Abstract
Since angiotensin (Ang) (1-7) injected into the brain blocked Ang II pressor actions in rats made hypertensive by aortic coarctation (CH), we examined systemic and tissue angiotensin peptide levels, specifically concentrating on the hypothalamic Ang-(1-7) levels. Plasma, heart and kidney isolated from CH rats showed increased levels of Ang I, Ang II and Ang-(1-7) compared with the normotensive group, with Ang II being the predominant peptide in heart and kidney. In the hypothalamus, equimolar amounts of Ang II and Ang-(1-7) were found in the sham group, whereas only Ang-(1-7) levels increased in CH rats. We conclude that aortic coarctation activates systemic and tissue renin-angiotensin system. The increased central levels of Ang-(1-7) in the CH rats suggest a potential mitigating role of this peptide in central control of the hypertensive process.
Keywords: angiotensin II, angiotensin-(1-7), aortic coarctation, hypertensive rats, tissue levels, plasma levels, hypothalamus
1. Introduction
The physiopathogenesis of acute aortic coarctation hypertension has been well documented [5,6,25–27,31]. Clamping the aorta between the renal arteries initially results in arterial and cardiac hypertrophy in response to the development of high blood pressure [5,6]. Involvement of angiotensin II (Ang II) in this hypertensive process has been demonstrated by blockade of Ang II receptors or measurement of plasma renin activity, which was significantly increased following aortic constriction [5,6,25–27,31]. On the other hand, the response of critical cardiovascular target organs to the increased renin-angiotensin system (RAS) in this model of hypertension has not been characterized. In addition, there is lacking information about RAS at the central level in this hypertensive model. It is not known whether the hypertension induced by renal hypoperfusion results from increased activity of the circulating or if other local tissue RAS are also activated. With this in mind, we have evaluated the participation of local tissue RAS systems (heart, kidney above the coarctation, and hypothalamus) by measuring plasma and tissue levels of Ang I, Ang II and Ang-(1-7) in rats made hypertensive by aortic coarctation. Furthermore, since a counterbalancing role on Ang II pressor and trophic actions has been suggested for Ang-(1-7) [10], specially in situations of increased Ang II activity as in hypertension, our aim was to investigate changes in plasma and tissue Ang-(1-7) levels. In fact, we have previously shown that Ang-(1-7) blocked Ang II actions at the hypothalamic level in coarcted hypertensive (CH) rats [12,15], so we hypothesize that Ang-(1-7) levels are increased in hypothalami from these rats to counterbalance the activated renal RAS.
2. Methods
2.1. Animal Preparation
Male Wistar rats (250–300 g) were anesthetized with ether and subjected to sham operation or complete ligation of the abdominal aorta between the renal arteries according to the method described by Rojo-Ortega and Genest [24]. Following surgery, rats were returned to their cages and penicillin (50000 UI) administered s.c. to prevent infections. Although they displayed lower limb paralysis during the first 20 h after surgery, normal range of motion returned within 24 h. All experimental procedures complied with the guidelines of the American Physiological Society, and the study was approved by our Institutional Review Board for Animal Experimentation.
2.2. Experimental Protocols
Experiments were performed 7 days after surgery. To measure mean arterial blood pressure (MAP), rats were anesthetized with ether and the left common carotid artery cannulated with a polyethylene tubing containing heparinized saline solution (25 U/ml). The cannula was externalized at the back of the neck and connected to a blood pressure transducer (Viggo Spectramed, Viggo, USA) coupled to a polygraph (Grass 79D, Grass, USA). After 3 h, arterial blood pressure was measured in conscious, freely moving animals. MAP was calculated according to the following formula: diastolic presure + [(systolic pressure − diastolic pressure)/3]. Heart rate (HR) was calculated by counting the pulsatile waves of arterial pressure using a tachograph.
24 h urine was collected in 6 N HCl 6 days after surgery and stored at −70°C until assay, as previously described [3,17].
Rats were killed by decapitation. Trunk blood was rapidly collected in glass tubes containing a mixture of 25 mmol/L potassium ethylene-diamine-tetraacetic acid (Sigma-Aldrich), 0.44 mmol/L o-phenanthroline (Sigma-Aldrich), 1 mmol/L Na+ para-chloromercuribenzoate (Sigma-Aldrich), 0.12 mmol/L pepstatin A (Sigma-Aldrich) and the renin inhibitor acetyl-His-Pro-Phe-Val-Statine-Leu-Phe (AnaSpeck Inc) (3 μmol/L), which inhibits in vitro production and degradation of angiotensin peptides [3,17]. Blood was centrifuged for 20 min at 3000 xg (4 °C) and plasma kept frozen at −70 °C until assay. The hypothalamus, right kidney (above the coarctation), and heart were rapidly dissected on ice, frozen and stored at −70°C until assay.
Ang peptides in plasma, urine, heart, kidney and hypothalamus were extracted and quantified as described previously [3,17]. Ang levels were not measured in the left kidney because it was completely necrotized due to complete ligation of the artery.
2.3. Statistical Analysis
All values are mean±SEM. Data were analyzed by one-way ANOVA or Student’s t test. Post hoc analysis was carried out using the Student-Newman-Keuls test, considering P< 0.05 significant.
