Abstract
Public concern about the presence of natural and anthropogenic compounds which affect human health by modulating normal endocrine functions is continuously growing. Fast and simple high-throughput screening methods for the detection of hormone activities are thus indispensable. During the last two decades, a panel of different in vitro assays has been developed, mainly for compounds with an estrogenic mode of action. Here we describe the development of an androgen transcription activation assay that is easy to use in routine screening. Recombinant yeast cells were constructed that express the human androgen receptor and yeast enhanced green fluorescent protein (yEGFP), the latter in response to androgens. Compared with other reporters, the yEGFP reporter protein is very convenient because it is directly measurable in intact living cells, i.e., cell wall disruption and the addition of a substrate are not needed. When yeast was exposed to 17β-testosterone, the concentration where half-maximal activation is reached (EC50) was 50 nM. The relative androgenic potencies, defined as the ratio between the EC50 of 17β-testosterone and the EC50 of the compound, of 5α-dihydrotestosterone, methyltrienolone, and 17β-boldenone are 2.3, 1.4, and 0.15 respectively. The results presented in this paper demonstrate that this new yeast androgen bioassay is fast, sensitive, and very specific and also suited to detect compounds that have an antiandrogenic mode of action.
Keywords: Antagonists, Brominated flame retardants, Crosstalk, Metabolism, Receptor, Saccharomyces cerevisiae
Introduction
There is concern that chemicals in our food, water, and environment affect human health by disrupting normal endocrine function, possibly leading to reproductive failure in humans and tumors in sensitive tissues [1, 2]. This relates to chemicals with previously unknown hormonal properties, like certain pesticides and plasticizers, but also to compounds used in pharmaceutical preparations, eg., oral contraceptives and tablets for hormone-replacement therapy, the endogenous steroids excreted in urine of man and domestic animals and potentially also compounds used for their growth-promoting properties in animals. Of all endocrine disruptors, environmental estrogens are the most studied [3]. However, recent studies show a crucial involvement of the androgen receptor in abnormal sex development. The presence of pollutants with adverse effects on human androgen receptor (hAR) has been reported from paper-mill effluents and as a result of intensive farming [4, 5]. Xenoandrogenic exposure-related disorders include testicular cancer, hypospadias, cryptorchidism, and poor sperm and very recently prepubertal gynecomastia was linked to both estrogenic and antiandrogenic effects of lavender and tea tree oil [6].
Chemical and immunological methods are commonly used to detect steroid hormones in food, clinical practice, environmental samples, or doping control. Owing to the great variety of chemicals with hormonelike activity, these methods have the drawback that they only quantify the compound of interest and are not able to determine biological activity of unknown compounds and their metabolites, this in contrast to biological assays. Receptor-based transcription activation assays can be used to detect all compounds having affinity for a given receptor [7, 8]. In contrast to receptor binding assays, receptor gene bioassays also include the transactivation step and can distinguish between receptor agonists and receptor antagonists [9]. This feature is very helpful in detecting both known and unknown compounds.
Several assays have been developed for this purpose, using both mammalian and yeast cells. In general, transcription activation assays based on mammalian, or more particular human, cell lines have been shown to be more sensitive than yeast-based assays, and may be able to identify compounds that require human metabolism for activation into their active state. Metabolic conversion can either activate or inactivate some compounds [10], whereas the relatively low metabolic capacity of yeast ensures that the test reflects the activity of the original compound. In addition, yeast-based assays have several other advantages. These include low costs, easy handling, lack of known endogenous receptors that may compete with the receptor activity under investigation (no crosstalk), and the use of media that are devoid of steroids [11–13]. Furthermore, yeast cell assays are extremely robust and survive extracts from dirty sample matrices such as sediments, urine, and feed [14–16]. Especially in the case of androgens, the lack of known endogenous receptors in yeast is a great advantage compared with mammalian cell lines, as androgen responsive elements (AREs) can also be activated by the progesterone receptor and the glucocorticoid receptor. To avoid potential crosstalk in mammalian cell lines, a lot of efforts was expended to construct an ARE that is specific and no longer inducible by the progesterone and glucocorticoid receptor [17–19]. However, up till now such an ARE does not exist and it is doubtful whether it will be found, as the consensus progesterone responsive element/glucocorticoid responsive element is equal to the consensus ARE. Moreover, the glucocorticoid receptor is normally expressed in all mammalian cell types. So far this has resulted in cell lines that are not specific for androgens and that also respond to gestagens or glucocorticoids [20–22].
This paper reports the development of a new yeast androgen bioassay by creating a stably transfected yeast strain that expresses yeast enhanced green fluorescent protein (yEGFP) as a measurable reporter protein in response to androgens. The lack of known endogenous receptors in yeast enabled us to use the strong nonspecific consensus ARE sequence, which is actually a common hormone responsive element that is recognized by the androgen, progesterone and glucocorticoid receptors and can therefore not be used in mammalian cell lines expressing more than one of these receptors. Exposures to 17β-testosterone, 17β-estradiol, progesterone, dexamethasone, and other compounds were performed in 96-well plates in order to demonstrate the suitability and specificity of this new yeast androgen bioassay. Additionally, flutamide and several brominated flame retardants were tested for their antagonistic mode of action and the results were compared with a yeast androgen bioassay expressing β-galactosidase as a reporter protein.
