Abstract
Peri-infarct increase of vascular density has been observed in animals and in humans with ischemic stroke. Increased peri-infarct vascular density correlates with improved functional outcome after stroke. We hypothesized that pre-treatment with estradiol will increase post-ischemic peri-infarct capillary density in a rat model of transient ischemic stroke. Estradiol, compared to placebo, augmented post-ischemic peri-infarct vascular density by 22% 10 days after stroke. Recovery of forelimb function was not improved with estradiol treatment on day three and nine post stroke. Loss of estradiol may limit repair in the peri-infarct region by limiting angiogenesis, but functional significance in stroke recovery requires further investigation.
Keywords: estradiol, angiogenesis, stroke, infarct, penumbra, rat
Introduction
Remodeling of peri-infarct cerebral vasculature resulting in increased density has been observed in humans and in animals. Increased peri-infarct (borderzone) vascular density correlates with improved functional recovery, but whether it is causative or an epiphenomenon is currently under investigation. Post-ischemic peri-infarct angiogenesis may enhance neurorepair by a variety of mechanisms, including augmentation of cerebral perfusion and stimulation of neurogenesis (Slevin, et al., 2006, Zhang, et al., 2005). Peri-infarct angiogenesis may also facilitate clean up and reorganization of necrotic tissue (Yu, et al., 2006).
Ischemia-induced cerebral angiogenesis can be enhanced by a variety of agents and manipulations in experimental animal models. Nitric oxide donors, VEGF, statins, heparin-binding epidermal growth factor-like growth factor, erythropoietin, adrenomedullin, kallikrein, tadalafil, bone marrow stromal cells, umbilical cord blood cells, neural progenitor cells, whisker stimulation and exercise, as well as angiotensin II type 1 receptor blockade have been shown most recently to augment post-ischemic angiogenesis in rodents (Chen, et al., 2004, Chen, et al., 2005, Chen, et al., 2003, Ding, et al., 2004, Forder, et al., 2005, Jiang, et al., 2005, Peterson, 2004, Sugiura, et al., 2005, Sun, et al., 2003, Taguchi, et al., 2004, Wang, et al., 2004, Whitaker, et al., 2006, Xia, et al., 2006, Xia, et al., 2006, Zhang, et al., 2006).
Estradiol is protective in animal models of focal cerebral ischemia through several different mechanisms, acting in different cell types including endothelium, neurons, and glia. Neuroprotective mechanisms attributed to estradiol include actions through estrogen receptor-mediated gene transcription and second messenger pathways, as well as direct (e.g. antioxidant) actions. In experimental focal cerebral ischemia, estradiol attenuates neuronal apoptosis and inflammation, and regulates neurogenesis and vasoreactivity (reviewed in (Krause, et al., 2006, McCullough and Hurn, 2003, Suzuki, et al., 2006)).
Estradiol modulates angiogenesis under physiologic and pathologic conditions, recently reviewed by Rubanyi et al. (Rubanyi, et al., 2002). We have previously found that estradiol augmented cerebral angiopoietin-1 (Ang-1) mRNA levels and capillary density prior to experimental stroke in rodents (Ardelt, et al., 2005). We now hypothesized that treatment with physiologic doses of 17 β-estradiol (E2) will augment peri-infarct capillary density and improve functional recovery from experimental stroke.
Materials and Methods
All procedures performed on rats were approved by the Johns Hopkins University Animal Care and Use Committee and were in accordance with the NIH guidelines for the use of animals in research.
Ovariectomy and pellet implantation
Female Wistar rats, 200-225grams, (Harlan Breeders, Indianapolis, IN), were anesthetized with isoflurane. The ovaries were removed via bilateral abdominal incisions using aseptic surgical technique. A placebo or E2-releasing pellet (25μg, 21-day release; Innovative Research of America, Sarasota, FL) was implanted in a subcutaneous pocket in the neck. Animals underwent middle cerebral artery occlusion (MCAO) approximately one week to 10 days after ovariectomy/pellet implantation.
