Abstract
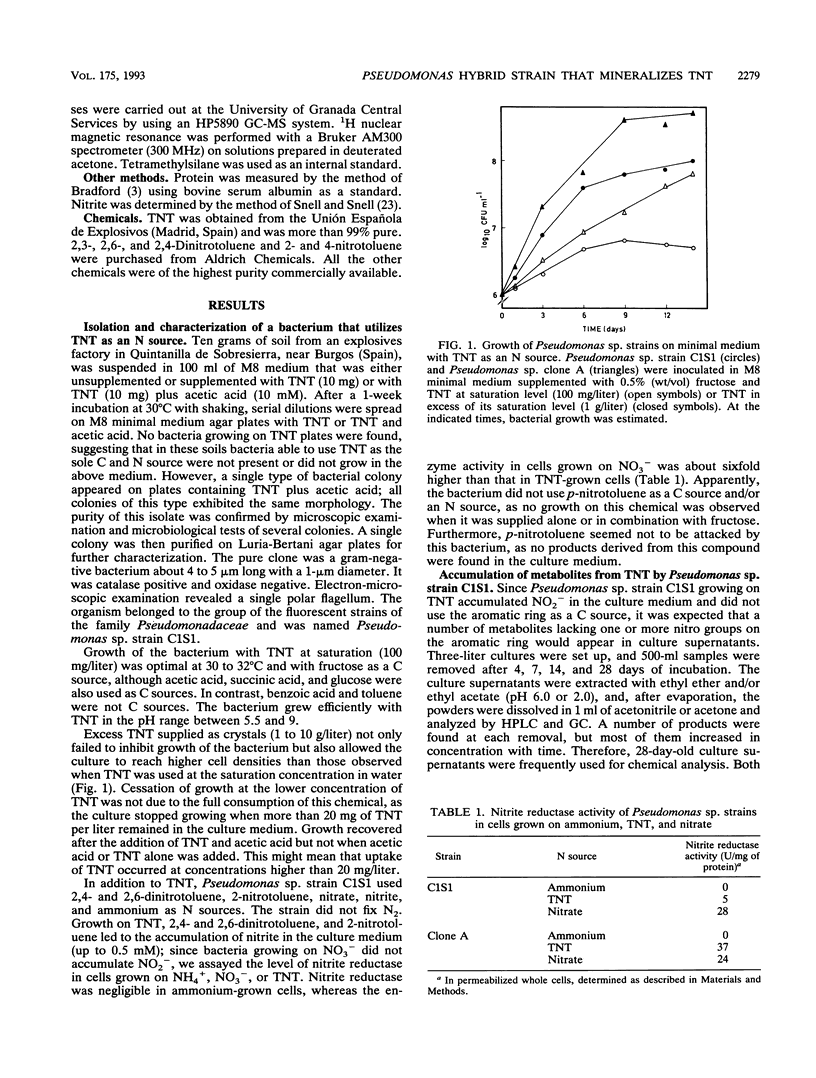
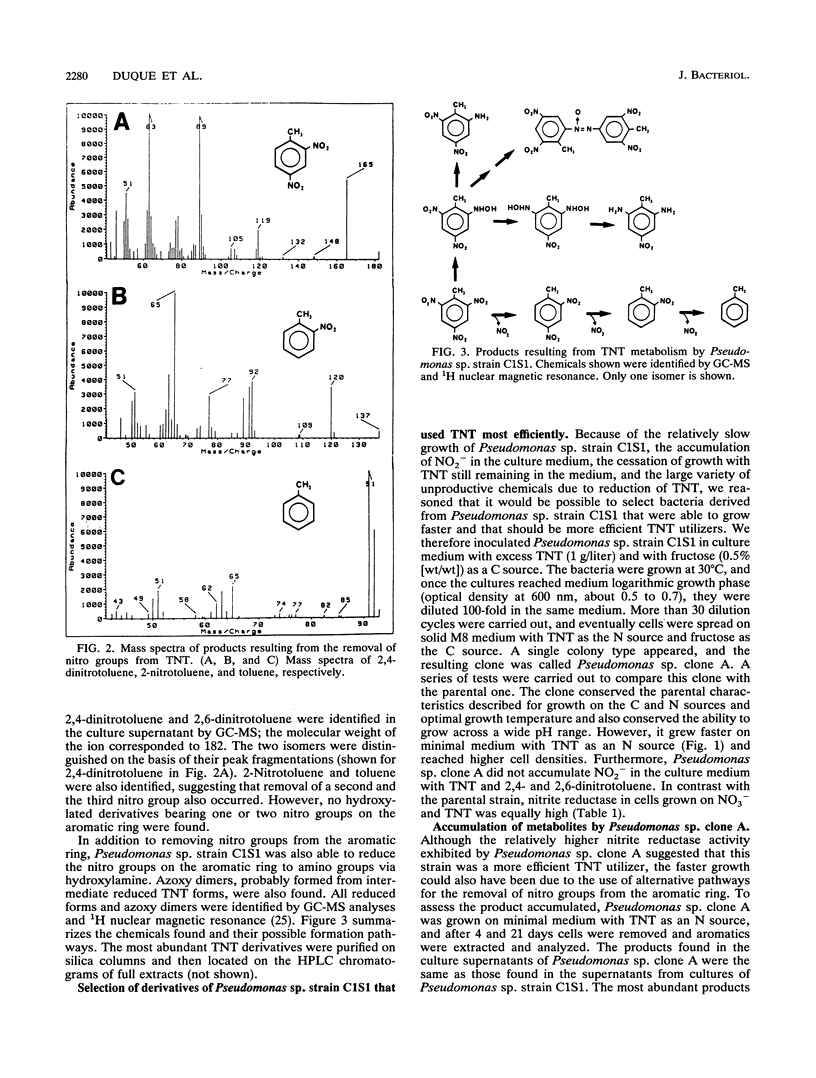
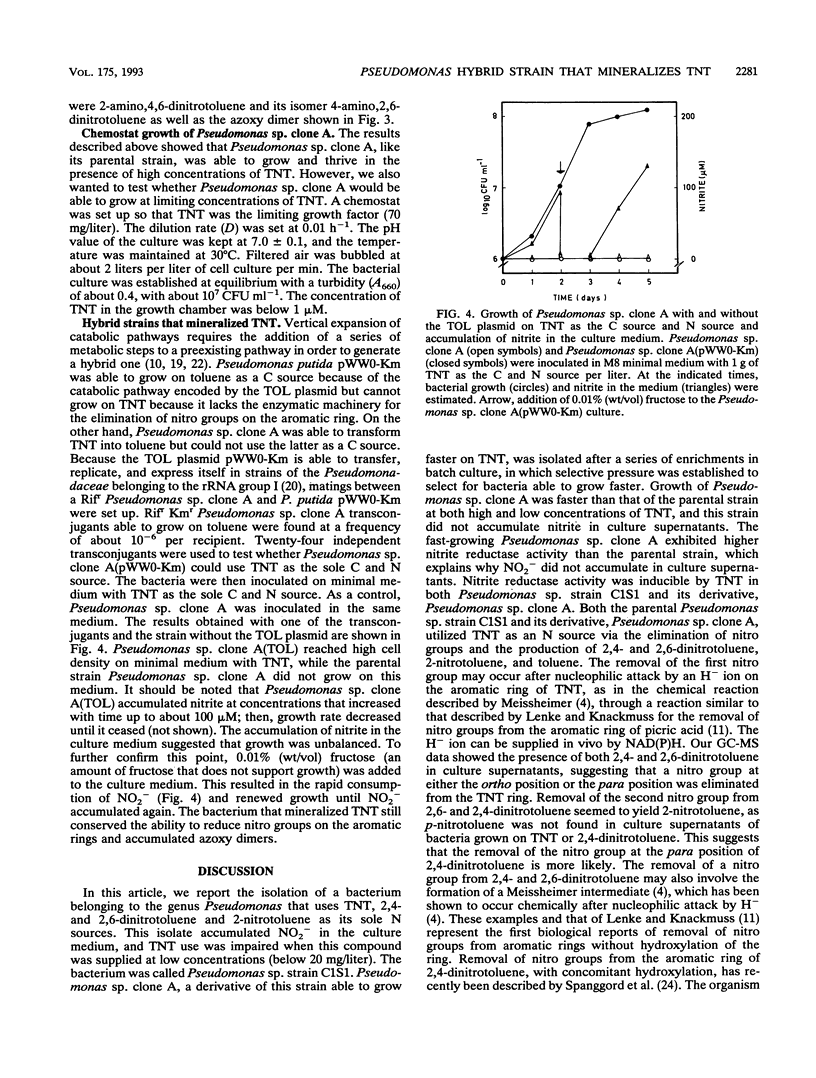
A bacterium, Pseudomonas sp. strain C1S1, able to grow on 2,4,6-trinitrotoluene (TNT), 2,4- and 2,6-dinitrotoluene, and 2-nitrotoluene as N sources, was isolated. The bacterium grew at 30 degrees C with fructose as a C source and accumulated nitrite. Through batch culture enrichment, we isolated a derivative strain, called Pseudomonas sp. clone A, which grew faster on TNT and did not accumulate nitrite in the culture medium. Use of TNT by these two strains as an N source involved the successive removal of nitro groups to yield 2,4- and 2,6-dinitrotoluene, 2-nitrotoluene, and toluene. Transfer of the Pseudomonas putida TOL plasmid pWW0-Km to Pseudomonas sp. clone A allowed the transconjugant bacteria to grow on TNT as the sole C and N source. All bacteria in this study, in addition to removing nitro groups from TNT, reduced nitro groups on the aromatic ring via hydroxylamine to amino derivatives. Azoxy dimers probably resulting from the condensation of partially reduced TNT derivatives were also found.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abril M. A., Michan C., Timmis K. N., Ramos J. L. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J Bacteriol. 1989 Dec;171(12):6782–6790. doi: 10.1128/jb.171.12.6782-6790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M. Biodegradation of chemicals of environmental concern. Science. 1981 Jan 9;211(4478):132–138. doi: 10.1126/science.7444456. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carpenter D. F., McCormick N. G., Cornell J. H., Kaplan A. M. Microbial transformation of 14C-labeled 2,4,6-trinitrotoluene in an activated-sludge system. Appl Environ Microbiol. 1978 May;35(5):949–954. doi: 10.1128/aem.35.5.949-954.