Materials and methods
Chemicals
Chemicals and methods to prepare the growth media, to perform PCR, to isolate DNA, and to transform bacteria and yeast were as described earlier [23]. Corticosterone, dexamethasone, 17α-estradiol, 17β-estradiol, estrone, flutamide, 4-hydroxytamoxifen, medroxyprogesterone 17-acetate, and progesterone were obtained from Sigma (St. Louis, MO, USA). The following compounds were obtained from Steraloids (Newport, RI, USA): 17β-boldenone, diethylstilbestrol, 5α-dihydrotestosterone, 17α-ethynylestradiol, 17β-testosterone and 17β-trenbolone. Tetrahydrogestrinone (THG) was a gift from M. Thevis (DSHS, Cologne, Germany). Copper sulfate and dimethyl sulfoxide (DMSO) were obtained from Merck (Darmstadt, Germany) and methyltrienolone was obtained from PerkinElmer (USA). All restriction endonucleases and corresponding buffers were obtained from New England Biolabs (Hitchin, UK) and the yeast β-galactosidase assay kit was from Pierce Biotechnology (Rockford, IL, USA). 2,4,6-Tribromophenol (TBP), BDE-39, and the hydroxyl derivative 4-OH-BDE-17 were synthesized at the Wallenberg Laboratory (Stockholm University, Sweden).
Yeast strains
The yeast Saccharomyces cerevisiae (CEN.PK 102-5B, K20, URA3−, HIS3−, LEU−) host strain was a gift from H. Silljé (University of Utrecht, The Netherlands). The yeast androgen bioassay with β-galactosidase as a marker was kindly provided by D.P. McDonnell (Duke University, USA).
Plasmids
The p403-GPD and p406-CYC1 yeast expression vectors were obtained from the American Type Culture Collection (ATCC, Rockville, Maryland, USA). The pyEGFP3 plasmid was a gift from A.J. Brown (Stanford University, USA).
Construction of the p403-GPD-hAR expression vector
The yeast cells provided by McDonnell were grown overnight and chromosomal DNA was isolated. This DNA was used to serve as a template for the PCR to obtain the complementary DNA (cDNA) of hAR. Full-length hAR cDNA was obtained using the Expand High Fidelity PCR system (Boehringer Mannheim) and an Eppendorf Mastercycler gradient. The sequence of the 5′-primer was 5′-GCTCTAGAATGGAAGTGCAGTTAGGGCTGGG-3′, containing a restriction site for XbaI just before the ATG start codon. The sequence of the 3′-primer was 5′-GCGGATCCTCACTGGGTGTGGAAATAGATGGG-3′, containing a restriction site for BamHI just after the TGA stop codon. This PCR generated a full-length double-stranded (ds) cDNA of 2,763 bp of the hAR gene with a 5′-XbaI and a 3′-BamHI restriction site.
The 2,763-bp full-length hAR PCR product was isolated from a 1% low-melt agarose gel, cleaved with XbaI and BamHI and ligated into the corresponding site of the p403-GPD yeast vector. Plasmid digestion and PCR controls revealed several good clones.
Construction of the p406-ARE2-CYC1-yEGFP reporter vector
A set of complementary oligonucleotides (a and b), each with two consensus ARE sequences (in bold), were synthesized. A solution with both cDNA oligonucleotides, 2.5 μM of each, was heated at 95 °C and cooled down to room temperature in 2 h. This set gave a ds DNA with a 5′-SacI sticky end and a 3′-MscI blunt end.Sa: 5′-AAAGTCAGAACAGCATGTTCTGATCAAATCTAGAAGATCCAAAGTCAGAACAGCATGTTCTGATCAAACTCGAGCAGATCCGCCAGGCGTGTATATATAGCGTGGATGG-3′Sb: 5′-CCATCCACGCTATATATACACGCCTGGCGGATCTGCTCGAGTTTGATCAGAACATGCTGTTCTGACTTTGGATCTTCTAGATTTGATCAGAACATGCTGTTCTGACTTTAGCT-3′.
This ds DNA was cloned into the corresponding site of the p406-CYC1 vector. Subsequently, yEGFP [24] obtained from a HindIII/SalI double digestion of pyEGFP was cloned in the corresponding HindIII/SalI sites of the p406-ARE2-CYC1 reporter construct. Plasmid digestion and PCR controls revealed several good clones.
Transformation of yeast cells
Transformation of yeast K20 host strain (Ura−, His−, and Leu−) was performed by the lithium acetate protocol as described earlier [23]. First, the yeast was transformed with the p406-ARE2-CYC1-yEGFP reporter vector, integrated at the chromosomal location of the uracil gene via homologous recombination. Therefore, prior to transformation, the reporter vector was linearized by cutting with StuI, which has a unique restriction site in the URA3 marker gene. Transformants were grown on minimal medium plates containing l-leucine and l-histidine (MM/L plates). This yeast reporter strain was then transformed with the p403-GPD-hAR expression vector, which was linearized by cleavage with NsiI, which has a unique restriction site in the HIS3 marker gene (histidine). Transformants were grown on MM/L plates and PCR controls were used to select clones that contain the p406-ARE2-CYC1-yEGFP reporter and the p403-GPD-hAR expression construct.