MCAO
The intra-luminal suture MCAO stroke model has been described previously (Ardelt, et al., 2005). All surgical procedures were carried out aseptically. Animals were anesthetized with isoflurane; a right femoral arterial line was placed for mean arterial pressure (MAP) monitoring and blood sampling; and the skull overlying the right middle cerebral artery territory was thinned for laser Doppler flow (LDF) monitoring (Moor Instruments, Axminster, UK). The right neck was dissected: the common and external carotid and pterygopalatine arteries were tied off; the occipital artery was cauterized; and a 4-0 monofilament suture was introduced into the common carotid artery and advanced until LDF dropped to less than 45% of baseline. After two hours of occlusion, the suture was removed, wounds closed, and the animal was awakened.
One cohort of animals was euthanized three days, and one cohort 10 days, after stroke. The 10-day survival cohort underwent evaluation of left upper extremity use with the cylinder test on day three and nine post stroke. Blood was sampled prior to euthanasia for plasma E2 measurement with radioimmunoassay (Diagnostic Systems, Webster, TX), as previously described (Alkayed, et al., 1998).
CD31 immunohistochemistry
Antibodies against CD31 (platelet endothelial cell adhesion molecule-1 or PECAM-1) primarily label vascular endothelium (Parums, et al., 1990). Brains were removed, placed in embedding medium-filled plastic molds, and flash-frozen in 2-methylbutane on dry ice. Brains were sectioned on the cryostat in the coronal plane. Five 5 μm-thick adjacent sections were collected every 100 μm, spanning the region between 1.70 and -0.40 mm from bregma. Sections were labeled with anti-CD31 antibody (Chemicon, Temecula, CA) at a dilution of 1:100. Signal was visualized with diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA). Adjacent sections were stained with eosin in order to determine infarct area and aid in sub-regional localization of infarct. Some sections processed for CD31 immunohistochemistry were also counterstained with hematoxylin to facilitate the evaluation of morphologic features of CD31-positive structures. Cortical peri-infarct regions of interest, as well as corresponding mirror-image contralateral regions, were photographed using a 10X lens with an additional 1.5X optical zoom by an investigator blinded to the hormonal status of the source animal.
Counting of CD31-positive structures
DAB-positive structures were counted in CD31-labeled, non-counterstained brain sections using Metamorph Offline version 4.6r4 software (Molecular Devices Corporation, Sunnyvale, CA) after manual threshold adjustment. The ischemic-to-nonischemic ratio was derived for each brain section, and the indices from a total of eight sections per animal were averaged in order to arrive at one vascular index value per animal.
In order to compare the sizes of peri-infarct CD31-positive structures between placebo-treated and estradiol-treated animals on day 10 after stroke, CD31-positive structures were first counted as described above. The counts were expressed as the proportion of the total number of CD31-positive structures falling within different size ranges: 50-250, 251-500, 501-750, 751-1000, and >1000 pixels. The proportional data were then averaged for the eight brain sections from each animal, and the ischemic-to-nonischemic difference was calculated for each size bin. All manual counts, measurements, and analysis were performed by an investigator blinded to the hormonal status of the source animals.
Determination of infarct area
Representative histologic infarct area was determined in an eosin-stained brain section at the level of bregma of each animal using Metamorph Offline version 4.6r4. Percent hemispheric infarct was calculated using the indirect method: [1 - (preserved ipsilateral hemisphere area/contralateral hemisphere area)]*100. Percent hemispheric swelling, (ischemic hemisphere area - contralateral hemisphere area)/contralateral area*100, and percent hemispheric atrophy, (contralateral hemisphere area - ischemic hemisphere area)/contralateral area*100, were calculated in the three and 10-day survival cohorts, respectively. The location of the medial edge of the infarct within the cerebral cortex was determined by measuring the distance in mm from the midline to the edge of the infarct in tracings of eosin-stained coronal sections at the level of bregma. The sub-regional localization of infarcts was established by comparing tracings of eosin-stained sections to diagrams in Paxinos and Watson’s rat brain atlas (Paxinos and Watson, 1998).
