Abstract
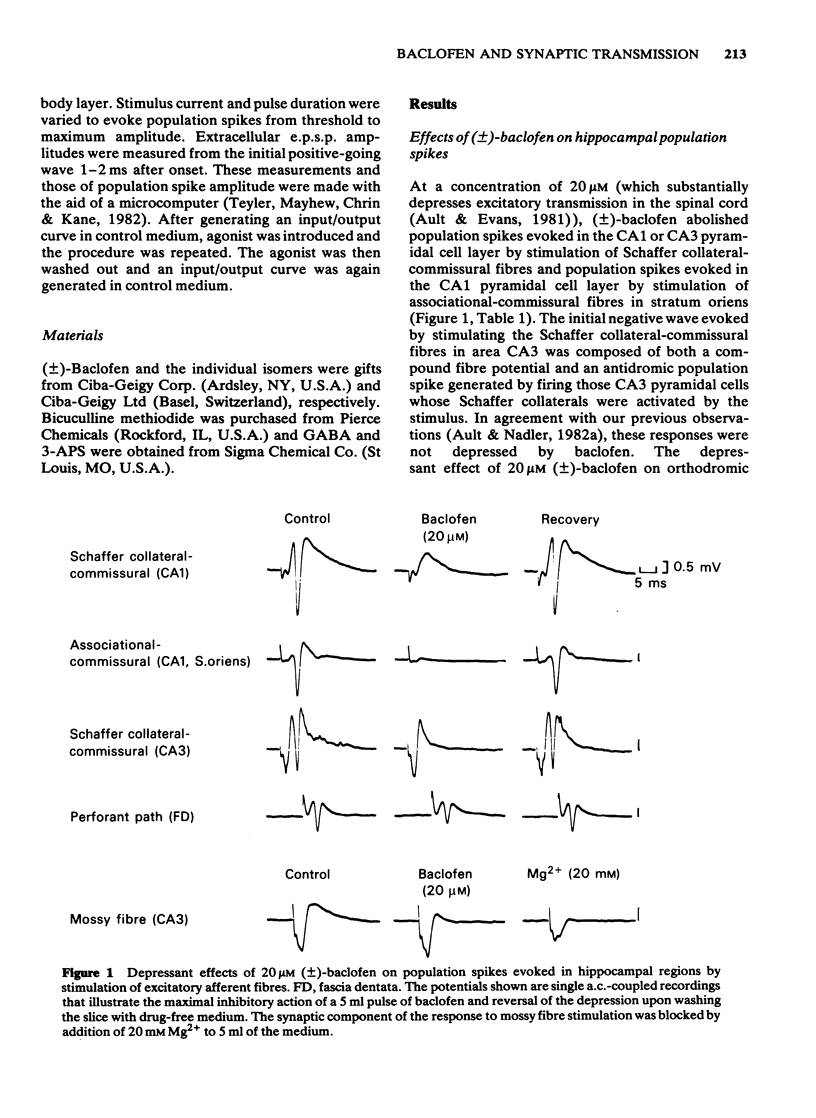
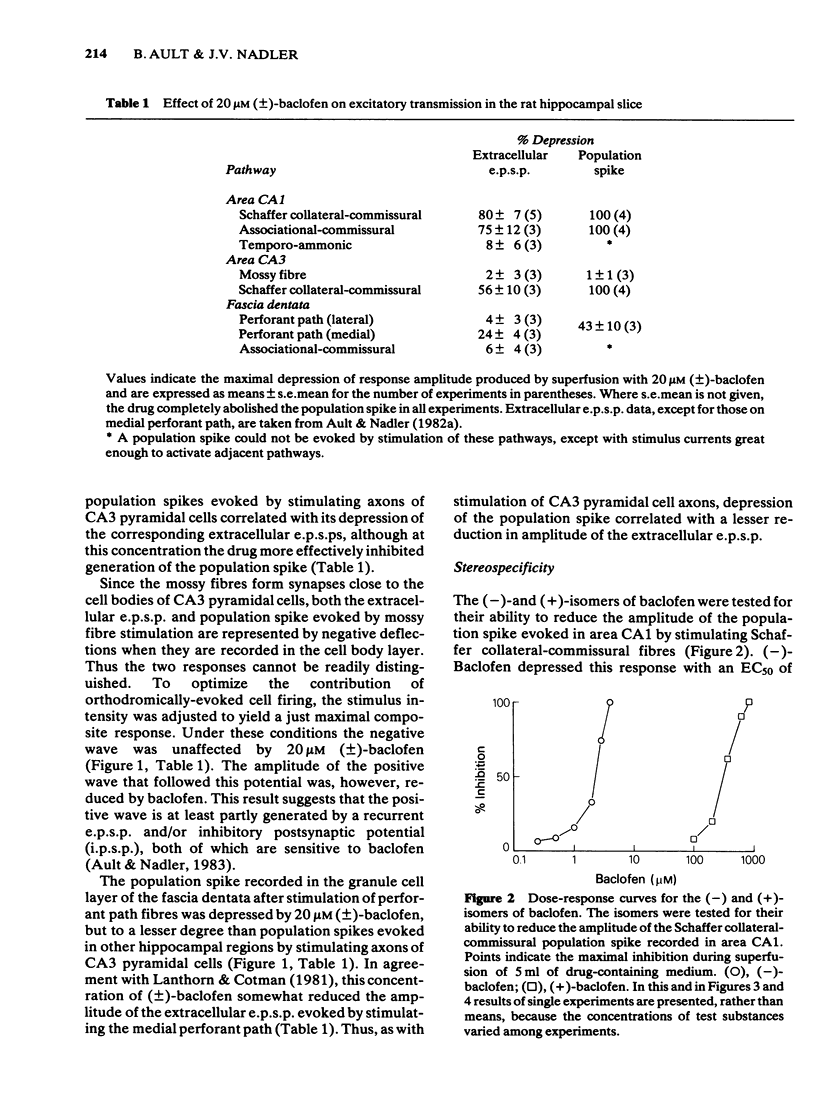
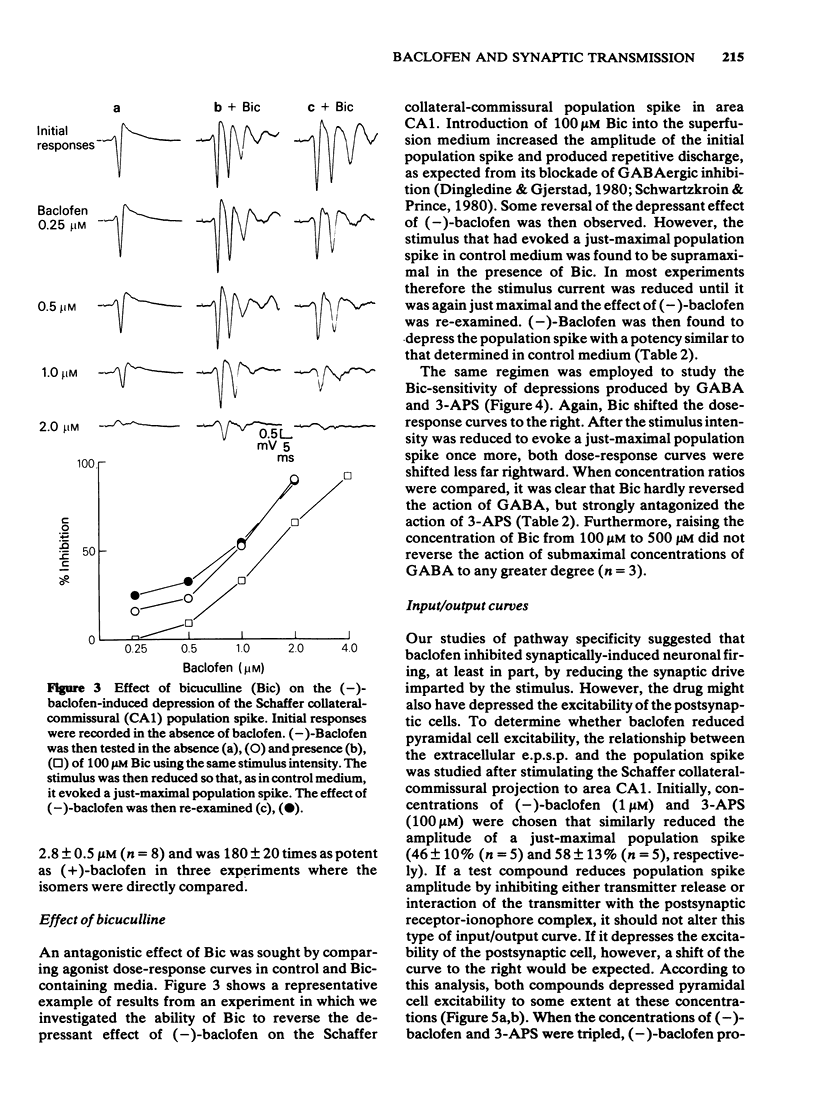
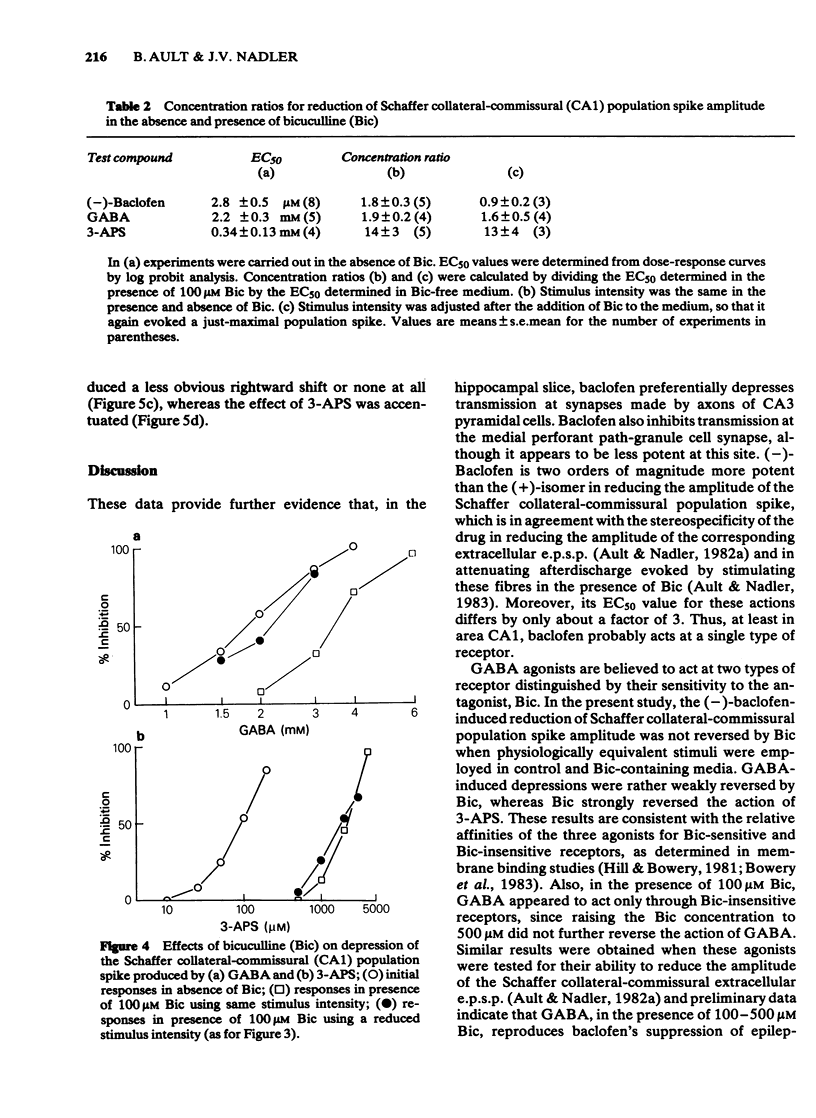
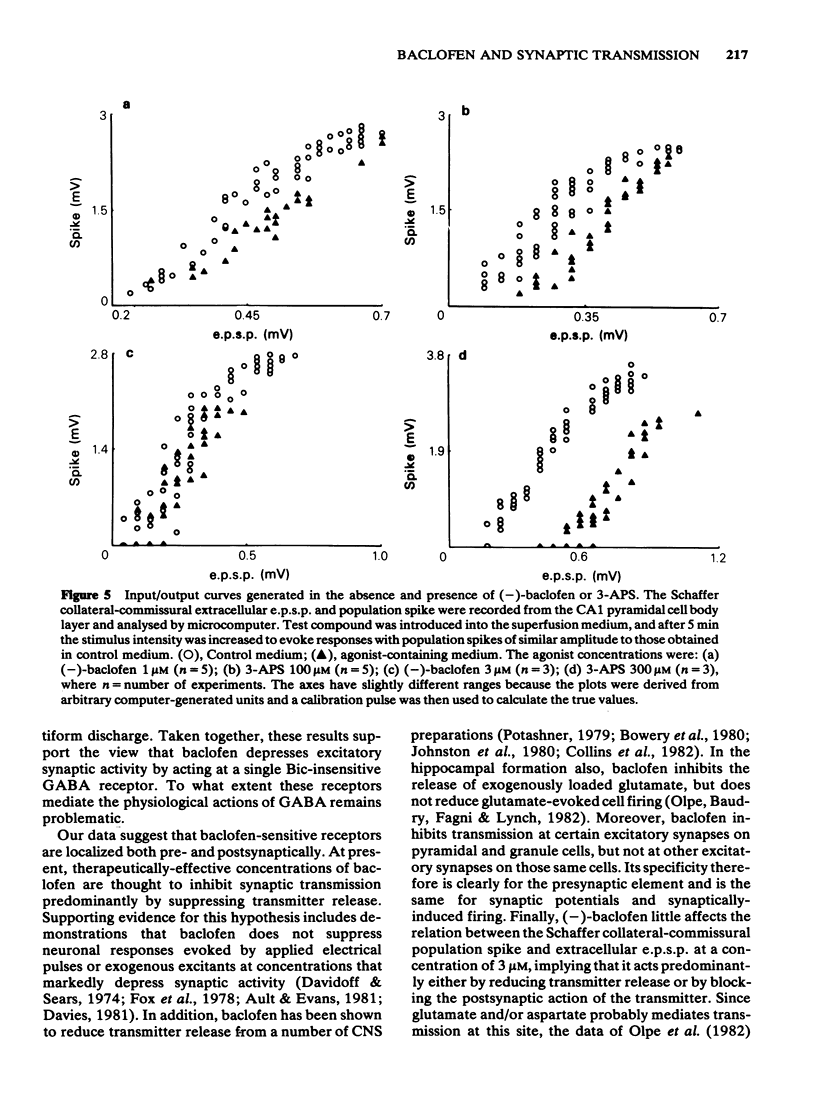
The effects of baclofen on the synaptically-induced firing of pyramidal and granule cell populations were tested in the rat hippocampal slice. Population spikes were evoked by stimulating excitatory pathways in the presence and absence of bath-applied drug. (+/-)-Baclofen (20 microM) completely blocked the firing of CA1 or CA3 hippocampal pyramidal cells subsequent to stimulation of projections that originate in area CA3. In contrast, the firing of dentate granule cells evoked by stimulation of the perforant path fibres was depressed by only 46% and baclofen did not affect the monosynaptic firing of CA3 pyramidal cells evoked by mossy fibre stimulation. These results are consistent with the effects of baclofen on the corresponding extracellularly-recorded excitatory postsynaptic potentials (e.p.s.ps). The Schaffer collateral-commissural population spike in area CA1 was depressed by (-)-baclofen (EC50 = 2.8 microM), GABA (EC50 = 2.2 mM) and 3-aminopropanesulphonic acid (3-APS) (EC50 = 0.34 mM). (-)-Baclofen was 180 times as potent as (+)-baclofen. Bicuculline methiodide (100 microM) did not reverse the depressant action of (-)-baclofen. GABA-induced depressions were antagonized to only a small degree, whilst