol Exp Ther. 1970 Nov;175(2):435–442. [PubMed] [Google Scholar]
- GILLIS C. N., LEWIS J. J. The pharmacology of nicotine monomethiodide. J Pharm Pharmacol. 1956 Jan;8(1):46–54. doi: 10.1111/j.2042-7158.1956.tb12130.x. [DOI] [PubMed] [Google Scholar]
- HENDERSHOT L. C., FORSAITH J. Antagonism of the frequency of phenylquinone-induced writhing in the mouse by weak analgesics and nonanalgesics. J Pharmacol Exp Ther. 1959 Mar;125(3):237–240. [PubMed] [Google Scholar]
- MCKENNIS H., Jr, BOWMAN E. R., HORVATH A., BEDERKA J. P., Jr METABOLIC RELEASE OF METHYL GROUPS FROM A SERIES OF N-METHYLPYRIDINIUM COMPOUNDS. Nature. 1964 May 16;202:699–700. doi: 10.1038/202699a0. [DOI] [PubMed] [Google Scholar]
- Mansner R. Relation between some central effects of nicotine and its brain levels in the mouse. Ann Med Exp Biol Fenn. 1972;50(4):205–212. [PubMed] [Google Scholar]
- Pearl J., Aceto M. D., Harris L. S. Prevention of writhing and other effects of narcotics and narcotic antagonists in mice. J Pharmacol Exp Ther. 1968 Mar;160(1):217–230. [PubMed] [Google Scholar]
- Pedigo N. W., Dewey W. L., Harris L. S. Determination and characterization of the antinociceptive activity of intraventricularly administered acetylcholine in mice. J Pharmacol Exp Ther. 1975 Jun;193(3):845–852. [PubMed] [Google Scholar]
- Phan D. V., Dóda M., Bite A., György L. Antin ociceptive activity of nicotine. Acta Physiol Acad Sci Hung. 1973;44(1):85–93. [PubMed] [Google Scholar]
- Sahley T. L., Berntson G. G. Antinociceptive effects of central and systemic administrations of nicotine in the rat. Psychopharmacology (Berl) 1979 Nov;65(3):279–283. doi: 10.1007/BF00492216. [DOI] [PubMed] [Google Scholar]
- Schechter M. D., Rosecrans J. A. Nicotine as a discriminative cue in rats: inability of related drugs to produce a nicotine-like cueing effect. Psychopharmacologia. 1972;27(4):379–387. doi: 10.1007/BF00429392. [DOI] [PubMed] [Google Scholar]
- Thompson J. H., Angulo M., Choi L., Roch M., Jenden D. J. The chronic effects of nicotine monomethiodide on gastric secretion in pylorus-ligated rats. Experientia. 1972 Oct 15;28(10):1176–1177. doi: 10.1007/BF01946153. [DOI] [PubMed] [Google Scholar]
- Tripathi H. L., Martin B. R., Aceto M. D. Nicotine-induced antinociception in rats and mice: correlation with nicotine brain levels. J Pharmacol Exp Ther. 1982 Apr;221(1):91–96. [PubMed] [Google Scholar]
- Tsujimoto A., Nakashima T., Tanino S., Dohi T., Kurogochi Y. Tissue distribution of ['H]nicotine in dogs and rhesus monkeys. Toxicol Appl Pharmacol. 1975 Apr;32(1):21–31. doi: 10.1016/0041-008x(75)90191-x. [DOI] [PubMed] [Google Scholar]
- Vincek W. C., Martin B. R., Aceto M. D., Bowman E. R. Synthesis and preliminary binding studies of 4,4-ditritio-(-)-nicotine of high specific activity. J Med Chem. 1980 Aug;23(8):960–962. doi: 10.1021/jm00182a028. [DOI] [PubMed] [Google Scholar]


