Abstract
Intracranial headaches such as that of migraine are generally accepted to be mediated by prolonged activation of meningeal nociceptors but the mechanisms responsible for such nociceptor activation are poorly understood. In this study, we examined the hypothesis that meningeal nociceptors can be activated locally through a neuroimmune interaction with resident mast cells, granulated immune cells that densely populate the dura mater. Using in vivo electrophysiological single unit recording of meningeal nociceptors in the rat we observed that degranulation of dural mast cells using intraperitoneal administration of the basic secretagogue agent compound 48/80 (2 mg/kg) induced a prolonged state of excitation in meningeal nociceptors. Such activation was accompanied by increased expression of the phosphorylated form of the extracellular signal-regulated kinase (pERK), an anatomical marker for nociceptor activation. Mast cell - induced nociceptor interaction was also associated with downstream activation of the spinal trigeminal nucleus as indicated by an increase in c-fos expression. Our findings provide evidence linking dural mast cell degranulation to prolonged activation of the trigeminal pain pathway believed to underlie intracranial headaches such as that of migraine.
1. Introduction
Migraine headache is one of the most common pain syndromes, affecting approximately 15% of the population (Lipton and Bigal 2005). Although mechanisms underlying the onset of a migraine attack are not completely understood, activation of pain fibers that innervate the dura mater (i.e., meningeal nociceptors) is believed to play a key role in promoting the intracranial pain of migraine (Pietrobon and Striessnig 2003; Strassman et al. 1996; Waeber and Moskowitz 2005). While the dura mater is the one intracranial structure most heavily innervated by pain fibers, it is also densely populated by immune cells. Among those are resident mast cells (MC), granulated immune cells that play a critical role in inflammation. MC are found throughout the intracranial dura mater in both humans (Artico and Cavallotti 2001) and rodents (Dimlich et al. 1991; Rozniecki et al. 1999; Strassman et al. 2004) where they reside near blood vessels and in close apposition to primary afferent nociceptive neurons (Dimlich et al. 1991; Rozniecki et al. 1999; Strassman et al. 2004).
Experimental work in animals has shown that electrical stimulation of the trigeminal ganglion, leading to activation of meningeal nociceptors, promotes the release of the granule content (i.e. degranulation) of dural MC (Buzzi et al. 1992; Dimitriadou et al. 1991). Such activation of meningeal nociceptors has been hypothesized to take place during migraine with aura in response to local release of protons, potassium ions, and glutamate (Bolay et al. 2002; Pietrobon and Striessnig 2003) in the wake of cortical spreading depression (CSD). Activated meningeal nociceptors appear to release neuropeptides such as substance P and calcitonin-gene-related peptide (CGRP) that induce the activation and degranulation of resident dural MC (Ottosson and Edvinsson 1997; Rozniecki et al. 1999). The local release of inflammatory molecules from degranulated MC during such neurogenic inflammation is believed to further stimulate meningeal nociceptors to promote a prolonged migraine headache. However, whether MC degranulation can promote such prolonged activation of meningeal nociceptors remains to be determined. In this study, using in vivo electrophysiological recording of meningeal nociceptors and immunocytochemical labeling of activated meningeal nociceptors we provide the first evidence that MC degranulation can produce a lasting activation of meningeal nociceptors. Using c-fos immunohistochemistry we further show that dural MC degranulation can also promote downstream activation of nociceptive neurons in the spinal trigeminal nucleus.
2. Materials and Methods
2. 1 Animals, induction of mast cell degranulation, and histological assessment
For all experiments, adult male rats (Sprague-Dawley. 250–350g, Taconic) were used. All experiments were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center and the standing committee on animals of the Harvard Medical School. Anesthesia was achieved using urethane (1.5 mg/kg i.p.). Dural MC degranulation was achieved using intraperitoneal administration of a sub-anaphylactic dose of the basic secretagogue agent compound 48/80 (2 mg/kg, in sterile saline, i.p., Biomol, Plymouth Meeting, PA). This agent promotes MC degranulation by directly activating a G-protein signaling pathway that mimics the activation of the high affinity IgE receptor in MC (Shefler and Sagi-Eisenberg 2001). For quantitative histological assessment, dural whole-mounts were prepared as described earlier (Strassman et al. 2004). Dural MC were visualized using acidified toluidine blue solution (TB, pH 2.4) and counted in a blinded manner under X200 magnification in 10 random fields. Cells were considered degranulated if there was an extensive dispersion of more than 15 extruded vesicles localized near the cell, or when there was an extensive loss of granule staining, giving the cell a “ghostly” look.