the effect of 3-APS was readily reversed. Raising the concentration of bicuculline from 100 microM to 500 microM did not further reverse the action of GABA. The effects of (-)-baclofen and 3-APS on the relationship between extracellular e.p.s.p. and population spike were tested by stimulation of the Schaffer collateral-commissural fibres in area CA1. (-)-Baclofen shifted the 'input/output' curve to the right at a concentration of 1 microM, but less or not at all at 3 microM. In contrast, increasing the concentration of 3-APS shifted this curve farther to the right.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Bliss T. V., Skrede K. K. Unit analysis of hippocampal polulation spikes. Exp Brain Res. 1971;13(2):208–221. doi: 10.1007/BF00234086. [DOI] [PubMed] [Google Scholar]
- Ault B., Evans R. H. The depressant action of baclofen on the isolated spinal cord of the neonatal rat. Eur J Pharmacol. 1981 May 22;71(4):357–364. doi: 10.1016/0014-2999(81)90179-5. [DOI] [PubMed] [Google Scholar]
- Ault B., Nadler J. V. Anticonvulsant-like actions of baclofen in the rat hippocampal slice. Br J Pharmacol. 1983 Apr;78(4):701–708. doi: 10.1111/j.1476-5381.1983.tb09423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault B., Nadler J. V. Baclofen selectively inhibits transmission at synapses made by axons of CA3 pyramidal cells in the hippocampal slice. J Pharmacol Exp Ther. 1982 