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando T., Bumpus J. A., Aust S. D. Biodegradation of TNT (2,4,6-trinitrotoluene) by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Jun;56(6):1666–1671. doi: 10.1128/aem.56.6.1666-1671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W., Hageman R. H. The purification and properties of nitrite reductase from higher plants, and its dependence on ferredoxin. Biochem J. 1966 Jul;100(1):263–273. doi: 10.1042/bj1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. L., Kaplan A. M. Thermophilic biotransformations of 2,4,6-trinitrotoluene under simulated composting conditions. Appl Environ Microbiol. 1982 Sep;44(3):757–760. doi: 10.1128/aem.44.3.757-760.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach P. R., Zeyer J., Reineke W., Knackmuss H. J., Timmis K. N. Enzyme recruitment in vitro: use of cloned genes to extend the range of haloaromatics degraded by Pseudomonas sp. strain B13. J Bacteriol. 1984 Jun;158(3):1025–1032. doi: 10.1128/jb.158.3.1025-1032.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenke H., Knackmuss H. J. Initial hydrogenation during catabolism of picric acid by Rhodococcus erythropolis HL 24-2. Appl Environ Microbiol. 1992 Sep;58(9):2933–2937. doi: 10.1128/aem.58.9.2933-2937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick N. G., Feeherry F. E., Levinson H. S. Microbial transformation of 2,4,6-trinitrotoluene and other nitroaromatic compounds. Appl Environ Microbiol. 1976 Jun;31(6):949–958. doi: 10.1128/aem.31.6.949-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nay M. W., Jr, Randall C. W., King P. H. Biological treatability of trinitrotoluene manufacturing wastewater. J Water Pollut Control Fed. 1974 Mar;46(3):485–497. [PubMed] [Google Scholar]
- Parrish F. W. Fungal transformation of 2,4-dinitrotoluene and 2,4,6-trinitrotoluene. Appl Environ Microbiol. 1977 Aug;34(2):232–233. doi: 10.1128/aem.34.2.232-233.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira W. E., Short D. L., Manigold D. B., Roscio P. K. Isolation and characterization of TNT and its metabolites in groundwater by gas chromatograph-mass spectrometer-computer techniques. Bull Environ Contam Toxicol. 1979 Mar;21(4-5):554–562. doi: 10.1007/BF01685469. [DOI] [PubMed] [Google Scholar]