PCR controls
PCR controls were performed on the reporter-receptor transformants. Yeast chromosomal DNA of transformants was isolated and PCR controls were performed. PCR I was performed with a 5′-primer on the backbone of the reporter plasmid and a 3′-primer on the ARE2 sequence. The sequence of the 5′-primer was 5′-AGCGAGTCAGTGAGCGAGGAAG-3′ and the sequence of the 3′-primer was 5′-TGCTGTTCTGACTTTGGATC-3′. PCR II was performed with a 5′-primer on the CYC1 (cytochrome c oxidase) promoter of the reporter plasmid and a 3′-primer on the CYC1 terminator. The sequence of the 5′-primer was 5′-TCTATAGACACACAAACACAA-3′ and the sequence of the 3′-primer was 5′-GGGAGGGCGTGAATGTAAG-3′. PCR III was performed with the primers that were also used to obtain the full length cDNA of the hAR (see “Construction of the p403-GPD-hAR expression vector”).
Streamlined yEGFP assay with the yeast androgen bioassay
The day before running the assay, a single colony from a MM/L agar plate was used to inoculate 10 mL of the selective MM/L medium. This culture was grown overnight at 30 °C with vigorous orbital shaking. At the late log phase, the yeast androgen receptor biosensor was diluted in the selective MM/L medium to an optical density (OD) at 604 nm between 0.08 and 0.12. For exposure, aliquots of 200 μL of this diluted yeast culture were pipetted into each well of a 96-well plate and 2 μL of a 17β-testosterone or other stock solution in DMSO was added. DMSO-only controls were included in each experiment and each sample concentration was assayed in triplicate. Exposure was performed for 24 h at 30 °C and orbital shaking at 125 rpm. Fluorescence was measured at 0 and 24 h directly in a CytoFluor multiwell plate reader (Series 4000, PerSeptive Biosystems) using excitation at 485 nm and measuring emission at 530 nm. The fluorescence signal was corrected with the signals obtained with MM/L containing DMSO solvent only. Densities of the yeast culture were determined by measuring the OD at 630 nm, but this was only done to check whether a sample was toxic for the yeast cells. For the calculation of the relative androgenic potency (RAP) of the compounds in the yeast androgen bioassay, the data of a complete dose–response curve were fitted using the equation 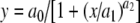 (Slide write Plus, version 6.00). This is equivalent to
(Slide write Plus, version 6.00). This is equivalent to 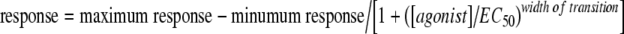 , where response is the measured fluorescence signal, [agonist] is the concentration of the test compound, and EC50 is the concentration of the test compound giving half-maximum response.
, where response is the measured fluorescence signal, [agonist] is the concentration of the test compound, and EC50 is the concentration of the test compound giving half-maximum response.
lacZ-based yeast androgen bioassay: β-galactosidase assay
An agar plate containing the selective growth medium, consisting of a yeast nitrogen base with dextrose (2%), lysine (36 mg/L), tryptophan (48 mg/L), uracil (24 mg/L), and adenine (41 mg/L), was inoculated with the yeast androgen receptor cytosensor from a frozen -80 °C stock (20% glycerol v/v). The plate was incubated at 30 °C for 24–48 h and then stored at 4 °C. The day before running the assay, a single colony of the yeast was used to inoculate 10 mL of the selective growth medium. This culture was grown overnight at 30 °C with vigorous orbital shaking at 225 rpm. At the late log phase, the yeast androgen receptor cytosensor was diluted in growth medium to an OD of 0.06 at 604 nm, and CuSO4 (0.05 mM) was added to induce the expression of the hAR. For exposure in 96-well plates, aliquots of 200 μL of this diluted yeast culture were pipetted into each well and 2 μL of stock solutions in DMSO was added. Exposure was performed for 24 h at 30 °C and 125 rpm, and the β-galactosidase activity was measured with a commercial yeast β-galactosidase assay kit from Pierce (Rockford, IL, USA). This kit uses o-nitrophenyl β-d-galactopyranoside as a substrate, and the solution turns yellow upon hydrolysis of β-d-galactopyranoside to o-nitrophenol and galactose. The yellow o-nitrophenol is measured in a Biotek (Winooski, VT, USA) model ELx 808 series ultra microplate reader at 405 nm. Densities of the yeast culture were determined by measuring the OD at 630 nm. The measured response at 405 nm was corrected for the OD at 630 nm.
Results and discussion
A recombinant yeast cell was constructed that expresses the hAR and yEGFP as a reporter protein in response to androgens. Both the receptor construct as well as the reporter construct were stably integrated into the yeast genome by the use of yeast-integrating plasmids. For the construction of the reporter vector the p406-CYC1 plasmid, containing the URA3 marker gene, was used. Two consensus AREs were placed in the SacI/MscI site of the truncated CYC1 promoter in a way that the −254 to −147 XhoI-SphI part of the CYC1 promoter was restored [23]. High expression levels of the androgen receptor were obtained by placing the cDNA of the hAR gene behind the strong constitutive yeast glyceraldehyde-3-phosphate dehydrogenase (GPD) promoter in the p403-GPD plasmid. This plasmid contains the HIS3 marker gene. Transfected strains were checked with PCR. The correct and specific functioning of the yeast androgen bioassay was studied by exposures to 17β-testosterone and other compounds and the results were compared with results obtained with the lacZ-based yeast androgen bioassay provided by McDonnell. In addition, the antiandrogenic properties of several brominated flame retardants were investigated.