3. Results
MAP was significantly higher in conscious rats with aortic coarctation-induced hypertension (CH) than in animals subjected to sham operation (134 ± 5 vs 98 ± 4 mm Hg, P<0.05), while the heart rate in CH rats was lower (409 ± 14 vs 459 ± 11 beats/min, P<0.05). The ratio of heart weight to body weight was significantly increased in the CH rats (4.8 ± 0.5 vs 3.2 ± 0.2 in the sham group, P<0.05), suggesting development of cardiac hypertrophy.
Plasma levels of Ang I, Ang II and Ang-(1-7) were increased 18-, 12- and 6-fold, respectively, in CH rats (Fig. 1A) with Ang I being the predominant peptide (levels in fmol/ml plasma of Ang I: 101.6±22.2 in sham vs 1538±376 in CH; Ang II: 31.2±1.8 in sham vs 352±119.6 in CH; Ang-(1-7): 14.2±3.6 in sham vs 88.8±8.3 in CH). Ang II/Ang I and Ang II/Ang-(1-7) ratios were not changed (Fig. 1B).
Fig. 1.
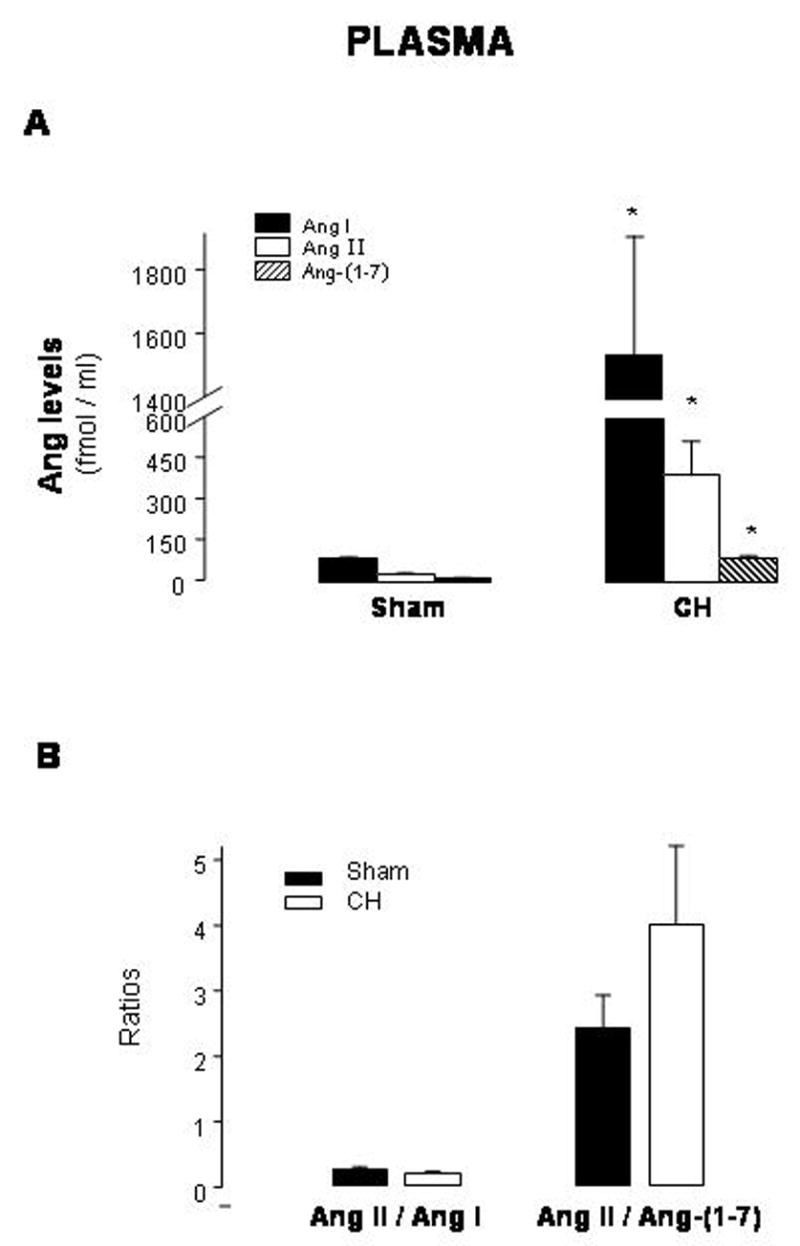
Plasma levels of angiotensin (Ang) I, Ang II and Ang-(1-7) (A) and Ang II/Ang I and Ang II/Ang-(1-7) ratios (B) in sham-operated rats (sham) and rats with aortic coarctation-induced hypertension (CH). *P<0.05 compared with sham. n=10–12
Figure 2A illustrates the effect of this form of hypertension on renal tissue concentrations of Ang I, Ang II, and Ang-(1-7) above the aortic ligature, that is in the right kidney. Ang levels were not measured in the left kidney (below the level of the aortic banding) because it was completely necrotized due to complete ligation of the artery. Compared with sham-operated rats, CH rats had increased renal tissue levels of Ang I (2-fold), Ang II (3.8-fold) and Ang-(1-7) (2-fold), with Ang II > Ang I > Ang-(1-7) (levels in fmol/g tissue of Ang I: 219.8±23.9 in sham vs 438.4±40.7 in CH; Ang II: 158.6±13.9 in sham vs 600.8±80.6 in CH; Ang-(1-7): 53.1±7.6 in sham vs 98.0±14.4 in CH). There was a shift to a higher Ang II/Ang-(1-7) ratio in CH compared to sham-operated rats, though the Ang II/Ang I ratio did not change (Fig. 2B).