2.2 Electrophysiological recording of meningeal nociceptors in vivo
2.2.1. Surgery
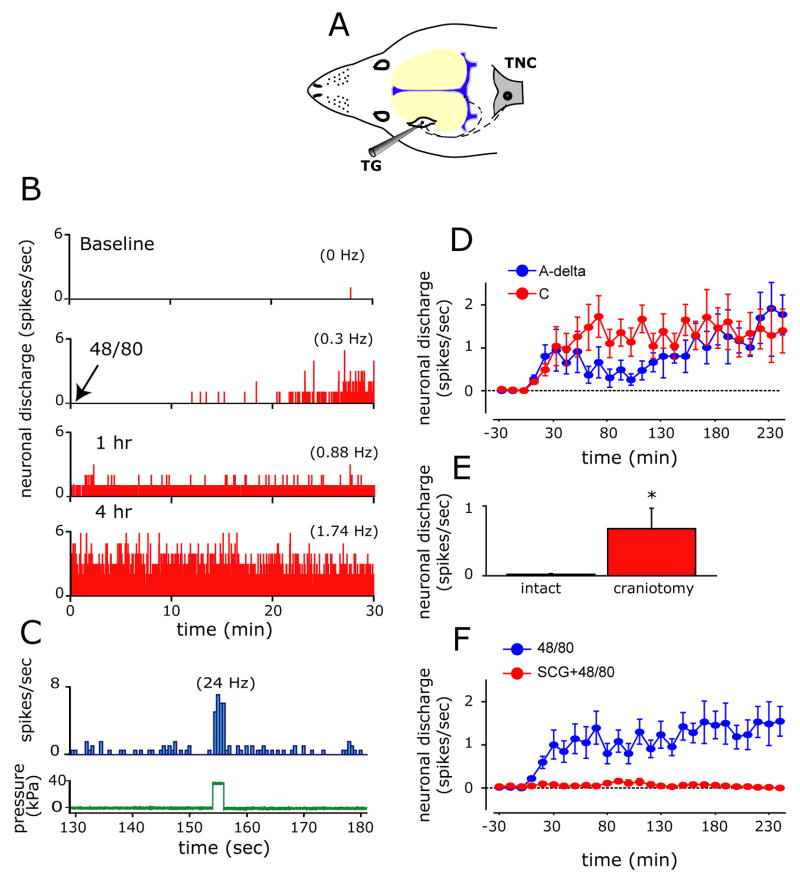
Anesthetized rats were fitted with cannulas (PE50) in the left femoral vein for injections of fluids (Ringer/0.9% saline solutions) and the femoral artery for gauging mean arterial pressure and heart rate with a transducer (Statham ID P23; Statham Instruments Inc., Osnard, CA). Rats were then placed in a stereotaxic head-holder. Core body temperature was maintained at 37°C using a feedback-controlled heating pad. In our previous electrophysiological studies of meningeal nociceptors we have exposed the dura mater following a craniotomy to examine the local effect of various algesic mediators on meningeal nociceptors. However, others (Markowitz et al. 1989) and we (this study) have noted that such surgical procedure results in a robust depletion of MC granules, as evidenced by the almost complete loss of TB staining in the underlying dura (see also Fig. 6A.). This effect precluded us from achieving a specific local controlled degranulation of dural MC, which is necessary to examine the effect of local MC degranulation on the activity of meningeal nociceptors. To circumvent this problem we used a novel surgical approach (see below) that allowed us to maintain an intact, non-degranulated population of MC within the dural receptive field territory of the recorded meningeal nociceptors. This procedure also limited the resulting local inflammation and potential nociceptor activation associated with such extensive craniotomy and dural exposure. Following exposure of the parietal and occipital bones two burr holes (1 mm diameter, 2 mm separation) were drilled to thin the cranial bone over the left transverse sinus using a saline-cooled dental drill. The minimal exposure was needed to allow the placement of the two silver wire stimulating electrodes needed for the identification of meningeal nociceptors without interrupting the underlying dura. The position of the stimulating electrode was chosen because it corresponds to the zone where the dural sensory fibers of the tentorial nerve first reach the dura in their course from the trigeminal ganglion before spreading out to innervate the supratentorial dura. For electrophysiological single-unit recordings of meningeal nociceptors a platinum-coated tungsten microelectrode (FHC, Bowdoin, ME) was advanced into the left trigeminal ganglia (TG) through a distant small craniotomy and durectomy (2 mm caudal to Bregma, 2–2.5 mm lateral, and 9.5–10 mm below the cortical surface). At the end of the experiment, in order to allow testing of neuronal mechanosensitivity and the effect of locally applied chemicals, a full craniotomy (~4 mm diameter) was made to expose the dural receptive field of the recorded neuron. Mechanosensitivity was then tested using von Frey hairs or a feedback-controlled mechanical stimulator as described previously (Levy and Strassman 2002b).
Figure 6.
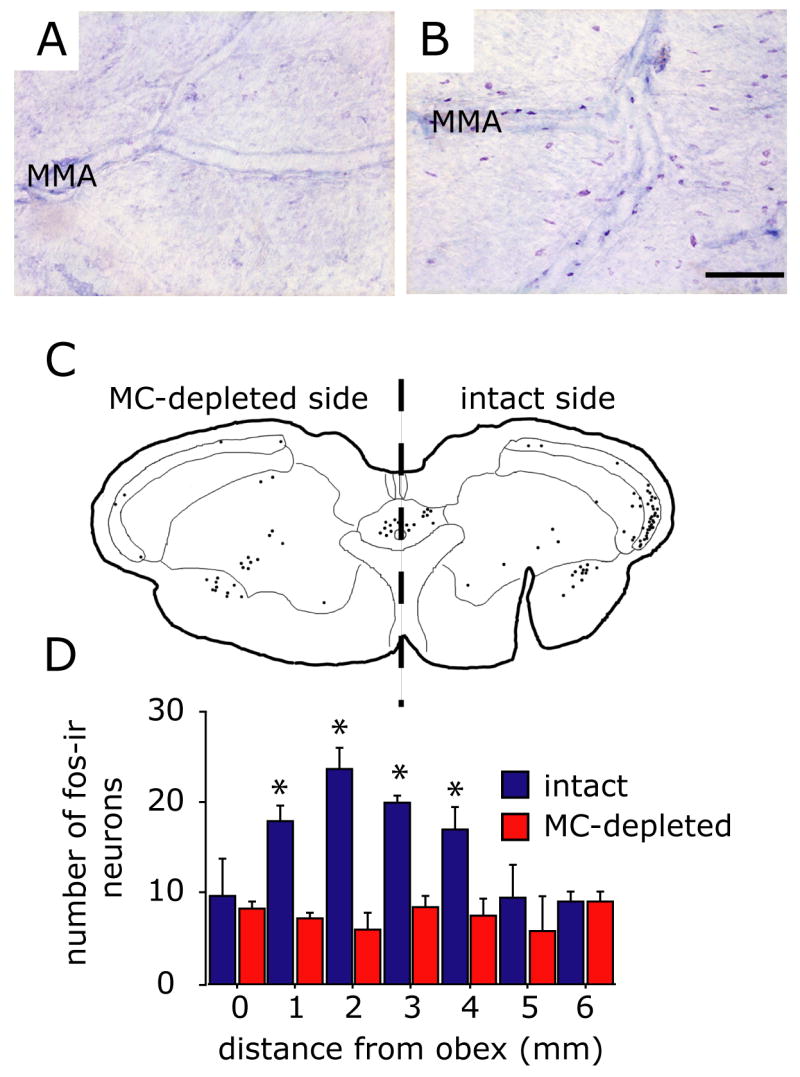
Depletion of dural mast cell granules blocks 48/80-induced fos expression in the TNC. (A and B) Samples of TB-stained dura mater taken from an animal undergoing a unilateral craniotomy 24 hours earlier. Note that on the craniotomized side (A) there is a complete loss of TB-staining of dural MC indicating granule depletion while the intact, non-craniotomized side (B) shows the typical level of MC degranulation following 48/80 treatment. (C) Cameral Lucida reconstruction of the anatomical location of fos-IR cells within the superficial dorsal horn (laminae I and II) at the level of the caudal TNC following MC degranulation in an animal that underwent a unilateral MC depletion using craniotomy. Each drawn section plots the location of fos-IR cells from 3 consecutive, alternate, 40 mm sections. Note that dural MC depletion blocked fos-IR only in the TNC ipsilateral to the depleted side. (D) Plot of the rostrocaudal distribution of the mean number of fos-IR cells in the dorsal horn ipsi- and contralateral to the MC depleted side in animals injected with 48/80 (n=4). Asterisks indicate a significant difference between the ipsi- and contralateral sides (p<0.05, Fisher PLSD test). Scale bar = 200 μm.