PCR controls
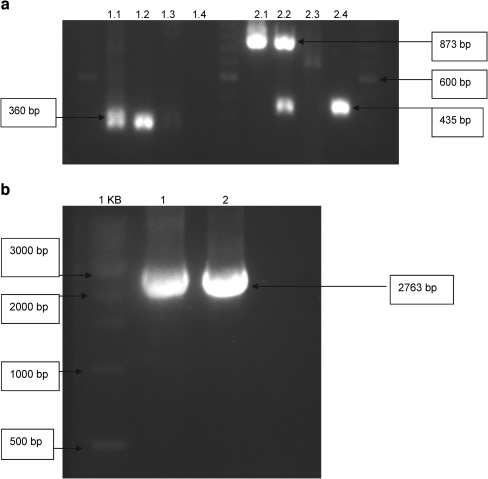
A number of different PCR controls were carried out to check the integration of the vectors into the yeast genome. Figure 1 shows the gel electrophoresis results of these PCR controls. PCR I (Fig. 1a) was performed with primers on the backbone of the p406 plasmid and on the ARE2 sequence. As expected, it gave the specific 360-bp band with the p406-ARE2-CYC1-yEGFP reporter vector and the DNA that was isolated from the yeast androgen biosensor. The negative controls, performed with the empty p406-CYC1 plasmid and with the DNA that was isolated from the empty yeast host (the nontransfected yeast cell), showed no PCR bands. PCR II (Fig. 1a) was performed with primers on the CYC1 promoter and the CYC1 terminator. As expected, it gave the specific 873-bp band with the reporter vector and the DNA that was isolated from the biosensor, because both contain the reporter construct with the yEGFP that was ligated between the CYC1 promoter and CYC1 terminator. The negative controls, performed with the empty p406-CYC1 plasmid and with the DNA that was isolated from the empty yeast host, did not show the reporter-specific 873-bp band. However, this PCR generated a 435-bp band with the DNA of the empty yeast host and the biosensor. This 435-bp band corresponds to the CYC gene of the yeast host itself and is therefore also a specific band. PCR III (Fig. 1b) was performed with the primers on the hAR gene. As expected, it gave the specific 2,763-bp band with the p403-GPD-hAR expression vector and the DNA that was isolated from the biosensor. In the negative control, performed with the DNA that was isolated from the yeast host, the receptor-specific 2,763-bp band was not present. These PCR controls demonstrate that all specific PCR bands can be seen, thus demonstrating that our yeast androgen bioassay contains the p403-GPD-hAR expression vector and the p406-ARE2-CYC1-yEGFP reporter vector, both stably integrated in the yeast genome.
Fig. 1.
PCR controls. The PCR controls were performed as described in “PCR controls.” a Lanes 1, 6 and 11 contain a 100-bp ladder. PCR I was performed with primers on the backbone of the p406 plasmid and on the ARE2 sequence. Lanes 2–5 are PCR I on the p406-ARE2-CYC1-yEGFP reporter vector, the DNA that was isolated from the yeast transformant, the empty p406-CYC1 plasmid, and the DNA that was isolated from the empty yeast host (the nontransfected yeast cells), respectively. PCR II was performed with primers on the CYC1 promoter and the CYC1 terminator. Lanes 7–11 are PCR II on the reporter vector, the DNA from the yeast transformant, the empty p406-CYC1 plasmid, and the DNA from the empty yeast host respectively. b Lane 1 contains a 1-kb ladder. PCR III was performed with the primers on the human androgen receptor gene. Lanes 2–4 are PCR III on the p403-GPD-hAR expression vector, the DNA from the yeast transformant, and the DNA from the empty yeast host, respectively. yEGFP yeast enhanced green fluorescent protein
Dose–response curves obtained with the new yeast androgen bioassay
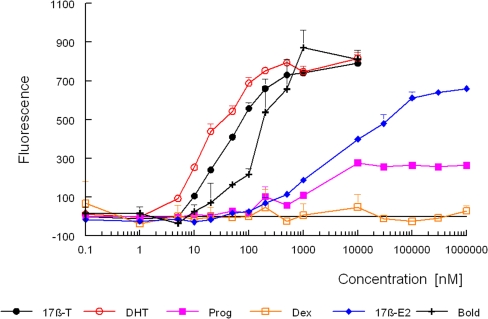
The dose–response curves for several natural and synthetic androgens are shown in Fig. 2. 5α-Dihydrotestosterone, 17β-testosterone and 17β-boldenone caused a dose-related increase in the production of yEGFP, demonstrating that these compounds are potent androgens. The bioassay showed a limit of detection of 3 nM for 5α-dihydrotestosterone with a dynamic range from 3 to 500 nM and very low standard deviations (les than 3%). The figure also shows that 17β-estradiol and progesterone give a response. The female hormone 17β-estradiol gives a full dose–response curve, but the maximum of the curve is reached at a 500 times higher concentration than that of 17β-testosterone and is less steep. Progesterone gives a response, but the maximum response is only about 35% of that of 17β-testosterone and is reached at a 25 times higher concentration. Both 17β-estradiol and progesterone are known to possess androgenic properties. Progesterone displays low binding to the androgen receptor [25] and shows androgenic effects in vivo [26]. According to [27], 17β-estradiol and progesterone showed androgenic activity in ten out of 11 and seven out of nine mammalian cell reporter gene (MCRG) systems, respectively. The corticosteroids corticosterone and dexamethasone showed no response in our assay.
