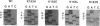Abstract
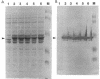
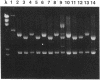
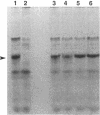
By a newly developed double-stranded mutagenesis technique, histidine (H), glutamate (E), arginine (R) and leucine (L) have been substituted for the lysyl 193 residue (K-193) in isocitrate lyase from Escherichia coli. The substitutions for this residue, which is present in a highly conserved, cationic region, significantly affect both the Km for Ds-isocitrate and the apparent kcat of isocitrate lyase. Specifically, the conservative substitutions, K-193-->H (K193H) and K193R, reduce catalytic activity by ca. 50- and 14-fold, respectively, and the nonconservative changes, K193E and K193L, result in assembled tetrameric protein that is completely inactive. The K193H and K193R mutations also increase the Km of the enzyme by five- and twofold, respectively. These results indicate that the cationic and/or acid-base character of K193 is essential for isocitrate lyase activity. In addition to the noted effects on enzyme activity, the effects of the mutations on growth of JE10, an E. coli strain which does not express isocitrate lyase, were observed. Active isocitrate lyase is necessary for E. coli to grow on acetate as the sole carbon source. It was found that a mutation affecting the activity of isocitrate lyase similarly affects the growth of E. coli JE10 on acetate when the mutated plasmid is expressed in this organism. Specifically, the lag time before growth increases over sevenfold and almost twofold for E. coli JE10 expressing the K193H and K193R isocitrate lyase variants, respectively. In addition, the rate of growth decreases by almost 40-fold for E. coli JE10 cells expressing form K193H and ca. 2-fold for those expressing the K193R variants. Thus, the onset and rate of E. coli growth on acetate appears to depend on isocitrate lyase activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeysinghe S. I., Baker P. J., Rice D. W., Rodgers H. F., Stillman T. J., Ko Y. H., McFadden B. A., Nimmo H. G. Use of chemical modification in the crystallization of isocitrate lyase from Escherichia coli. J Mol Biol. 1991 Jul 5;220(1):13–16. doi: 10.1016/0022-2836(91)90376-h. [DOI] [PubMed] [Google Scholar]
- Atomi H., Ueda M., Hikida M., Hishida T., Teranishi Y., Tanaka A. Peroxisomal isocitrate lyase of the n-alkane-assimilating yeast Candida tropicalis: gene analysis and characterization. J Biochem. 1990 Feb;107(2):262–266. doi: 10.1093/oxfordjournals.jbchem.a123036. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Comai L., Dietrich R. A., Maslyar D. J., Baden C. S., Harada J. J. Coordinate expression of transcriptionally regulated isocitrate lyase and malate synthase genes in Brassica napus L. Plant Cell. 1989 Mar;1(3):293–300. doi: 10.1105/tpc.1.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Sal G., Manfioletti G., Schneider C. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. Biotechniques. 1989 May;7(5):514–520. [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D. F., Johnson M. S., Doolittle R. F. Aligning amino acid sequences: comparison of commonly used methods. J Mol Evol. 1984;21(2):112–125. doi: 10.1007/BF02100085. [DOI] [PubMed] [Google Scholar]
- Fernández E., Moreno F., Rodicio R. The ICL1 gene from Saccharomyces cerevisiae. Eur J Biochem. 1992 Mar 15;204(3):983–990. doi: 10.1111/j.1432-1033.1992.tb16720.x. [DOI] [PubMed] [Google Scholar]
- Ko Y. H., McFadden B. A. Alkylation of isocitrate lyase from Escherichia coli by 3-bromopyruvate. Arch Biochem Biophys. 1990 May 1;278(2):373–380. doi: 10.1016/0003-9861(90)90273-2. [DOI] [PubMed] [Google Scholar]
- Ko Y. H., Vanni P., Munske G. R., McFadden B. A. Substrate-decreased modification by diethyl pyrocarbonate of two histidines in isocitrate lyase from Escherichia coli. Biochemistry. 1991 Jul 30;30(30):7451–7456. doi: 10.1021/bi00244a012. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., McFadden B. A. Isolation, hyperexpression, and sequencing of the aceA gene encoding isocitrate lyase in Escherichia coli. J Bacteriol. 1988 Oct;170(10):4528–4536. doi: 10.1128/jb.170.10.4528-4536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- RAYMOND S. ACRYLAMIDE GEL ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:350–365. doi: 10.1111/j.1749-6632.1964.tb14208.x. [DOI] [PubMed] [Google Scholar]
- Rieul C., Bleicher F., Duclos B., Cortay J. C., Cozzone A. J. Nucleotide sequence of the aceA gene coding for isocitrate lyase in Escherichia coli. Nucleic Acids Res. 1988 Jun 24;16(12):5689–5689. doi: 10.1093/nar/16.12.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E. F., Hoyt J. C., Reeves H. C. Evidence of histidine phosphorylation in isocitrate lyase from Escherichia coli. J Biol Chem. 1988 Feb 15;263(5):2477–2482. [PubMed] [Google Scholar]
- Turley R. B., Choe S. M., Trelease R. N. Characterization of a cDNA clone encoding the complete amino acid sequence of cotton isocitrate lyase. Biochim Biophys Acta. 1990 Jun 21;1049(2):223–226. doi: 10.1016/0167-4781(90)90045-4. [DOI] [PubMed] [Google Scholar]
- Vanni P., Giachetti E., Pinzauti G., McFadden B. A. Comparative structure, function and regulation of isocitrate lyase, an important assimilatory enzyme. Comp Biochem Physiol B. 1990;95(3):431–458. doi: 10.1016/0305-0491(90)90002-b. [DOI] [PubMed] [Google Scholar]