Fig. 2.
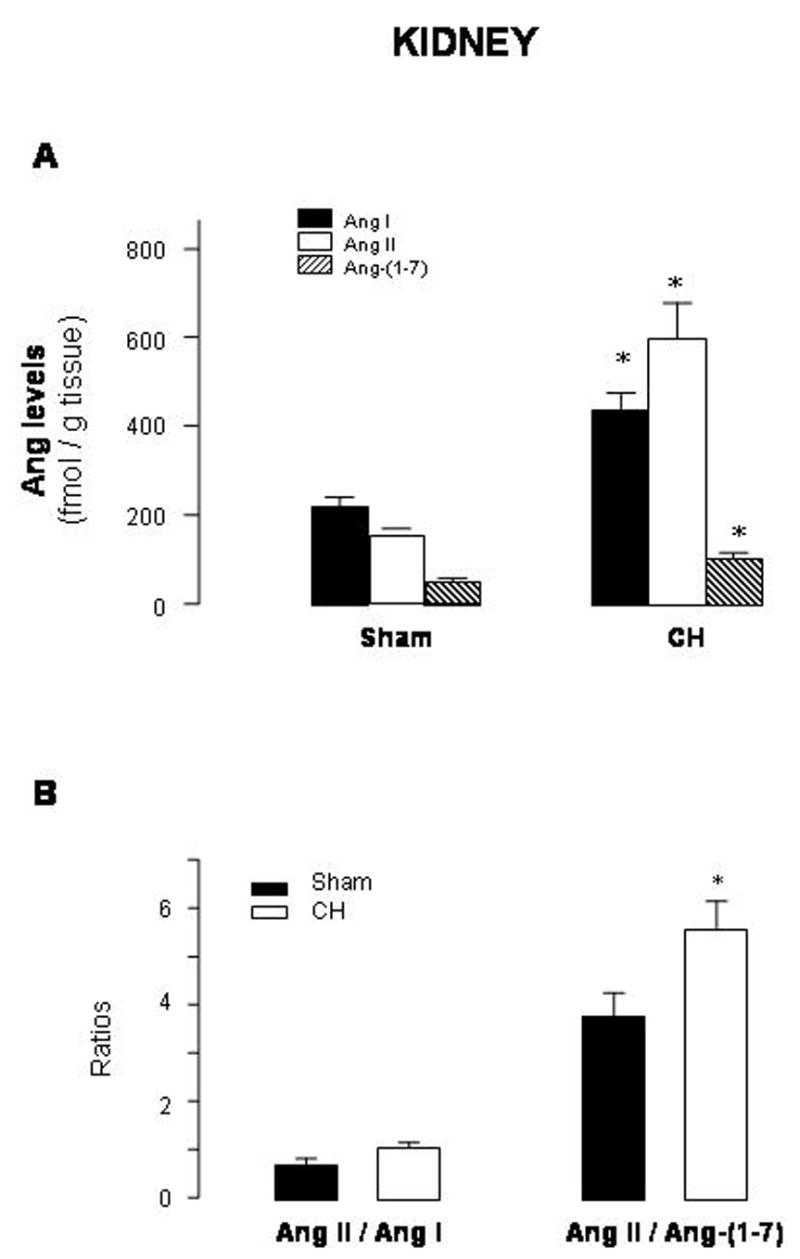
Renal levels of angiotensin (Ang) I, Ang II and Ang-(1-7) (A) and Ang II/Ang I and Ang II/Ang-(1-7) ratios (B) in sham-operated rats (sham) and rats with aortic coarctation-induced hypertension (CH). *P<0.05 compared with sham rats. n=10–12
Ang peptides were also detected in the urine. Urinary concentrations of Ang I, Ang II and Ang-(1-7) were increased in CH rats (levels in fmol/24 h of Ang I: 794.2±82.9 in sham vs 5365.6±1420.0 in CH; Ang II: 1087.8±174.2 in sham vs 2908.4±555.0 in CH; Ang-(1-7): 1031.6±289.2 in sham vs 2818.3±641.8 in CH) (Fig. 3A). Both Ang II/Ang I and Ang II/Ang-(1-7) ratios were diminished in CH rats (Fig. 3B).
Fig. 3.