Fig. 2.
Response of the yeast androgen biosensor to different substances. Exposure to 17β-testosterone, 5α-dihydrotestosterone, progesterone, dexamethasone, 17β-estradiol, and 17β-boldenone was started by adding to 200 μL of a yeast culture a 2-μL aliquot of a stock solution of the compound in dimethyl sulfoxide (DMSO). Fluorescence was determined after 24 h as described in “Streamlined yEGFP assay with the yeast androgen bioassay.” Fluorescence signals are the mean of a triplicate with the standard deviation (SD). 17β-T 17β-testosterone, DHT 5α-dihydrotestosterone, Prog progesterone, Dex dexamethasone, 17β-E2 17β-estradiol, Bold 17β-boldenone
Table 1 shows the calculated EC50, ie., the concentration giving a half-maximum response, and the RAP, defined as the ratio between the EC50 of 17β-testosterone and the EC50 of the compound, for several compounds. The yeast androgen bioassay showed good sensitivity towards all androgens tested, with the following range of potencies: 5α-dihydrotestosterone > 17β-trenbolone > methyltrienolone > tetrahydrogestrinone > 17β-testosterone > 17β-boldenone > medroxyprogesterone acetate > 17β-estradiol > progesterone. Steroids representative for other hormone receptors, like estrone, 17α-estradiol, 17α-ethynylestradiol, and diethylstilbestrol for the estrogen receptor and corticosterone and dexamethasone for the glucocorticoid receptor, showed no agonistic response. Only 17β-estradiol, progesterone and medroxyprogesterone acetate gave a clear agonistic response. However, these compounds are known to exert androgenic effects.
Table 1.
EC50 concentrations and relative androgenic potencies (RAP) of compounds in the yeast androgen biosensor expressing yeast enhanced green fluorescent protein in response to androgens
| Compound | Qualitative response for AR agonisma | Commentsb | EC50 (nM) in the yeast androgen bioassayc | RAPd |
|---|---|---|---|---|
| 17β-Testosterone | Positive (11/11) | Strong AR agonist | 76 | 1.0 |
| 5α-Dihydrotestosterone | Positive (21/21) | Strong AR agonist, weak ER agonist | 33 | 2.3 |
| 17β-Boldenone | 510 | 0.15 | ||
| 17β-Trenbolone | Positive | Binds strongly to AR | 52 | 1.5 |
| Methyltrienolone | Positive (8/8) | AR agonist | 54 | 1.4 |
| Tetrahydrogestrinone | AR agonist | 65 | 1.2 | |
| 17β-Estradiol | Positive (10/11) | AR agonist and antagonist, strong ER agonist | 9,000 | 0.0084 |
| Estrone | Positive (2/2) | AR agonist, strong ER agonist | NR | NR |
| 17α-Estradiol | Negative (1/1) | ER agonist | NR | NR |
| 17α-Ethynylestradiol | Negative (1/1) | Strong ER agonist | NR | NR |
| Diethylstilbestrol | Negative (2/2) | Strong ER agonist | NR | NR |
| 4-Hydroxytamoxifene | Negative (1/1) | ER antagonist | NR | NR |
| Progesteronef | Positive (7/9) | 1,700 | 0.045 | |
| Medroxyprogesterone acetate | Positive (4/4) | Weak AR agonist | 1,500 | 0.051 |
| Corticosterone | Negative (1/1) | Binds weakly to AR | NR | NR |
| Dexamethasone | Positive (3/4) | AR agonist | NR | NR |
| Flutamide | Negative (5/5) | AR antagonist | NR | NR |
| 2,4,6-Tribromophenol | NR | NR | ||
| BDE-19 | NR | NR | ||
| 4-OH-BDE-17 | NR | NR |
AR androgen receptor, ER estrogen receptor, NR no response
aQualitative response for AR agonism across all mammalian cell reporter gene studies (data obtained from [27])
bComments obtained from [27]
cThe EC50 is the concentration giving half-maximum response.
dThe RAP is defined as the ratio between the EC50 of 17β-testosterone and the EC50 of the compound.
eThis compound was toxic to yeast above 30 μM.
fThese compounds reach a maximum response that is lower than 70% of the maximum response obtained with 17β-testosterone. The maxima obtained with 4-androstenedione and progesterone are about 40 and 35%, respectively.
Compared with the NIH publication [27], there are a few discrepancies. According to [27], estrone is an androgen receptor agonist that showed androgenic activity in two out of two MCRG systems. However, some mammalian cells are able to convert estrone into 17β-estradiol and vice versa. This conversion is ascribed to 17β-hydroxysteroid dehydrogenase 3 and this enzyme is responsible for the high relative estrogenic potency (REP) of estrone in the estrogen bioassay with the T47-D breast cancer cells (ER-CALUX test). In that test, the estrogenic potency of estrone was equal to that of 17β-estradiol and a REP of 1.0 was reported for estrone [10]. This probably explains why estrone gave a positive result for androgenic activity in two MCRG systems, but gave a negative result in our yeast androgen bioassay. Yeast is obviously not able to convert estrone into 17β-estradiol. The reported REP of 0.2 in our yeast estrogen biosensor corresponds nicely with the in vivo potency of this compound [28]. Dexamethasone was also described as an androgen receptor agonist in the NIH publication, showing androgenic activity in three out of four mammalian assays, but gave a negative result in our yeast androgen bioassay. However, these MCRG systems use an MMTV-Luc reporter construct or an ARE-Luc reporter construct. Both the MMTV and the ARE sequence are recognized by the glucocorticoid receptor and this means that the response found in these MCRG systems is probably due to crosstalk, as the glucocorticoid receptor is normally expressed in all cell types.