- Ramos-Gonzalez M. I., Duque E., Ramos J. L. Conjugational transfer of recombinant DNA in cultures and in soils: host range of Pseudomonas putida TOL plasmids. Appl Environ Microbiol. 1991 Oct;57(10):3020–3027. doi: 10.1128/aem.57.10.3020-3027.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J. L., Stolz A., Reineke W., Timmis K. N. Altered effector specificities in regulators of gene expression: TOL plasmid xylS mutants and their use to engineer expansion of the range of aromatics degraded by bacteria. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8467–8471. doi: 10.1073/pnas.83.22.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J. L., Timmis K. N. Experimental evolution of catabolic pathways of bacteria. Microbiol Sci. 1987 Aug;4(8):228–237. [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Construction of haloaromatics utilising bacteria. Nature. 1979 Feb 1;277(5695):385–386. doi: 10.1038/277385a0. [DOI] [PubMed] [Google Scholar]
- Rojo F., Pieper D. H., Engesser K. H., Knackmuss H. J., Timmis K. N. Assemblage of ortho cleavage route for simultaneous degradation of chloro- and methylaromatics. Science. 1987 Dec 4;238(4832):1395–1398. doi: 10.1126/science.3479842. [DOI] [PubMed] [Google Scholar]
- Spanggord R. J., Spain J. C., Nishino S. F., Mortelmans K. E. Biodegradation of 2,4-dinitrotoluene by a Pseudomonas sp. Appl Environ Microbiol. 1991 Nov;57(11):3200–3205. doi: 10.1128/aem.57.11.3200-3205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. Catalysis of pentose phosphate pathway reactions by cytoplasmic fractions from muscle, uterus and liver of the rat, and the presence of a reduced nicotinamide-adenine dinucleotide phosphate-triose phosphate oxidoreductase in rat muscle. Biochem J. 1974 Jan;138(1):71–76. doi: 10.1042/bj1380071. [DOI] [PMC free article] [PubMed] [Google Scholar]