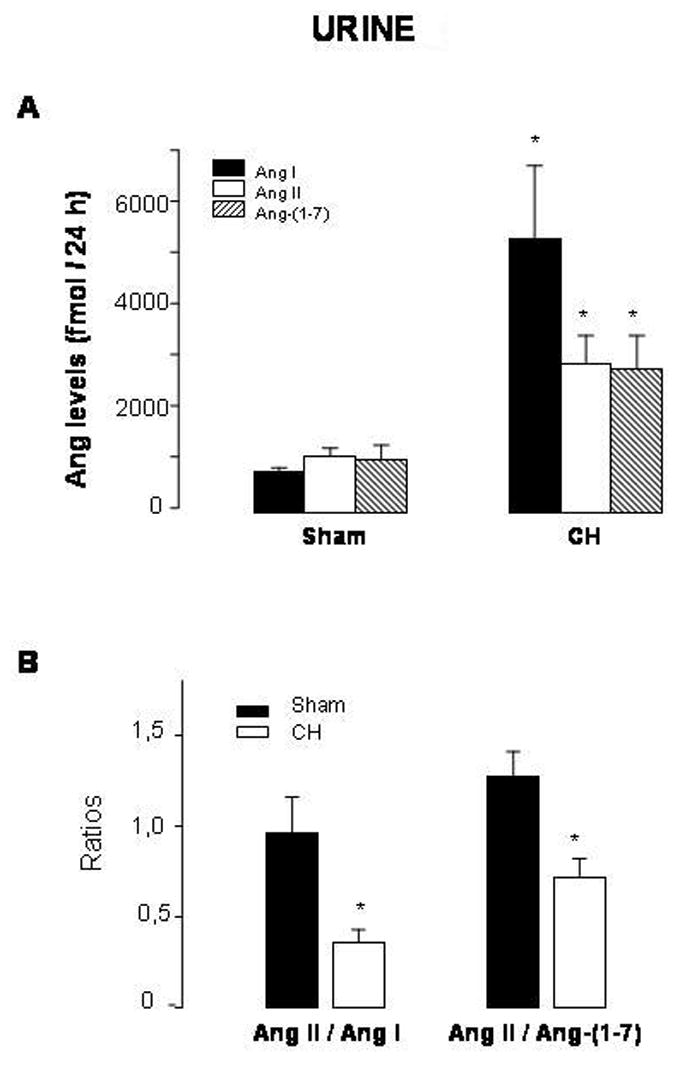
Levels of angiotensin (Ang) I, Ang II and Ang-(1-7) (A) and Ang II/Ang I and Ang II/Ang-(1-7) ratios (B) in urines from sham-operated rats (sham) and rats with aortic coarctation-induced hypertension (CH). *P<0.05 compared with sham rats. n=10–12
Cardiac levels of Ang II and Ang-(1-7) averaged 100% and 69%, respectively, above sham values (levels in fmol/g tissue of Ang II: 32.3±1.9 in sham vs 65.6±12.7 in CH; Ang-(1-7): 16.3±0.7 in sham vs 27.5±2.3 in CH)(Fig. 4A). The Ang II/Ang-(1-7) ratio was higher in CH than in sham-operated rats, while the Ang II/Ang I ratio did not change (Fig. 4B).
Fig. 4.
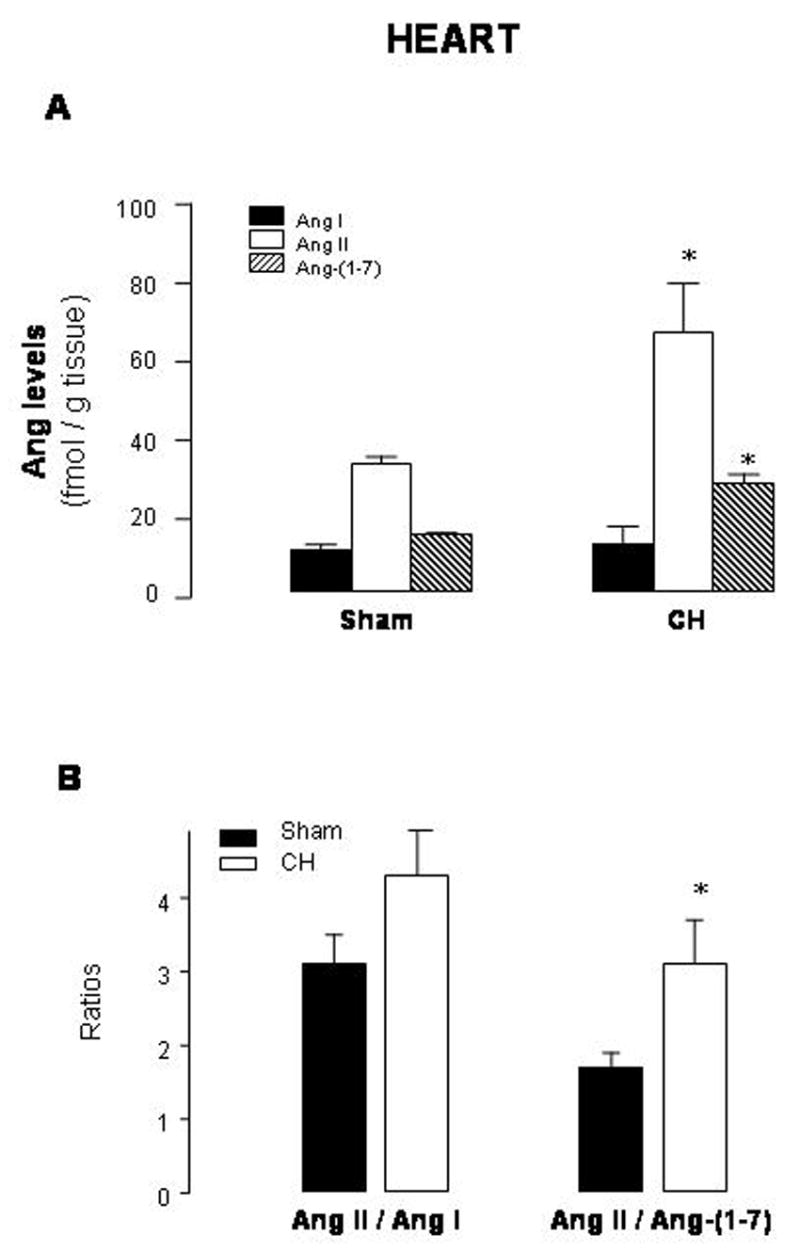
Cardiac levels of angiotensin (Ang) I, Ang II and Ang-(1-7) (A) and Ang II/Ang I and Ang II/Ang-(1-7) ratios (B) in sham-operated rats (sham) and rats with aortic coarctation-induced hypertension (CH). *P<0.05 compared with sham rats. n=10–12