2.2.2. Identification of meningeal nociceptors
Identification of meningeal nociceptors was made as reported (Levy and Strassman 2002a; Strassman et al. 1996) by delivering single-shock electrical stimuli (0.5 Hz, 0.05–0.5 ms pulse duration, 0.5–5 mA) through the bipolar silver stimulating electrodes. The slow rate and relatively short pulse duration were chosen to avoid possible afferent activity-evoked dural MC degranulation prior to 48/80 administration. Meningeal nociceptors were then identified by their constant latency response to single shock stimulation. Response latencies were used to calculate conduction velocity (CV) based on a 12.5 mm distance to the TG (Strassman et al. 1996) and nociceptors were classified as either C-units (CV≤1.5 m/sec) or A-delta units (CV>1.5 m/sec). A waveform of the action potential evoked by the electrical stimuli was stored as a template using a real-time waveform discriminator (Spike 2, CED, Cambridge, UK) which was used to acquire experimental data and perform on- and off-line analyses. In all experiments, only one unit was tested in each animal.
2.3 pERK expression in meningeal nociceptors
Fifteen minutes following administration of 48/80 the dura mater was removed as described above. Dura specimens were processed free-floating and stained with either a monoclonal or a polyclonal anti-pERK Abs (#4376, 1:5000 or #9101, 1:1000 respectively; Cell Signaling Technology, Danvers, MA). pERK expression was visualized using the ABC method (Vector Laboratories, Burlingame, CA). After immunolabeling, dura specimens were counterstained with toluidine blue to visualize dural MC. Antibodies specificity was tested by omission of the primary antibody or by preadsorption with a blocking peptide (#1150, Cell Signaling Technology). Changes in pERK neuronal expression in the dura were determined by counting pERK positive nerve fibers under X200 magnification in 15 visual fields in an area bordered by the transverse sinus, superior sagittal sinus and the middle meningeal artery. The number of pERK-positive fibers that were localized in the vicinity (<100μm) of a degranulated MC was further determined under the same magnification. For double immunofluorescence detection, dura specimens were incubated with a mixture of the monoclonal anti-pERK and a polyclonal goat anti-CGRP antibodies (T-5027, 1:500, Bachem, San Carlos, CA ) followed by incubating with a mixture of appropriate Alexa Fluor 488 (Invitrogen, Carlsbad, CA) and Cy3 (Sigma, St. Louis, MO) conjugated secondary antibodies. The percentage of pERK immunoreactive (pERK-IR) fibers that also demonstrated CGRP-IR was determined under X200 magnification in 10 random visual fields in the same dural areas mentioned above.
2.4. C-Fos immunohistochemistry and cell counts
Two and a half hours after administration of compound 48/80, animals were perfused and transverse 40 μm sections of the caudal medulla and upper cervical spinal cord were prepared as described (Strassman et al. 1994). Fos-IR was detected using a polyclonal rabbit antibody to C-fos (Ab-2, 1:20,000, Calbiochem, San Diego, California), and the ABC method followed by DAB and 0.02% Nickel sulfate to enhance staining. Specificity was tested as above. Cell nuclei that were labeled with c-fos were counted in alternate sections (every other 40 μm section) through a 6-mm long segment of the medullary dorsal horn starting at the obex (−14.3 from Bregma according to (Paxinos G. 1998). This 6-mm continuum thus covered the following structures: the transition zone between trigeminal subnucleus interpolaris and subnucleus caudalis (Vi/Vc), the subnucleus caudalis zone (Vc), C1, and the rostral part of C2 (Strassman et al. 1994). Data were expressed as mean number of labeled cell nuclei counted per 1-mm block in the above continuum.
2.5 Data analysis and statistics
MC-degranulation and Fos-IR data were analyzed using 1-way ANOVA followed by post hoc Fisher’s PLSD test. Electrophysiological and hemodynamic (mean arterial pressure and heart rate) data were analyzed using Repeated Measures ANOVA and Fisher’s PLSD post-hoc comparisons. The Mann-Whitney U test was used to analyze changes in the number of fibers expressing pERK. p<0.05 was considered significant for all studies.