Nov;223(2):291–297. [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol. 1983 Jan;78(1):191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Collins G. G., Anson J., Kelly E. P. Baclofen: effects on evoked field potentials and amino acid neurotransmitter release in the rat olfactory cortex slice. Brain Res. 1982 Apr 29;238(2):371–383. doi: 10.1016/0006-8993(82)90111-1. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D., Bornstein J. C., Peet M. J. Selective effects of (-)-baclofen on spinal synaptic transmission in the cat. Exp Brain Res. 1981;42(2):158–170. doi: 10.1007/BF00236902. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A., Sears E. S. The effects of Lioresal on synaptic activity in the isolated spinal cord. Neurology. 1974 Oct;24(10):957–963. doi: 10.1212/wnl.24.10.957. [DOI] [PubMed] [Google Scholar]
- Davies J. Selective depression of synaptic excitation in cat spinal neurones by baclofen: an iontophoretic study. Br J Pharmacol. 1981 Feb;72(2):373–384. doi: 10.1111/j.1476-5381.1981.tb09137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. The action of beta-phenyl-GABA derivatives on neurones of the cat cerebral cortex. Brain Res. 1974 Apr 26;70(3):501–505. doi: 10.1016/0006-8993(74)90258-3. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Gjerstad L. Reduced inhibition during epileptiform activity in the in vitro hippocampal slice. J Physiol. 