In the hypothalamus, equimolar amounts of Ang II and Ang-(1-7) were found in the sham group, whereas only Ang-(1-7) levels were significantly increased in CH rats (levels in fmol/g tissue of Ang I: 50.0±5.6 in sham vs 74.4±12.3 in CH; Ang II: 196.9±9.6 in sham vs 220.7±37.3 in CH; Ang-(1-7): 142.6±22.5 in sham vs 232.9±33.1 in CH) (Fig. 5A). Ang II/Ang I and Ang II/Ang-(1-7) were not changed (Fig. 5B).
Fig. 5.
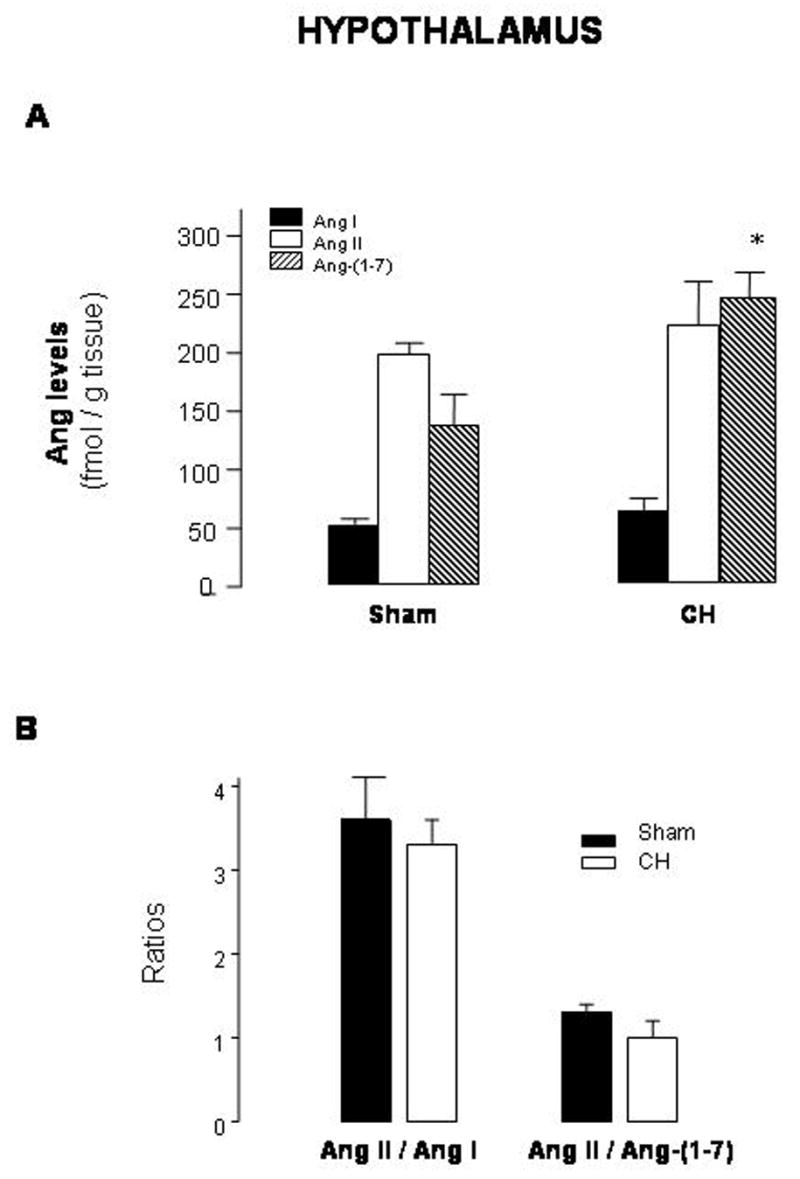
Levels of angiotensin (Ang) I, Ang II and Ang-(1-7) (A) and Ang II/Ang I and Ang II/Ang-(1-7) ratios (B) in hypothalami from sham-operated rats (sham) and rats with aortic coarctation-induced hypertension (CH). *P<0.05 compared with sham rats. n=10–12
4. Discussion
The key observations derived from the present work is that a renin-dependent model of hypertension induced by ligation of the aorta between the renal arteries is associated with a significant increase in not only plasma but also tissue concentrations of Ang II and Ang-(1-7) in the kidney and heart. Ang II was the predominant peptide in both tissues, shifting the balance of Ang peptides toward the vasoconstrictor peptide. Conversely, in hypothalamus from these hypertensive rats only Ang-(1-7) levels were augmented.