Cylinder test
Rats in the 10-day survival cohort were placed in a Plexiglas cylinder, 20 cm in diameter. They were videotaped for 10 minutes, and the number of times they reared up touching the cylinder wall was recorded (Li, et al., 2004). Only initial paw placement was noted; paw placement was recorded as left, right, or both. Each animal was videotaped on three occasions by an investigator blinded to the hormonal status of the animals: before stroke, on day three after stroke, and on day nine after stroke. Limb use asymmetry scores [(right - left) / (right + left + both)] were calculated.
Statistics
Statistical analysis was performed using SigmaStat 3.0 (SPSS, Chicago, IL). One or two-way ANOVA with Holm-Sidak post-hoc analysis, ANOVA on ranks with Dunn’s comparison method, or Student’s t-test were utilized where appropriate. Results are reported as mean±s.d.
Results
Physiology and plasma estradiol
Rats with normal intra-ischemic physiology and metabolism (MAP, rectal and temporalis temperature, arterial blood gas values, hemoglobin concentration and glucose levels), and documented cerebral reperfusion after filament removal were included (data not shown). Between 10 and 14 days after ovariectomy and pellet implantation, plasma E2 values were: placebo, 1.5±0.30 and E2, 30.3±20.1 pg/ml; and average weight gain was: placebo, 57±1.4 and E2, 11±2.8 g.
Vascular density
CD31-positive structures were counted in the cortical peri-infarct region (Figure 1) on day three and 10 after transient ischemia. The morphology of CD31-positive structures in intact brain and peri-infarct regions was consistent with that of capillaries (Figure 2). The ischemic-to-nonischemic ratio of CD31-positive structures three days after stroke was close to unity and not different between placebo and E2-treated animals (Figure 3A). Ten days after stroke, the ischemic-to-nonischemic CD31-positive structure ratio was greater than one in both placebo and E2-treated animals: in E2-treated animals compared to placebo, the index was 22% higher. The percent increase in vascular density index between the day three and 10 post-stroke cohorts was 27 in placebo-treated and 42 in E2-treated animals.
Figure 1.

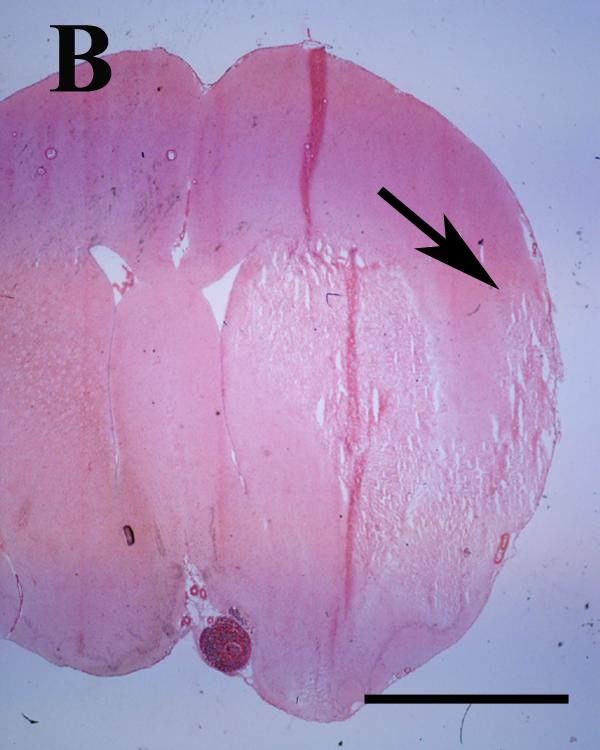
A and B. Eosin-stained coronal rat brain section photographed at 1.25X illustrating the approximate peri-infarct region analyzed (arrows) in rats with the largest (A) and smallest (B) infarcts on post stroke day three. Infarct dimensions and sub-regional localization of the cortical portion of the lesion were heterogeneous within and between cohorts. Scale bar = 5 mm.