3. Results
3.1 Excitation of meningeal nociceptors by mast cell degranulation in vivo
We first investigated whether MC degranulation promotes activation of primary afferent nociceptive neurons innervating the cerebral meninges in vivo using single unit electrophysiological recording from the trigeminal ganglion. In 22 animals, 22 neurons were tested, including 12 C-units (CV 0.95±0.1 m/sec, range 0.37–1.25 m/sec) and 10 A-delta units (CV 4.57±0.6 m/sec, 1.6–6.9 m/sec). At baseline, prior to MC degranulation, all neurons were silent or had a very low rate of ongoing discharge (range 0.01–0.05 Hz). Administration of the secretagogue caused a prominent degranulation in more than half of the dural MC population (Table 1, Fig. 1B and D) without significantly effecting either the mean arterial pressure (101.3±3.6 mmHg at baseline vs. 105.6±4.9 mmHg at 30 min after 48/80, p=0.49) or heart rate (382.9±27.6 beats/min at baseline vs. 391.7±24.7 beats/min at 30 min after 48/80, p=0.52). Such MC degranulation was associated with neuronal excitation in 10/12 (83%) of the C-units tested and 3/10 (30%) of the A-delta units tested. Regardless of their conduction velocity, in neurons affected by MC degranulation, the onset of increased neuronal activity was observed 10–20 minutes following administration of the secretagogue (Fig. 2B and D). This initial excitation was followed by a progressive increase in the ongoing discharge rate (Fig. 2D) such that increased neuronal activity (1.5±0.3 Hz, range 0.4–3.6 Hz) was still significantly elevated at 4 hours in 10 out of the 13 excited neurons. Most of the neurons (11/13) that responded to MC degranulation were deemed mechanosensitive when tested by direct dural stimulation following surgical exposure of the dura at the end of the experiment (Fig. 2C). In addition to their greater propensity to become excited by the MC degranulation, C-units exhibited a different pattern of excitation, exhibiting higher firing rates 30–110 minutes after their initial activation as compared to the A-delta units (Fig. 2D).
Table 1.
Effects of systemic treatment with vehicle (saline), 48/80, 48/80 following sodium cromoglycate (SCG+48/80) and 48/80 following sumatriptan on the level of mast cells degranulation within the intracranial dura mater. Data is presented as mean ± SEM.
| Treatment group | Degranulation (%) | Number of total counted cells per group |
|---|---|---|
| Saline (n=7) | 19±6 | 4828 |
| 48/80 (n=10) | 65±10* | 8830 |
| 48/80+SCG (n=8) | 25±4# | 7032 |
| No treatment (n=8) | 17±17 | 4958 |
| 48/80+Sumatriptan (n=5) | 59±15* | 3012 |
p<0.01 compared with saline control
p<0.05, compared with 48/80 treatment, Fisher’s PLSD test
Figure 1.
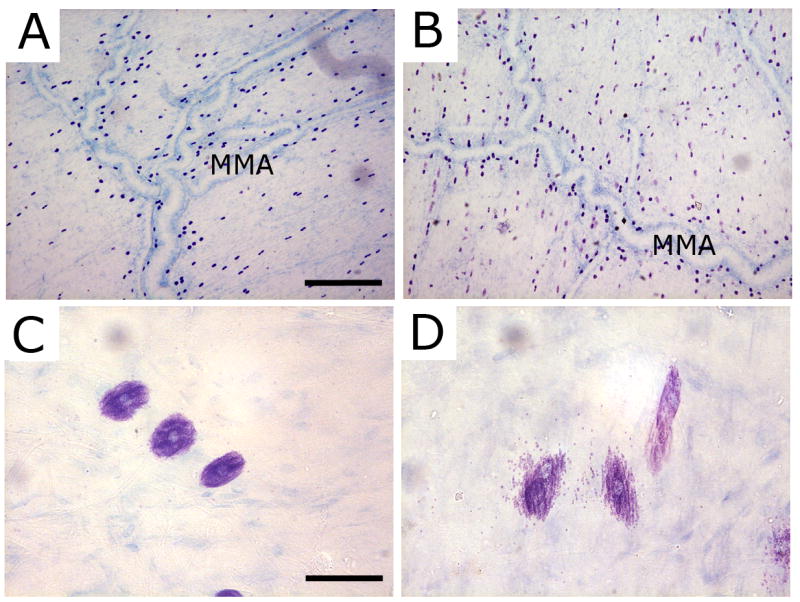
Degranulation of dural mast cells by compound 48/80. (A and C) Representative TB-stained dura whole-mount taken from a control animal treated with saline. Note in high magnification (C) the normal appearance of dural MC. (B and D) Toluidine blue - stained dura whole-mount taken 20 minutes following the administration of 48/80. Note the robust signs of degranulation in D. MMA - middle meningeal artery. Scale bar = 500 μm in A and B and 50 μm in C and D.
Figure 2.
Mast cell degranulation triggers prolonged excitation of meningeal nociceptors. (A) Schematic localization of the recording site for meningeal nociceptors in the trigeminal ganglion (TG). The figure also illustrates the trigeminovascular pathway from the meninges to the central trigeminal nucleus caudalis (TNC). (B) Selected 30 minutes recording periods demonstrating the neuronal activity (bin size 1 sec) of an affected C-unit meningeal nociceptor at baseline (top) and then at various time points following MC degranulation with 48/80 (bottom traces). Average discharge rates are given in parentheses. (C) Peri-stimulus time histogram showing the response of the same meningeal nociceptor (top trace, bin size 0.5 sec) to a suprathreshold 38kPa mechanical stimulus (bottom trace) applied to the neuron’s dural receptive field following bone removal at the end of the experiment. (D) Time course changes (mean ± SE) in neuronal discharge rate of the affected A-δ and C-units following MC degranulation. (E) The effect of local craniotomy and mast cell depletion on baseline neuronal ongoing discharge level. Note the high level of neuronal activity in neurons in which their receptive field was interrupted by a craniotomy. (F) Time course effect of MC degranulation on meningeal nociceptors showing the increase in ongoing discharge rate (mean ± SEM) and its blockade by prior administration of the MC stabilizer SCG. * p<0.01, Fisher PLSD test.