Our observation of increased plasma Ang I, Ang II and Ang-(1-7) levels is in agreement with the elevated plasma renin activity reported in CH rats [5,6] and confirms that the systemic RAS is activated. The elevated plasma Ang levels may result from the augmented plasma renin activity arising from the reduced perfusion pressure of the kidney below the constriction previously reported in CH rats [5,6,25–27,31], suggesting that activation of the enzyme contributes to the increased production of Ang peptides. Similarly, augmented Ang I and Ang II levels as a result of increased plasma renin activity have also been reported in two-kidney, one-clip (2K, 1C) hypertensive rats [13], another renovascular hypertensive rat model characterized by RAS hyperactivity. Plasma conversion of Ang I to Ang II as reflected by the Ang II/Ang I ratio, which is an index of angiotensin-converting enzyme (ACE) activity [21], was similar in normotensive and CH rats (present results), suggesting that ACE activity was not modified. In agreement with this, Guan at al. [13] have shown that ACE activity did not change 7 days after clipping one renal artery in 2K, 1C rats.
In the present study, we found that renal content of Ang peptides was elevated in CH rats compared to the normotensive group, with Ang II being the predominant peptide. Due to exposure of this kidney to increased blood pressure, one might anticipate that juxtaglomerular renin would be reduced. Since we did not measure renin activity or concentration in renal tissue, the markedly elevated tissue levels of Ang I and Ang II suggest no suppression of the local tissue RAS. The increase in Ang peptides may result from local renin secretion, outside the juxtaglomerular cells. However, Wang et al. [29] reported finding no difference in either renal renin activity or renin mRNA expression above the coarctation in normotensive and CH rats. In agreement with our results, Guan et al.[13] described elevated Ang levels with no change in renin activity in the non-clipped kidney from 2K,1C rats. A possible explanation may be that the increased renal levels of Ang II (present results) may be due to renal uptake of circulating renin, as suggested by Campbell et al. in kidneys from transgenic hypertensive rats [2]. In fact, Ang formation mediated by renin uptake has been demonstrated in cardiac and coronary vascular tissues [8,20] and in the endothelium [14]. Our results indicate that conversion of Ang I to Ang II was not changed, that is, the Ang II/Ang I ratio in the CH rat kidney was similar to that of the sham-operated rats. On the other hand, conversion of Ang II to Ang-(1-7) was decreased in the CH kidney suggesting diminished activity of an enzyme involved in Ang-(1-7) generation. One may speculate that ACE2, which efficiently degrades Ang II to Ang-(1-7) [1,7,22], may be reduced. In fact, in rat models of hypertension, renal ACE2 mRNA and protein expression are markedly reduced [7]. Thus, diminished renal ACE2 activity in CH rats may lead to increased Ang II with resultant promotion of Na+ retention. Although the ratios may provide an index of ACE and ACE2 activity they do not definitely measure the activities of the enzymes. Furthermore, other enzymes such us prolyl endopeptidases, thimet oligopeptidases or neutral endopeptidases 24.11 may be involved [4,30]. The contribution of these enzymes to Ang-(1-7) generation may be directed by the tissue-specific distribution of the appropriate enzyme. Further studies will have to be done to characterize the involvement of specific enzymes.
Urine excretion of Ang I, Ang II and Ang-(1-7) were several-fold higher in CH rats compared to normotensive controls, with Ang I being the predominant peptide. This may not be due to increased plasma filtration in the kidney, since Ang concentrations were substantially greater than in plasma of CH rats, implicating the kidney as a site for Ang peptide production. In agreement with this, augmented urinary Ang excretion has been reported in spontaneously and transgenic hypertensive rats [32] and in animals exposed to chronic treatment with either an ACE inhibitor or Ang II receptor blocker [16].
Cardiac levels of Ang I were not modified in CH rats, whereas Ang II and Ang-(1-7) content were significantly increased, suggesting that Ang II and Ang-(1-7) production may be compartmentalized in the rat heart, as previously demonstrated in the dog heart [9]. The existence of a cardiac RAS has been a matter of debate since a controversy on the question of local synthesis versus uptake from the circulation exists [23]. However, it has been recently demonstrated an intracellular synthesis of Ang II by isolated cardiac myocytes and fibroblasts [28] supporting the idea of local cardiac RAS synthesis.
Our results showed that the cardiac Ang II/Ang-(1-7) ratio was increased in CH rats, suggesting that the Ang-(1-7) generation pathway is diminished, favoring Ang II accumulation. This increase in Ang II formation in the heart may contribute to the pathogenesis of cardiac hypertrophy observed in this model of hypertension (present results and those of Chatelain et al. [5]). In fact, in transgenic mice overexpressing angiotensinogen exclusively in the heart, it was shown that a locally activated RAS induces cardiac hypertrophy [19].