Dose–response curves obtained with the lacZ-based yeast androgen bioassay
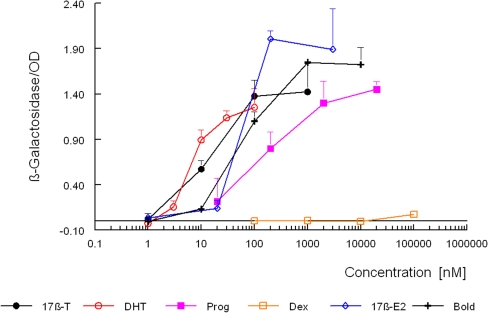
Figure 3 shows the dose–response curves for several natural and synthetic androgens obtained with the lacZ-based yeast androgen bioassay. The assay was simplified in our laboratory by scaling it down to a 96-well format and the use of a β-galactosidase assay kit. With increasing β-galactosidase activity, the density of the yeast culture, measured at 630 nm, dropped to about 50% (data not shown). Therefore, the measured â-galactosidase activity was corrected for the OD of the yeast culture. Table 2 shows the calculated EC50 and the corresponding RAP values. The data demonstrate that there is a good correlation between the EC50 values for androgens determined in our laboratory and those reported by Gaido et al. [29] in 1997. It seems that the lacZ-based yeast androgen bioassay, in terms of EC50 values, is 5–10 times more sensitive than our new bioassay. However, there was almost no difference in the limit of detection. Although the curves in the β-galactosidase assay go up at around 0.3 nM and in the yEGFP assay at around 1 nM, the limit of detection is about 3 nM for 5α-dihydrotestosterone in both assay types. This is mainly because the standard deviations are much higher in the β-galactosidase assay. However, the curves in the β-galactosidase assay are much steeper and are thus responsible for the lower EC50 values. The steeper curves are probably due to using an enzyme as a marker, β-galactosidase compared with a yEGFP marker protein, and the expression of the RSP5 cofactor that enhances transcription activation in the lacZ-based yeast androgen bioassay. The dynamic range for 5α-dihydrotestosterone in the McDonnell assay was from 3 to 100 nM and is slightly smaller than the range of the new bioassay (3–500 nM). There were also no great differences in the RAP values determined, although methyltrienolone was more potent in the lacZ-based bioassay. However, the new yEGFP bioassay is less sensitive for 17β-estradiol and progesterone: RAPs of 0.008 and 0.045, respectively, and for the latter no full dose–response curve, compared with RAPs of 0.1 and 0.068 and full dose–response curves in the lacZ-based bioassay. This means that our new bioassay is more specific for detecting compounds with a pure androgenic mode of action. The main difference between the lacZ-based and our yEGFP biosassay is that protein RSP5, which is a counterpart of the mammalian RPF1, is overexpressed in the lacZ-based bioassay in order to enhance transcriptional efficacy. However, this cannot explain the observed difference with methyltrienolone , as according to Imhof and McDonnell [30], this did not alter the potency or specificity of the assay. In addition and in contrast to the lacZ-based yeast androgen bioassay there were no differences in the density of the yeast culture measured at 630 nm upon exposure to different compounds that induced yEGFP expression, which indicates a decreased growth of yeast cells exposed to androgens in the lacZ-based yeast androgen bioassay. The only other known yeast androgen bioassay, one that uses luciferase as a reporter protein, displays similar characteristics in terms of specificity and was 5–10 times more sensitive in terms of EC50 values [15]. However, this assay needs the correction of the same yeast strain that only and stably expressed luciferase as an external control to correct for and normalize the aspecific responses caused by variation in cell vitality due to matrix and analyte toxicity. Unorrected dose–response curves for 17β-testosterone displayed more than 10 times higher EC50 values. An earlier assay described by Lee et al. [31] in 2003 uses β-galactosidase, but only expresses the hinge-ligand binding domain of the androgen receptor.
Fig. 3.
Response of the McDonnell yeast androgen bioassay to different substances. Exposure to 17β-testosterone, 5α-dihydrotestosterone, progesterone, dexamethasone, 17β-estradiol, and 17β-boldenone was started by adding to 200 μL of a yeast culture a 2-μL aliquot of a stock solution of the compound in DMSO. The β-galactosidase activity was determined after 24 h and corrected for the optical density at 630 nm as described in “lacZ-based yeast androgen bioassay: β-galactosidase assay.” Signals are the mean of a triplicate with the SD
Table 2.