The systemic administration of 48/80 potentially affects MC throughout the body. We therefore tested next whether the meningeal nociceptor activation we observed was dependent specifically on degranulation of the dural MC population rather than other MC populations located elsewhere. We therefore repeated the experiments in animals in which dural MC had been locally depleted of their granules by performing a craniotomy and exposing the dural receptive field of the studied meningeal nociceptors (See Methods and Materials section). Meningeal nociceptors (n=9, 5 C-units, 4 A-delta) isolated immediately following this procedure displayed a baseline level of ongoing discharge (mean 0.7±0.3, range 0.6–2.6 Hz) that was significantly higher (p=0.002) than that found in neurons isolated from animals with intact dural MC population (Fig. 2E). The high level of ongoing discharge rate diminished significantly over a 4 hours recording period (mean 0.15±0.2, range 0.02–0.3 Hz, p=0.023) while the dura was constantly superfused with synthetic interstitial fluid to remove local MC/inflammatory mediators. Subsequent administration of 48/80, either systemically (n=8) or topically (10 μg/ml, n=6), to the MC-depleted dura failed to promote nociceptor excitation (not depicted), indicating that the 48/80-evoked neuronal discharge is dependent on the presence of intact dural MC. This lack of response following craniotomy-induced MC depletion was also not due to a generalized loss of responsiveness, because activation could still be produced by mechanical stimulation (Figure 2C), or by administration of a mixture of inflammatory mediators containing histamine, 5HT, prostaglandin E2 and bradykinin which produced robust activation in 7/8 neurons (not depicted), similar to that observed in our previous studies (Levy and Strassman 2002a; Strassman et al. 1996). This indicates that craniotomy and the resulting local MC granule depletion itself do not preclude further nociceptor activation and that dural MC are not required for such activation.
To further verify that the excitation produced by 48/80 resulted specifically from its MC degranulating effect rather than other non-specific actions, we examined whether it could be blocked by the MC stabilizer sodium cromoglycate (SCG, 10 mg/kg i.p. Sigma). SCG blocks MC secretion by modifying the protein phosphorylation pattern of MC (Theoharides et al. 1980) and inhibiting Ca2+ uptake-mediated MC secretion (Hemmerich et al. 1991). Pretreatment with SCG resulted in a significant inhibition of 48/80 - induced dural MC degranulation (Table 1). In 8 meningeal nociceptors tested (4 C and 4 A-delta units), SCG also abrogated the 48/80-mediated neuronal excitation (Fig. 2F). In another control study, vehicle control injections of systemic saline (n=6, 3 C-units 3 A-delta) also failed to promote excitation of meningeal nociceptors (not depicted).
3.2 Mast cell degranulation promotes pERK expression in meningeal nociceptors
Noxious stimulation promotes a number of biochemical changes in primary afferent nociceptive neurons. Phosphorylation of the MAP kinase ERK (pERK) within the peripheral terminals of these neurons has been shown to be an important anatomical marker of such nociceptive activation (Dai et al. 2002; Seino et al. 2006). Using pERK expression in the meninges we next investigated the extent of meningeal nociceptor activation following MC degranulation. Fifteen minutes following administration of 48/80 (n=6), there was a fourfold increase in the number of nerve fibers expressing pERK (Fig. 3A) with many pERK-positive fibers seen in close proximity to degranulated MC (Fig. 3C). Out of 307 counted pERK-positive fibers (n=4), 269 (87.6%) were localized in the vicinity (<100μm) of at least one degranulated MC along their axonal process.
Figure 3.
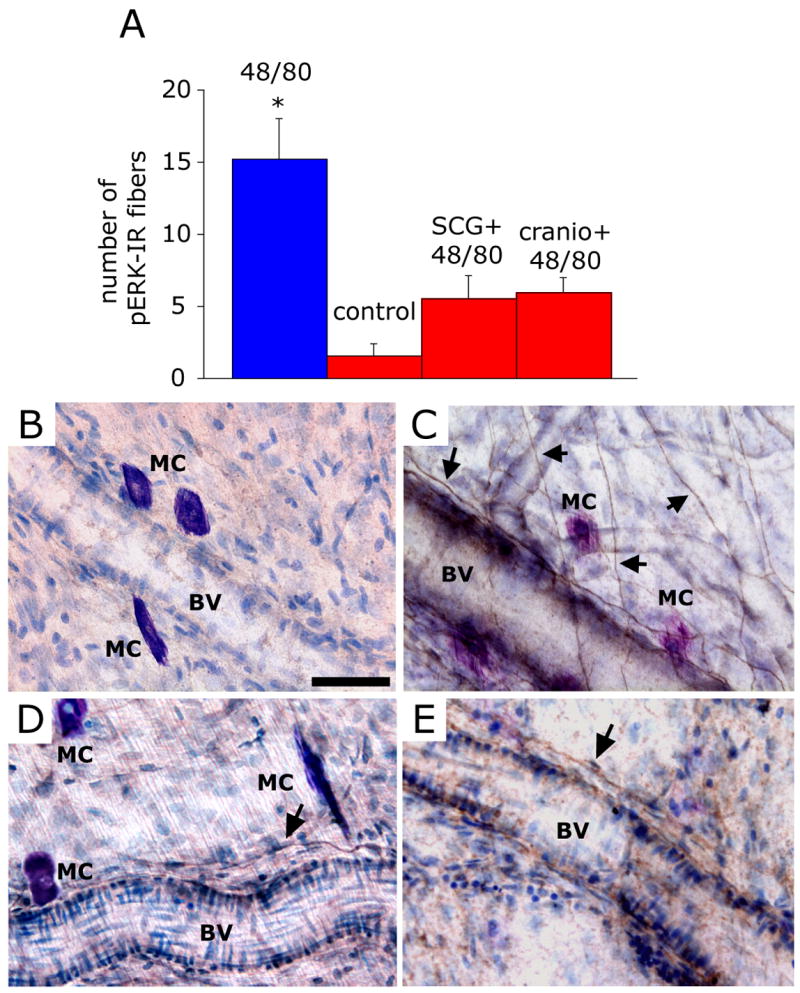
Mast cell degranulation evokes pERK expression in meningeal nerve fibers. (A) Number (mean ± SEM) of pERK-positive fibers per observation field in the dura of animals treated with 48/80 (n=6), saline (n=4), SCG+48/80 (n=4) and 48/80 (n=4) following local mast cell depletion using craniotomy (cranio + 48/80). * p<0.05 Mann Whitney U test compared with control. (B) Dura whole-mount section taken from control (saline-treated) animal showing non-degranulated MC and lack of pERK expression. (C) An example of typical pERK expression (arrows) in perivascular and non-vascular dural nerve fibers 15 minutes following administration of the MC degranulating agent 48/80. Note the proximity between pERK-positive fibers and the TB-stained degranulated MC. (D) Dura whole-mount section taken from animal treated with SCG prior to 48/80 showing intact MC and very few perivascular pERK-labeled fibers. (E) Dura whole-mount section taken from an animal that underwent a craniotomy prior to 48/80 treatment showing lack of MC staining and very few perivascular pERK-labeled fibers. BV= Blood Vessel. Scale bar = 50 μm
Inhibition of dural MC degranulation with either SCG (n=4, Fig, 3D), or through their depletion by prior craniotomy (n=4, Fig. 3E), prevented the increased neuronal pERK expression (p=0.04, Fig. 3A). While both SCG and craniotomy pretreatments were associated with a mild increase in the number of pERK-labeled fibers, values were not statistically different than those obtained in the control experiments (p=0.149 for SCG+48/80 group vs. control, and p=0.086 for craniotomy+48/80 group vs. control).