The present results indicate that in contrast to plasma and peripheral tissues, the hypothalamus in CH rats experienced a specific increase in Ang-(1-7) content with no change in Ang I and Ang II levels, suggesting that the heptapeptide may play a mitigating role in the central control of blood pressure regulation in this hypertensive process. This is the first report demonstrating increased levels of Ang-(1-7), which in addition are slightly higher than those of Ang II, at the central level in a renovascular hypertensive model. These augmented Ang-(1-7) levels in hypothalami from these rats support the fact that the heptapeptide not only diminished NE release but also blocked Ang II-enhanced norepinephrine outflow from hypothalami isolated from CH rats [12], thus diminishing sympathetic nervous system activity and contributing to reduce blood pressure. Furthermore, we found that intrahypothalamic injections of Ang-(1-7) in conscious CH rats blocked the increase in blood pressure caused by Ang II [15].
5. Conclusions
In summary, the present study demonstrates that Ang levels are increased in plasma and local tissues of CH rats and provides further evidence that RAS hyperactivity promotes elevated arterial pressure in the acute state of this model of renovascular hypertension. Nevertheless, the increasing complexity of the RAS requires further detailed analysis of the expression and/or activity of tissue angiotensin forming enzymes. Our results show that Ang II is the predominant peptide found in the heart and kidney, and these data provide direct support for an Ang II-dependent mechanism of hypertension and cardiac hypertrophy in this model. Despite the fact that a cardioprotective [11, 18] and a counterregulatory role of Ang II actions have been suggested for Ang-(1-7) [10], its increased levels may not be enough to overcome the Ang II-induced cardiac hypertrophy and Na+-enhanced retention observed in this hypertensive model. On the other hand, the increased central levels of Ang-(1-7) in CH rats, together with the fact that Ang-(1-7) blocked central Ang II actions in these hypertensive rats [12,15], suggest a potential mitigating role of this peptide in central control of the hypertensive process. The new information on the brain levels in conjunction with the peripheral tissues provide a more complete assessment of the RAS in this model of hypertension.
Acknowledgments
This work was supported by grants from University of Buenos Aires, Agencia Nacional de Promoción Científica y Técnica, CONICET and NIH/NHLBI HL67363.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burrell LM, Johnston CI, Tikellis C, Cooper ME. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab. 2004;15:166–169. doi: 10.1016/j.tem.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell DJ, Rong P, Kladis A, Rees B, Ganten D, Skinner SL. Angiotensin and bradykinin peptides in the TGR(mRen-2)27 rat. Hypertension. 1995;25:1014–1020. doi: 10.1161/01.hyp.25.5.1014. [DOI] [PubMed] [Google Scholar]
- 3.Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin-(1-7) in rat brain. J Biol Chem. 1989;264:16518–16523. [PubMed] [Google Scholar]
- 4.Chappell MC, Tallant EA, Brosnihan KB, Ferrario CM. Conversion of angiotensin I to angiotensin-(1-7) by thimet oligopeptidase (EC 3.4.24.15) in vascular smooth muscle cells. J Vasc Med Biol. 1994;5:129–137. [Google Scholar]
- 5.Chatelain RE, Ehrhart LA, DiBello PM, Dardik BN, Shainoff JR, Ferrario CM. Impaired arterial collagen and elastin metabolism in experimental malignant hypertension. J Lab Clin Med. 1981;97:700–717. [PubMed] [Google Scholar]
- 6.Chatelain RE, Muñoz-Ramirez H, Dardik BN, Ehrhart LA, Shainoff JR, Ferrario CM. Contribution of humoral factors to vascular damage during the acute phase of renal hypertension. In: Villarreal H, editor. Hypertension. John Wiley & Sons, Inc; New York: 1983. pp. 149–158. [Google Scholar]
- 7.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 8.Danser AHJ. Local renin-angiotensin systems: the unanswered questions. Int J Biochem Cell Biol. 2003;35:759–768. doi: 10.1016/s1357-2725(02)00178-4. [DOI] [PubMed] [Google Scholar]
- 9.Dell’Italia LJ, Meng QC, Balcells E, Wei C, Palmer R, Hageman GR, Durand J, Hankens GH, Oparil S. Compartmentalization of angiotensin II generation in the dog heart. J Clin Invest. 1997;100:253–258. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1-7) Hypertension. 1997;30(part 2):535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 11.Ferrario CM. Does angiotensin-(1-7) contribute to cardiac adaptation and preservation of endothelial function in heart failure? Circulation. 2002;105:1523–1525. doi: 10.1161/01.cir.0000013787.10609.dc. [DOI] [PubMed] [Google Scholar]