Figure 2.


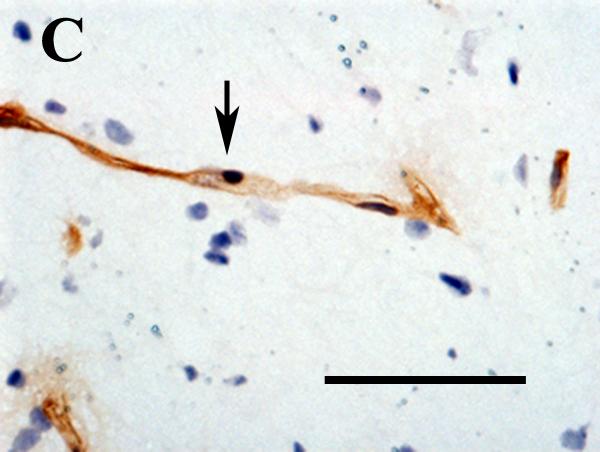

A and B. Low magnification views of CD31-labeled structures in the non-ischemic cortex (A) and peri-infarct region (B) photographed 10 days after experimental ischemic stroke. Counts of these structures in the peri-infarct and contralateral mirror-image regions were used to derive ischemic/nonischemic vascular indices. The structures located in the peri-ischemic region were more numerous, larger, and torturous. Scale bar = 200μm.
C and D. High magnification views of CD31-labeled structures (arrows) in intact brain (C) and in the peri-infarct region (D) 10 days after experimental stroke. In intact brain, the morphology is typical of a normal parenchymal capillary: the endothelial cells form a narrow lumen, with relatively few nuclei oriented along the axis of the vessel. In the injured area, the morphology somewhat resembles that of angiogenic blood vessels: the lumen is wide, and there are numerous endothelial cell nuclei clustered around the lumen. CD31 outlines the surface membranes. Nuclei are labeled with hematoxylin. Scale bar = 50μm.
Figure 3.
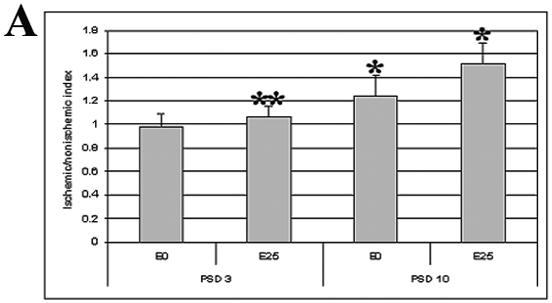

A. Peri-infarct vascular density indices were determined in brain sections labeled with anti-CD31 antibody from placebo (E0) and estradiol-treated (E25) animals on post stroke day three and 10 (PSD 3 and PSD 10, respectively). *p=0.011 for E0 vs. E25 on PSD 10; **p=0.010 for E25 on PSD 3 vs. E25 on PSD 10. One-way ANOVA. Sample size: E0, PSD 3, n=4; E25, PSD 3, n=4; E0, PSD 10, n=6; E25, PSD 10, n=5.
B. Ischemic/nonischemic difference in the proportions of CD31-positive structures of different sizes was derived 10 days post stroke. CD31-positive structures were counted in placebo (E0) and estradiol (E25)-treated rats and sorted into five bins by pixel area. The percent difference in proportion of structures within each bin between the peri-infarct area and the contralateral matched region was determined. *p<0.017 for E0 vs. E25 in >1000 pixel bin; **p<0.005 for >1000 versus 50-250 pixel bin for estradiol-treated animals. Two-way ANOVA.