1980 Aug;305:297–313. doi: 10.1113/jphysiol.1980.sp013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S., Krnjević K., Morris M. E., Puil E., Werman R. Action of baclofen on mammalian synaptic transmission. Neuroscience. 1978;3(6):495–515. doi: 10.1016/0306-4522(78)90016-7. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G., Haas H. L. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature. 1982 Dec 2;300(5891):448–450. doi: 10.1038/300448a0. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Hailstone M. H., Freeman C. G. Baclofen: stereoselective inhibition of excitant amino acid release. J Pharm Pharmacol. 1980 Mar;32(3):230–231. doi: 10.1111/j.2042-7158.1980.tb12902.x. [DOI] [PubMed] [Google Scholar]
- Lanthorn T. H., Cotman C. W. Baclofen selectively inhibits excitatory synaptic transmission in the hippocampus. Brain Res. 1981 Nov 23;225(1):171–178. doi: 10.1016/0006-8993(81)90326-7. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L. Evidence for two physiologically distinct perforant pathways to the fascia dentata. Brain Res. 1980 Oct 13;199(1):1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Baudry M., Fagni L., Lynch G. The blocking action of baclofen on excitatory transmission in the rat hippocampal slice. J Neurosci. 1982 Jun;2(6):698–703. doi: 10.1523/JNEUROSCI.02-06-00698.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpe H. R., Koella W. P., Wolf P., Haas H. L. The action of baclofen on neurons of the substantia nigra and of the ventral tegmental area. Brain Res. 1977 Oct 14;134(3):577–580. doi: 10.1016/0006-8993(77)90834-4. [DOI] [PubMed] [Google Scholar]