EC50 concentrations and RAPs of compounds in the yeast androgen bioassay expressing β-galactosidase in response to androgens
| Compound | EC50 (nM)a by Gaido et al. [29] | EC50 (nM)b in our laboratory | RAP |
|---|---|---|---|
| 17β-Testosterone | 4.7 | 11.5 | 1.0 |
| 5α-Dihydrotestosterone | 3.5 | 4.9 | 2.3 |
| 17β-Boldenone | ND | 70 | 0.2 |
| 17β-Trenbolone | ND | 13 | 0.9 |
| Methyltrienolone | ND | 3.7 | 3.1 |
| Tetrahydrogestrinone | ND | 11.5 | 1.0 |
| 17β-Estradiol | 86.1 | 95 | 0.1 |
| 17α-Estradiol | ND | NR | NR |
| Progesterone | 89.3 | 170 | 0.068 |
| Corticosterone | ND | NR | NR |
| Dexamethasone | ND | NR | NR |
ND not determined
aValues obtained by Gaido et al. [29], using the McDonnell yeast androgen bioassay
bValues determined in our laboratory, using the McDonnell yeast androgen bioassay (see “Dose–response curves obtained with the lacZ-based yeast androgen bioassay”)
Antiandrogenic activity
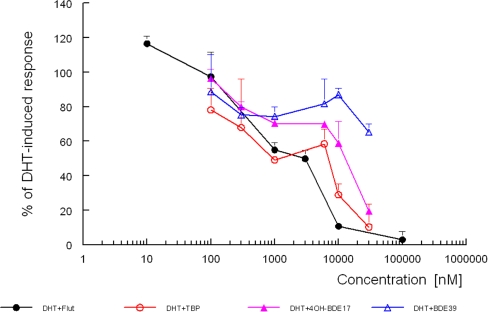
The specificity of the new yeast androgen bioassay was further demonstrated by the ability of antiandrogens to suppress the induction of yEGFP. Figure 4 shows the antiandrogenic activity of the known antagonist flutamide and three brominated flame retardants, BDE-39, TBP, and 4-OH-BDE-17. The antagonistic properties were examined by coexposure with a concentration of 5α-dihydrotestosterone that induced a submaximal response (50 nM). None of these four compounds were able to show an agonistic response (Table 1), but Fig. 4 clearly shows that all three were able to inhibit the response induced by 5α-dihydrotestosterone . The IC50 value was about 1 μM for flutamide, and TBP and 4-OH-BDE-17 were about as potent, while BDE-39 was clearly less antiandrogenic. Similar results were obtained with a human cell line. Only TBP was less potent in that test, but cytotoxicity of TBP could not be excluded [32].
Fig. 4.
Inhibition of a submaximal response obtained by 5α-dihydrotestosterone with flutamide and three brominated flame retardants (BFRs). Coexposure to a concentration of 5α-dihydrotestosterone that induced a submaximal response was started by adding to 200 μL of a yeast culture, 1 μL of a 5α-dihydrotestosterone and 1 μL of the BFR stock solution in DMSO. Fluorescence was determined after 24 h as described in “Streamlined yEGFP assay with the yeast androgen bioassay.” Fluorescence signals are the mean of a triplicate with the SD. TBP 2,4,6-tribromophenol
Conclusions
A recombinant yeast cell was constructed that expresses the hAR and yEGFP as a reporter protein in response to androgens. Compared with other yeast androgen bioassays, this new biosassay showed a similar limit of detection and dynamic range. However, the measurement of the fluorescence (yEGFP) can be followed as a function of incubation time and is easier, quicker, and cheaper than the measurement of the β-galactosidase or the luciferase activity, which needs cell wall disruption and/or the addition of expensive substrates. Owing to the ease of the yEGFP measurement, standard deviations are generally less than 3%. Moreover, the assay seems to be more robust and more specific for detecting compounds with a pure androgenic mode of action.
Brominated flame retardants with suspected antiandrogenic properties were able to inhibit the response obtained with 5α-dihydrotestosterone, the most potent endogenous androgen, demonstrating that this yeast androgen bioassay is suited to detect compounds with both agonistic and antagonistic characteristics.
As all compounds tested were able to show either their agonistic or their antagonistic properties, neither the cell wall nor the cell membrane seemed to be an obstacle. As for the yeast estrogen bioassay, we validated this new yeast androgen bioassay according to international criteria, eg., the determination of the decision limit (CCα) and the detection capability (CCβ). The decision limit is then used to distinguish negative and suspect samples [16] (unpublished results). The assay was proven to be useful to detect the new designer steroid tetrahydrogestrinone in human urine [33] while prohormones with an androgenic mode of action, e.g., dehydroepiandrosterone, are not active in yeast-based bioassays and need metabolic activation before they can be detected [34]. Future work will include the validation of the assay for urine and feed and the screening of prohormones with and without metabolic activation. To mimic the in vivo metabolic activation, liver slices, liver cell lines, liver S9 enzymes, and pure enzymes will be used.
Acknowledgement
This project was supported financially by the Dutch Ministry of Agriculture, Nature and Food Quality.