Ten days after cerebral ischemia, CD31-positive structures within the peri-infarct area were larger than those in the contralateral cortex (Figure 2). The proportion of large CD31-positive structures in the peri-infarct area was significantly higher in E2-treated, compared to placebo-treated, animals (Figure 3B).
Infarct area and functional recovery
Histologic infarct area in a representative eosin-stained section at the level of bregma was variable between animals (Figure 1). Overall infarct area and hemispheric swelling were not different between placebo and E2-treated rats in the three day survival cohort (Table 1). In the 10-day survival cohort, infarct area was smaller in placebo-treated animals. Hemispheric atrophy, although not statistically significant, showed a trend toward decrease in placebo-treated animals (Table 1).
Table 1.
| Post stroke day 3 | Post stroke day 10 | |||
|---|---|---|---|---|
| Infarct area, % hemisphere | Swelling, % | Infarct area, % hemisphere | Atrophy, % | |
| Placebo | 47±8 | 18±19 | 37±6 | 11±14 |
| Estradiol | 50±9 | 16±4 | 53±9 | 21±12 |
| p value | 0.88 | 0.79 | 0.012 | 0.27 |
Sub-regional localization of the medial edge of the infarct in eosin-stained sections at the level of bregma was also variable, but all animals showed involvement of the corpus striatum (Figure 1). In the three day post-stroke cohort, the anatomic primary motor cortex was involved in one of four placebo-treated animals, and none of E2-treated animals, while forelimb sensory cortex was involved in three of four placebo-treated animals and one of four E2-treated animals. In the 10-day cohort, the situation was reversed: none of the placebo-treated animals showed histologic involvement of the primary motor cortex and forelimb sensory area, while one of five E2-treated animals had damage to the primary motor cortex and four of five had involvement of the forelimb sensory fields (data not shown).
Left forelimb use was evaluated before stroke and three and nine days post stroke in the 10-day survival cohort. Contralateral limb use, as determined by calculation of limb asymmetry scores, was not different between placebo-treated and E2-treated animals (Figure 4).
Figure 4.
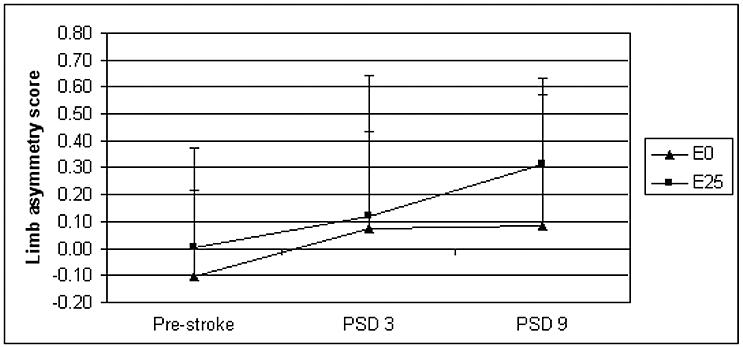
Forelimb use asymmetry scores were derived for estradiol (E2) and placebo (E0)-treated animals using the cylinder test pre stroke and on post stroke day three (PSD 3) and 10 (PSD 10). Limb asymmetry score = (right - left) / (right + left + both); thus, positive scores reflect decreased left forelimb use (as expected with a right hemispheric stroke).