Immunohistochemical double labeling further showed that 60±1.5% (n=4) of the pERK-positive neurons also co-localized CGRP (Fig. 4E), a neuropeptide marker of meningeal nociceptive neurons (Keller and Marfurt 1991; Strassman et al. 2004)
Figure 4.
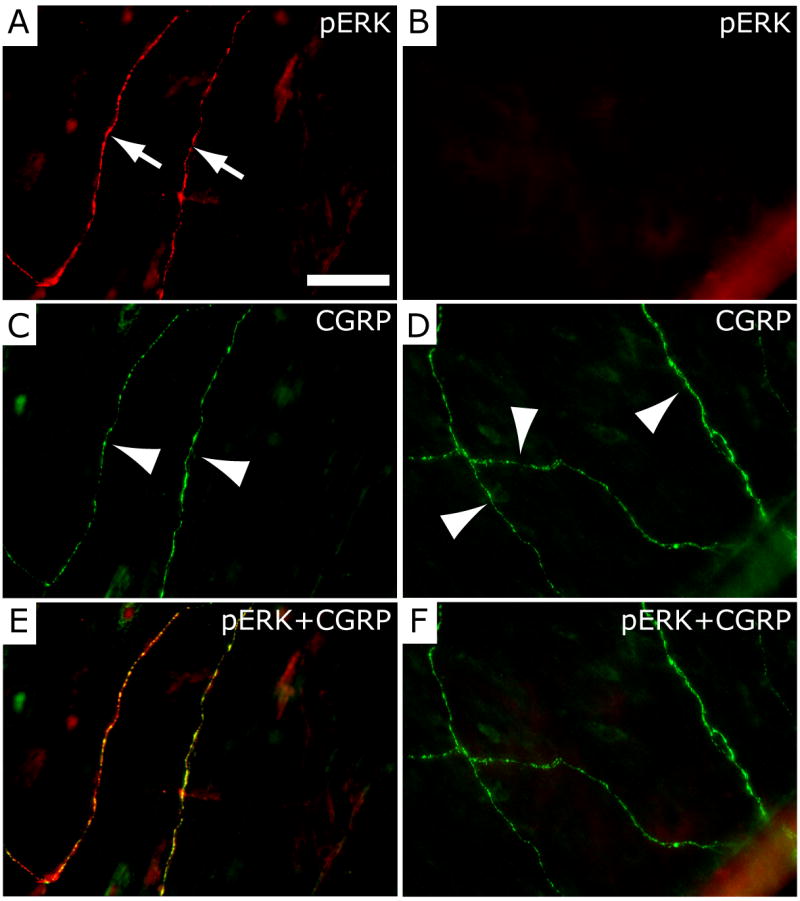
Localization of pERK in CGRP-immunoreactive dural nerve fibers following mast cell degranulation with 48/80. (A) Dural whole-mount section showing pERK immunofluorescence in two dural nerve fibers (arrows) following treatment with 48/80. The same two fibers (arrowheads) showing CGRP-immunofluorescence are shown in C. The merged image (pERK+CGRP) in E shows that the pERK and CGRP labeling is overlapping and thus localized within the same fibers. The dural whole-mount section shown on the right (B, D, F) was obtained from an animal treated with sodium cromoglycate (SCG) prior to 48/80 and shows lack of pERK-immunofluorescence (B), but preservation of CGRP labeling in 3 dural fibers (arrowheads in D). Panel F represents a merged image of B and D. Scale bar = 50 μm.
3.3 Mast cell degranulation promotes activation of nociceptive trigeminovascular brainstem neurons
Previously we have shown that chemical stimulation of meningeal nociceptors promotes activation of nociceptive neurons in the trigeminal nucleus caudalis (TNC) (Burstein et al. 1998) which is well correlated with c-fos immunoreactivity (fos-IR) at the same anatomical location (Strassman et al. 1994). We therefore investigated whether MC degranulation also promotes activation of TNC neurons by examining fos expression. Following MC degranulation, fos-IR was increased in the superficial laminae of the TNC (medullary dorsal horn), as well as the upper cervical dorsal horn (n=10). The labeled cells had a distinct ventrolateral distribution within the dorsal horn (Fig. 5A, B), similar to that found after direct mechanical or chemical stimulation of the dura mater (Malick et al. 2001; Strassman et al. 1994). This area contains second order neurons that receive convergent sensory input from the dura and the ophthalmic facial region (Burstein et al. 1998; Strassman and Vos 1993; Strassman et al. 1994). The 48/80-induced increases in fos expression was almost completely blocked by prior administration of SCG (n=6, p=0.007, Fig. 5C, D, I).