- 12.Gironacci MM, Yujnovsky I, Gorzalczany S, Taira C, Peña C. Angiotensin-(1-7) inhibits the angiotensin II-enhanced norepinephrine release in coarcted hypertensive rats. Regul Pept. 2004;118:45–49. doi: 10.1016/j.regpep.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension. 1992;20:763–767. doi: 10.1161/01.hyp.20.6.763. [DOI] [PubMed] [Google Scholar]
- 14.Hilgers KF, Veelken R, Müller DN, Kohler H, Hartner A, Botkin SR, Stumpf C, Schmieder RE, Gomez RA. Renin uptake by the endothelium mediates vascular angiotensin formation. Hypertension. 2001;38:243–248. doi: 10.1161/01.hyp.38.2.243. [DOI] [PubMed] [Google Scholar]
- 15.Höcht C, Opezzo JAW, Gironacci MM, Peña C, Taira CA. Efectos hipotalámicos de la angiotensina- (1-7) en ratas con coartación aórtica. Revista Argentina de Cardiología. 2005;73:1–5. [Google Scholar]
- 16.Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of angiotensin-(1-7) unmasked during combined treatment with lisinopril and losartan. Hypertension. 1998;31:699–705. doi: 10.1161/01.hyp.31.2.699. [DOI] [PubMed] [Google Scholar]
- 17.Kohara K, Tabuchi Y, Senanayake P, Brosnihan KB, Ferrario CM. Reassessment of plasma angiotensin measurement: effects of protease inhibitors and sample handling procedures. Peptides. 1991;12:1135–1141. doi: 10.1016/0196-9781(91)90070-6. [DOI] [PubMed] [Google Scholar]
- 18.Loot AE, Roks AJM, Henning RH, Tio RA, Suurmeijer AJH, Boomsma F, van Gilst WH. Angiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- 19.Mazzolai L, Pedrazzini T, Nicoud F, Gabbiani G, Brunner H, Nussberger J. Increased cardiac angiotensin II levels induce right and left ventricular hypertrophy in normotensive mice. Hypertension. 2000;35:985–991. doi: 10.1161/01.hyp.35.4.985. [DOI] [PubMed] [Google Scholar]
- 20.Müller DN, Fischli W, Clozel JP, Hilgers KF, Bohlender J, Ménard J, Busjahn A, Ganten D, Luft FC. Local angiotensin II generation in the rat heart. Role of renin uptake. Circ Res. 1998;82:13–20. doi: 10.1161/01.res.82.1.13. [DOI] [PubMed] [Google Scholar]
- 21.Nussberger J, Brunner D, Keller I, Brunner HR. Measurement of converting enzyme activity by antibody-trapping of generated angiotensin II: comparison with two other methods. Am J Hypertens. 1992;5:393–398. doi: 10.1093/ajh/5.6.393. [DOI] [PubMed] [Google Scholar]
- 22.Oudit GY, Crackower MA, Backx PH, Penninger JM. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13:93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 23.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 24.Rojo-Ortega JM, Genest J. A model for production of experimental hypertension in rats. Can J Physiol Pharmacol. 1968;241:H273–H278. doi: 10.1139/y68-137. [DOI] [PubMed] [Google Scholar]
- 25.Salgado HC, Fazan R, Salgado MCO. Vasopressor mechanisms in acute aortic coarctation hypertension. Braz J Med Biol Res. 1997;30:447–452. doi: 10.1590/s0100-879x1997000400003. [DOI] [PubMed] [Google Scholar]
- 26.Salgado HC, Salgado MCO. Acute aortic coarctation hypertension: role of vasopressin and angiotensin II. Am J Phsyiol. 1989;257:H1480–H1484. doi: 10.1152/ajpheart.1989.257.5.H1480. [DOI] [PubMed] [Google Scholar]
- 27.Salgado HC, Skelton MM, Salgado MCO, Cowley AW. Physiophathogenesis of acute aortic coarctation hypertension in conscious rats. Hypertension. 1994;23(suppl I):I-78–I81. doi: 10.1161/01.hyp.23.1_suppl.i78. [DOI] [PubMed] [Google Scholar]
- 28.Singh VP, Baker KM, Kumar R. Evidence of intracellular synthesis of angiotensin II in cardiac myocytes and fibroblasts. Hypertension. 2006;48:e42. abstract. [Google Scholar]
- 29.Wang J, Cheng H, Harris RC. Cyclooxygenase-2 inhibition decreases renin content and lowers blood pressure in a model of renovascular hypertension. Hypertension. 1999;34:96–101. doi: 10.1161/01.hyp.34.1.96. [DOI] [PubMed] [Google Scholar]
- 30.Welches WR, Santos RAS, Chappell MC, Brosnihan KB, Greene LJ, Ferrario CM. Evidence that prolyl endopeptidase participates in the processing of brain angiotensin. J Hypertens. 1991;9:631–638. doi: 10.1097/00004872-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Whitlow PL, Katholi RE. Neurohumoral mechanisms in acute aortic coarctation in conscious and anesthetized dogs. Am J Physiol. 1983;244:H614–H621. doi: 10.1152/ajpheart.1983.244.4.H614. [DOI] [PubMed] [Google Scholar]
- 32.Yamada K, Iyer SN, Chappell MC, Brosnihan KB, Fukuhara M, Ferrario CM. Differential response of angiotensin peptides in the urine of hypertensive animals. Regul Pept. 1999;80:57–66. doi: 10.1016/s0167-0115(99)00005-1. [DOI] [PubMed] [Google Scholar]