Discussion
Ischemia-induced angiogenesis and augmentation of cerebral vascular density may contribute to neurorepair and improved functional outcome after stroke. We now show that E2 replacement to physiologic levels in ovariectomized female rats prior to experimental cerebral ischemia augments peri-infarct vascular density 10 days after stroke. In previous experiments, we observed that E2 increased cerebral levels of Ang-1 mRNA before stroke induction (Ardelt, et al., 2005). Ang-1 is potent modulator of vascular remodeling and frequently acts in concert with VEGF to stimulate robust angiogenesis (Patan, 2004). E2 also induces VEGF (Mueller, et al., 2000) as well as endothelial nitric oxide synthase (eNOS) activity, mRNA and protein (reviewed in (McCullough and Hurn, 2003)). Recently, a nitric oxide donor was observed to upregulate expression of Ang-1 and Tie-2 (the receptor for Ang-1) in the peri-infarct region in rats with experimental stroke (Zacharek, et al., 2006). It is possible that in our model, E2 upregulates eNOS in cerebral endothelium resulting in increased nitric oxide, which in turn upregulates vascular endothelial Ang-1/Tie-2 in the peri-infract area. E2 or ischemia-induced VEGF may then act in concert with Ang-1/Tie-2 to augment peri-infarct vascular density.
Despite increased peri-infarct vascular density with E2 replacement in the 10-day survival cohort, we did not observe improved functional recovery using the cylinder test, which had been found to differentiate E2 and placebo-treated mice recovering from experimental stroke (Li, et al., 2004). Some possible reasons for our not observing an effect in rats may have been a true species difference, time points too early to detect behavioral differences in rats, or insensitivity to detect small behavioral changes resulting from tissue salvaged by increased peri-infarct vascularity in rats. Additionally, the relationship between histologic infarct size, involvement of specific functional regions, and sensorimotor recovery deserves further investigation. In our study, despite meticulous standardization of surgical technique and control of intra-ischemic physiology, representative histologic infarct areas and involvement of cortical motor and sensory forelimb regions were variable from animal to animal within each cohort, possibly relating to intrinsic variability of collateral vascular anatomy between animals. Averaged histologic infarct area and degree of hemispheric swelling were similar with E2 and placebo treatment in the three day survival cohort. In the 10-day survival cohort, infarcts and degree of atrophy tended to be smaller with placebo treatment. Infarct size does not always correlate with functional outcome: a study in mice showed E2-mediated improvement in post stroke recovery but no difference in total infarct volume between E2 and placebo-treated animals two weeks after stroke (Li, et al., 2004).
Very little is known about the evolution of ischemic brain injury over time with E2 treatment, as most prior investigations reporting smaller infarcts with E2 were performed only one day after stroke. The results of our study and those of Li et al. in mice suggest that E2 treatment may delay, not prevent, sequelae of ischemic injury (manifested as histologic infarct size) in the acute period. However, our experiment was designed to assess vascular density as the primary endpoint; infarct area was a secondary endpoint. It is possible that analyzing multiple sections from the entire brain to measure infarct volume would have produced a different result than measurement of area on a single section. In addition, infarct volume is variable. The small sample size of five to six, which was adequate for vascular density measurements, was underpowered for infarct size analysis and may have resulted in a false-positive difference on day 10 post stroke. Nevertheless, our observation of E2-mediated increase in peri-infarct vascular density suggests that in the subacute post-stroke period E2 affects neurorepair independently of infarct size. The results of the Li et al. study suggest that E2 affects functional recovery independently of infarct size (Li, et al., 2004). Thus, to understand the effects of E2 in post stroke recovery, individual rats must be investigated longitudinally with imaging and behavioral testing in order to clarify the relationship of histologic infarct size, peri-infarct blood flow, functional cortical reorganization, and sensorimotor recovery.