References
- 1.Pike MC, Spicer DV, Dahmoush L, Press MF (1993) Epidemiol Rev 15:17–35 [DOI] [PubMed]
- 2.Skakkebæk NE, Jørgensen N, Main KM, Rajpert-DeMeyts E, Leffers H, Andersson A, Juul A, Carlsen E, Mortensen GK, Jensen TK, Toppari J (2006) Int J Androl 29:2–11 [DOI] [PubMed]
- 3.Jobling S, Reynolds T, White R, Parker MG, Sumpter JP (1995) Environ Health Perspect 103:582–587 [DOI] [PMC free article] [PubMed]
- 4.Jenkins RL, Wilson EM, Angus RA, Howell WM, Kirk M (2003) Toxicol Sci 73:53–59 [DOI] [PubMed]
- 5.Lemaire G, Terouanne B, Mauvais P, Michel S, Rahmani R (2004) Toxicol Appl Pharmacol 196:235–246 [DOI] [PubMed]
- 6.Henley DV, Lipson N, Korach KS, Bloch CA (2007) N Engl J Med 356:479–485 [DOI] [PubMed]
- 7.Garcia-Reyero N, Grau E, Castillo M, Lopez De Alda MJ, Barcelo D, Pina B (2001) Environ Toxicol Chem 20:1152–1158 [DOI] [PubMed]
- 8.Mueller SO (2002) J Chromatogr B 777:155–165 [DOI] [PubMed]
- 9.Sonneveld E, Riteco JAC, Jansen HJ, Pieterse B, Brouwer A, Schoonen WG, Van der Burg B (2006) Toxicol Sci 89:173–187 [DOI] [PubMed]
- 10.Hoogenboom LAP, De Haan L, Hooijerink D, Bor G, Murk AJ, Brouwer A (2001) Acta Pathol Microbiol Immunol Scand 109:101–107 [DOI] [PubMed]
- 11.Graumann K, Breithofer A, Jungbauer A (1999) Sci Total Environ 225:69–79 [DOI] [PubMed]
- 12.Witters HE, Vangenechten C, Berckmans P (2001) Water Sci Technol 43:117–123 [PubMed]
- 13.Dhooge W, Arijs K, D’Haese I, Stuyvaert S, Versonnen B, Janssen C, Verstaete W, Comhaire F (2006) Anal Bioanal Chem 386:1419–1428 [DOI] [PubMed]
- 14.Rehmann K, Schramm K, Kettrup AA (1999) Chemosphere 38:3303–3312 [DOI] [PubMed]
- 15.Michelini E, Leskinen P, Virta M, Karp M, Roda A (2005) Biosens Bioelectron 20:2261–2267 [DOI] [PubMed]
- 16.Bovee TFH, Bor G, Heskamp HH, Hoogenboom LAP, Nielen MWF (2006) Food Addit Contam 23:556–568 [DOI] [PubMed]
- 17.Haelens A, Verrijdt G, Callewaert L, Christiaens V, Schauwaers K, Peeters B, Rombauts W, Claessens F (2003) Biochemistry 369:141–151 [DOI] [PMC free article] [PubMed]
- 18.Shaffer PL, Jivan A, Dollins DE, Cleassens F, Gewirth DT (2004) Proc Natl Acad Sci USA 101:4758–4763 [DOI] [PMC free article] [PubMed]
- 19.Brodie J, McEwan IJ (2005) J Mol Endocrinol 34:603–615 [DOI] [PubMed]
- 20.Schoonen WG, Deckers G, De Gooyer ME, De Ries R, Kloosterboer HJ (2000) J Steroid Biochem Mol Biol 74:213–222 [DOI] [PubMed]
- 21.Blankvoort BMG, De Groene EM, Van Meeteren-Kreikamp AP, Witkamp RF, Rodenburg RJT, Aarts, JMMJG (2001) Anal Biochem 298:93–102 [DOI] [PubMed]
- 22.Willemsen P, Scippo M, Maghuin-Rogister G, Martial JA, Muller M (2005) Anal Bioanal Chem 382:894–905 [DOI] [PubMed]
- 23.Bovee TFH, Helsdingen JR, Koks PD, Kuiper HA, Hoogenboom LAP, Keijer J (2004) Gene 325:187–200 [DOI] [PubMed]
- 24.Cormack BP, Bertram G, Egerton M, Gow NAR, Falkow S, Brown AJP (1997) Microbiology 143:303–311 [DOI] [PubMed]
- 25.Fuhrman U, Krattenmacher R, Slater EP, Fritzmeier KH (1996) Contraception 54:243–251 [DOI] [PubMed]
- 26.Collins DC (1994)Am J Obstet Gynecol 170:1508–1513 [DOI] [PubMed]
- 27.NIH (2003) NIH publication no 03-4503
- 28.Bovee TFH, Helsdingen JR, Rietjens IMCM, Keijer J, Hoogenboom LAP (2004) J Steroid Biochem Mol Biol 91:99–109 [DOI] [PubMed]
- 29.Gaido KW, Leonard LS, Lovell S, Gould JC, Babaï D, Portier CJ, McDonnell DP (1997) Toxicol Appl Pharmacol 143:205–212 [DOI] [PubMed]
- 30.Imhof MO, McDonnell DP (1996) Mol Cell Biol 16:2594–2605 [DOI] [PMC free article] [PubMed]
- 31.Lee HJ, Lee YS, Kwon HB, Lee K (2003) In Vitro Toxicol 17:237–244 [DOI]
- 32.Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MHA, Andersson PL, Legler J, Brouwer A (2006) Toxicol Sci 92:157–173 [DOI] [PubMed]
- 33.Nielen MWF, Bovee TFH, Van Engelen MC, Rutgers P, Hamers ARM, Van Rhijn JA, Hoogenboom LAP (2006) Anal Chem 78:424–431 [DOI] [PubMed]
- 34.Rijk J, Groot M, Peijnenburg A, Bovee T, Van Engelen M, Nielen M (2006) Paper presented at the 5th international symposium on hormone and veterinary drug residue analysis, Antwerp, Belgium