Figure 5.
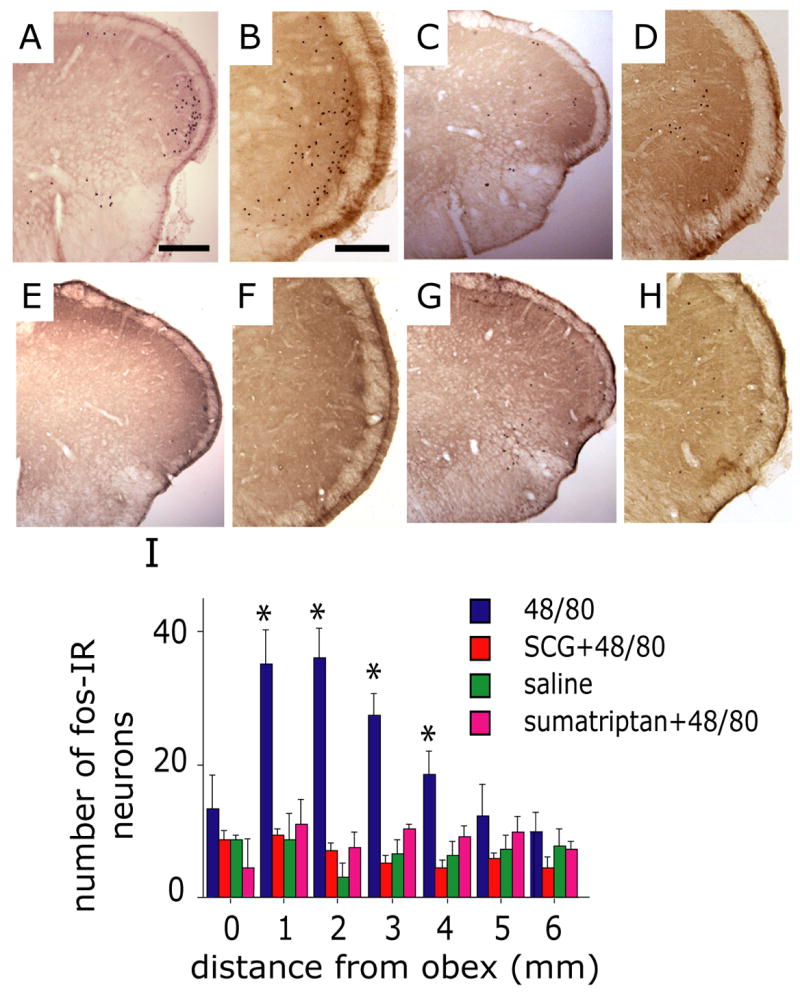
Mast cell degranulation evokes activation of brainstem trigeminovascular nociceptive neurons. Representative low (A, C, E, G) and high (B, D, F, H) magnification photomicrographs demonstrating c-fos IR in the TNC (medullary dorsal horn) taken from animals treated with 48/80 (A, B), SCG prior to 48/80 (C, D), saline control (E, F) and sumatriptan prior to 48/80 (G, H). Note the distinct distribution of fos in the ventrolateral part of TNC in the 48/80 treated animal. (I) Histogram comparing the number (mean ± SEM) of fos-IR cells in the upper cervical and medullary dorsal horn from animals receiving 48/80, saline, SCG 30 minutes prior to 48/80 or sumatriptan. (* p<0.05, Fisher PLSD test compared with saline control). Scale bar = 1000 μm for (A, C, E, G) and 200 μm for (B, D, F, H).
We have shown previously that triptans, the most effective anti-migraine therapy, confer their anti-migraine effect by disrupting communication between meningeal nociceptors and second-order trigeminovascular neurons in the TNC (Levy et al. 2004). We therefore examined the effect of sumatriptan on the MC-induced increase in fos expression. Pretreatment with sumatriptan (1 mg/kg i.p., n=5) had a significant inhibitory effect (p=0.003) on the MC degranulation-induced increase in fos expression in the TNC (Fig. 5G, H, I). Sumatriptan, however, failed to inhibit the 48/80-induced MC degranulation, consistent with its effect on the central terminals of meningeal nociceptors in the TNC rather than peripheral site of action on meningeal nociceptors.
To further test whether the increased fos resulted specifically from degranulation of dural MC, we repeated these experiments in a separate set of animals in which a localized depletion of dural MC had been produced by a unilateral craniotomy prior to 48/80 administration (n=4, Fig 6 A). The increase in fos expression was specifically abolished in the dorsal horn ipsilateral to the craniotomy (Fig. 6C and D, p=0.02 compared to intact side) further suggesting the importance of dural MC to 48/80-induced C-fos in the TNC.
4. Discussion
The cranial dura, a peripheral tissue covering the brain, is invested with a large number of connective tissue-like MC, many of which are found in close apposition to nerve endings of meningeal nociceptors. Previous studies have shown that electrical stimulation of the trigeminal nerve, a model of intracranial pain, evokes degranulation of dural MC (Buzzi et al. 1992; Dimitriadou et al. 1991), a process likely to contribute to local meningeal inflammation. We present here for the first time evidence for the reverse process, whereby degranulation of dural MC can promote a prolonged state of excitation of neighboring trigeminal meningeal nociceptors. A similar MC - nerve interaction has been hypothesized in the pathogenesis of allergic airway disease (Undem et al. 1995) and food allergies (Wood 2006). Release of MC-derived inflammatory mediators is also believed to contribute to the pain hypersensitivity associated with visceral inflammation (Ribeiro et al. 2000) such as that of irritable bowel syndrome (Barbara et al. 2007). Our findings, however, are the first demonstration, in any tissue, that MC degranulation per se can promote a prolonged state of excitation in primary afferent nociceptors.