- Ono H., Fukuda H., Kudo Y. Mechanisms of depressant action of baclofen on the spinal reflex in the rat. Neuropharmacology. 1979 Aug-Sep;18(8-9):647–653. doi: 10.1016/0028-3908(79)90030-3. [DOI] [PubMed] [Google Scholar]
- Pierau F. K., Zimmermann P. Action of a GABA-derivative on postsynaptic potentials and membrane properties of cats' spinal motoneurones. Brain Res. 1973 May 17;54:376–380. doi: 10.1016/0006-8993(73)90064-4. [DOI] [PubMed] [Google Scholar]
- Potashner S. J. Baclofen: effects on amino acid release and metabolism in slices of guinea pig cerebral cortex. J Neurochem. 1979 Jan;32(1):103–109. doi: 10.1111/j.1471-4159.1979.tb04516.x. [DOI] [PubMed] [Google Scholar]
- Saito K., Konishi S., Otsuka M. Antagonism between Lioresal and substance P in rat spinal cord. Brain Res. 1975 Oct 24;97(1):177–180. doi: 10.1016/0006-8993(75)90928-2. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A. Further characteristics of hippocampal CA1 cells in vitro. Brain Res. 1977 Jun 3;128(1):53–68. doi: 10.1016/0006-8993(77)90235-9. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Prince D. A. Changes in excitatory and inhibitory synaptic potentials leading to epileptogenic activity. Brain Res. 1980 Feb 3;183(1):61–76. doi: 10.1016/0006-8993(80)90119-5. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Slawsky M. Probable calcium spikes in hippocampal neurons. Brain Res. 1977 Oct 21;135(1):157–161. doi: 10.1016/0006-8993(77)91060-5. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Wyler A. R. Mechanisms underlying epileptiform burst discharge. Ann Neurol. 1980 Feb;7(2):95–107. doi: 10.1002/ana.410070202. [DOI] [PubMed] [Google Scholar]
- Taylor C. P., Dudek F. E. Synchronous neural afterdischarges in rat hippocampal slices without active chemical synapses. Science. 1982 Nov 19;218(4574):810–812. doi: 10.1126/science.7134978. [DOI] [PubMed] [Google Scholar]
- Teyler T. J., Mayhew W., Chrin C., Kane J. Neurophysiological field potential analysis by microcomputer. J Neurosci Methods. 1982 Mar;5(3):291–303. doi: 10.1016/0165-0270(82)90081-4. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- White W. F., Nadler J. V., Cotman C. W. A perfusion chamber for the study of CNS physiology and pharmacology in vitro. Brain Res. 1978 Sep 8;152(3):591–596. doi: 10.1016/0006-8993(78)91115-0. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A., Basbaum A. I. Intradendritic recordings from hippocampal neurons. Proc Natl Acad Sci U S A. 1979 Feb;76(2):986–990. doi: 10.1073/pnas.76.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A. Participation of calcium spikes during intrinsic burst firing in hippocampal neurons. Brain Res. 1978 Dec 29;159(2):385–390. doi: 10.1016/0006-8993(78)90544-9. [DOI] [PubMed] [Google Scholar]