In summary, we have shown that E2 treatment augments post-ischemic peri-infarct vascular density in a transient focal ischemia model of experimental stroke in rats, but further studies are necessary to elucidate the functional effect in stroke recovery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD.Gender-linked brain injury in experimental stroke Stroke 199829159–165. discussion 166 [DOI] [PubMed] [Google Scholar]
- 2.Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, Hurn PD. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke. 2005;36:337–341. doi: 10.1161/01.STR.0000153795.38388.72. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, Lu M, Zhang Z, Chopp M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005:21–28. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 6.Ding YH, Luan XD, Li J, Rafols JA, Guthinkonda M, Diaz FG, Ding Y. Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke. Curr Neurovasc Res. 2004;1:411–420. doi: 10.2174/1567202043361875. [DOI] [PubMed] [Google Scholar]
- 7.Forder JP, Munzenmaier DH, Greene AS. Angiogenic protection from focal ischemia with angiotensin II type 1 receptor blockade in the rat. Am J Physiol Heart Circ Physiol. 2005;288:H1989–1996. doi: 10.1152/ajpheart.00839.2004. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Q, Zhang ZG, Ding GL, Zhang L, Ewing JR, Wang L, Zhang R, Li L, Lu M, Meng H, Arbab AS, Hu J, Li QJ, Pourabdollah Nejad DS, Athiraman H, Chopp M. Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using MRI. Neuroimage. 2005;28:698–707. doi: 10.1016/j.neuroimage.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 9.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. 2006;101:1252–1261. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 11.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 12.Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci U S A. 2000;97:10972–10977. doi: 10.1073/pnas.200377097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parums DV, Cordell JL, Micklem K, Heryet AR, Gatter KC, Mason DY. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol. 1990;43:752–757. doi: 10.1136/jcp.43.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patan S. Vasculogenesis and angiogenesis. Cancer Treat Res. 2004;117:3–32. doi: 10.1007/978-1-4419-8871-3_1. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson C. The Rat Brain in Stereotactic Coordinates. Academic Press; 1998. [Google Scholar]
- 16.Peterson DA. Umbilical cord blood cells and brain stroke injury: bringing in fresh blood to address an old problem. J Clin Invest. 2004;114:312–314. doi: 10.1172/JCI22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubanyi GM, Johns A, Kauser K. Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol. 2002;38:89–98. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 18.Slevin M, Kumar P, Gaffney J, Kumar S, Krupinski J. Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clin Sci (Lond) 2006;111:171–183. doi: 10.1042/CS20060049. [DOI] [PubMed] [Google Scholar]
- 19.Sugiura S, Kitagawa K, Tanaka S, Todo K, Omura-Matsuoka E, Sasaki T, Mabuchi T, Matsushita K, Yagita Y, Hori M. Adenovirus-mediated gene transfer of heparin-binding epidermal growth factor-like growth factor enhances neurogenesis and angiogenesis after focal cerebral ischemia in rats. Stroke. 2005;36:859–864. doi: 10.1161/01.STR.0000158905.22871.95. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 22.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 24.Whitaker VR, Cui L, Miller S, Yu SP, Wei L. Whisker stimulation enhances angiogenesis in the barrel cortex following focal ischemia in mice. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600318. [DOI] [PubMed] [Google Scholar]
- 25.Xia CF, Yin H, Borlongan CV, Chao J, Chao L. Postischemic infusion of adrenomedullin protects against ischemic stroke by inhibiting apoptosis and promoting angiogenesis. Exp Neurol. 2006;197:521–530. doi: 10.1016/j.expneurol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Xia CF, Yin H, Yao YY, Borlongan CV, Chao L, Chao J. Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis. Hum Gene Ther. 2006;17:206–219. doi: 10.1089/hum.2006.17.206. [DOI] [PubMed] [Google Scholar]
- 27.Yu SW, Friedman B, Cheng Q, Lyden PD. Stroke-evoked angiogenesis results in a transient population of microvessels. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600378. [DOI] [PubMed] [Google Scholar]
- 28.Zacharek A, Chen J, Zhang C, Cui X, Roberts C, Jiang H, Teng H, Chopp M. Nitric oxide regulates Angiopoietin1/Tie2 expression after stroke. Neurosci Lett. 2006;404:28–32. doi: 10.1016/j.neulet.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Zhang Z, Zhang RL, Cui Y, Lapointe MC, Silver B, Chopp M. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res. 2006 doi: 10.1016/j.brainres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–416. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]