We found that MC degranulation induced by the receptor mimetic compound 48/80 triggered in meningeal nociceptors a rapid increase of neuronal firing. Although there is some evidence suggesting that 48/80 may affect neurons directly by activating phospholipase D (Palomaki and Laitinen 2006) we have found that the immediate induction of neuronal excitation induced by 48/80 was completely blocked by locally depleting the dural MC content, using a surgical craniotomy. Such MC depletion, however, did not affect the excitatory response of meningeal nociceptors to topical application of a mix of inflammatory mediators or to mechanical stimulation of the dura suggesting that it does not interfere with the overall responsiveness of meningeal nociceptors. Further, 48/80 failed to activate meningeal nociceptor when applied topically to the MC-depleted dura. The effect of 48/80 was also prevented by pretreatment with the MC stabilizer SCG which has no known direct effect on neurons, particularly primary afferent nociceptors. Taken together, our findings suggest that 48/80 promotes meningeal nociceptor activation by promoting local MC degranulation, likely through the excitatory action of locally released prepackaged MC-derived algesic mediators. Such MC-meningeal nociceptor interaction is further supported by the finding that 48/80-induced MC degranulation also produced a rapid increase in the expression of pERK, a surrogate marker of nociceptor activation in dural CGRP-positive fibers.
Previous studies in both humans and rodents have shown that mediator release following local degranulation of cutaneous MC is associated only with an itch sensation (pruritus) rather than overt pain (Rukwied et al. 2000; Ui et al. 2006). Pruritus is mediated primarily by the activation of a distinct class of primary afferent neurons which consist of slow conducting mechano-insensitive C-units that are not nociceptors and do not mediate pain (Ikoma et al. 2005; Schmelz et al. 1997). In this work we found that MC degranulation induced excitation chiefly in mechano-nociceptive C-units, suggesting that MC mediators could trigger pain only when acting on deep tissue nociceptors such as those innervating the meninges.
It should be noted that in our study the use of a novel surgical preparation to maintain an intact intracranial milieu was essential for the present findings. In previous studies, activation of meningeal nociceptors was produced by a surgical exposure of the dura and direct application of exogenous stimuli, including inflammatory mediators. Our present findings suggest that in the absence of a dural exposure, MC can serve as an endogenous source for algesic mediators, and can release sufficient quantities to produce suprathreshold excitation of high intensity and prolonged duration. This is the first demonstration that meningeal nociceptors can in fact be activated without requiring direct application of external stimuli to the exposed dura, and so represents a critical breakthrough in establishing the possible role of these neurons in physiological or pathophysiological conditions.
We found that MC degranulation also produced downstream activation of brainstem nociceptive neurons that participate in the central transmission of migraine pain, as evidenced by increased fos expression specifically in the ventrolateral part of the TNC, in a distinctive distribution that matches that found following direct dural stimulation. The finding that 48/80-induced fos expression was inhibited by local MC depletion further supports a specific role of dural MC in producing such neural effects. Furthermore, the inhibition of fos expression was restricted to the side ipsilateral to the MC depletion, and so was not due to a generalized effect of the craniotomy on neural responsiveness. We also show that the anti-migraine drug sumatriptan, a prototypical 5HT1B/D agonist, also abrogated the MC degranulation evoked c-fos expression in the TNC without blocking the 48/80-induced dural MC degranulation. Although previous studies indicated that sumatriptan can inhibit trigeminal nerve activation-induced meningeal inflammation and MC degranulation (Buzzi and Moskowitz 1990), this was shown to result from its inhibitory action on neuropeptide release from peripheral nerve endings, and so it would not be expected to affect 48/80-induced MC degranulation. Our results are consistent with previous findings that sumatriptan specifically disrupts transmission between peripheral and central trigeminovascular neurons without inhibiting peripheral activation and sensitization of meningeal nociceptors (Levy et al. 2004). These results thus point to its central neuronal action in the brainstem and provide further evidence for a specific link between dural MC and the activation of the trigeminovascular system.
In view of the potential ability of dural MC to promote persistent activation of meningeal nociceptors during migraine with aura, a question remains as to whether dural MC could also play a role in migraine without aura or in other types of headaches. Activation of meningeal nociceptors is presumably the substrate of other headaches with intracranial origin (Strassman et al. 1996). It is therefore tempting to speculate that such headaches also involve a local neurogenic inflammation component. Such neurogenic induction of dural MC degranulation could therefore serve as a potential mechanism playing, at least in part, a role in sustaining such headaches.
Currently the exact MC-related mediator or mediators that may influence meningeal nociceptors is unknown. Historically, histamine is considered the main effector molecule underlying MC-related actions. Clinical evidence suggests the involvement of histamine in the pain of some migraine patients as evidenced by elevated histamine levels (Heatley et al. 1982) and the prophylactic effect of antihistamines in a subset of migraine patients (Lewis et al. 2004; Rossi et al. 2003; Togha et al. 2006). Although histamine has been shown to activate and sensitize nociceptors (Koda et al. 1996; Koda and Mizumura 2002) other molecules known to be released from MC can potentially activate meningeal nociceptors. A short list of such MC mediators includes prostanoids, leukotrienes, cytokines, chemokines, and tryptase (Metcalfe et al. 1997). Among those, MC-derived leukotrienes (Sheftell et al. 2000), the cytokines TNF-alpha and IL-6 (Sarchielli et al. 2006) and endothelin-1 (Hasselblatt et al. 1999) have been implicated in migraine pathophysiology. Further studies are needed to identify MC mediators that could play a role in the activation of meningeal nociceptors.
Acknowledgments
This study was supported by the NIH (National Institute of Neurological Disorders and Stroke grants R01NS046502 to D.L., R01DE013347 to R.B., and R02NS032534 to A.M.S.) and The National Headache Foundation (A.M.S.)
Abbreviations
- MC
Mast cells
- pERK
Phosphorylated Extracelular-Related Kinase
- SCG
sodium cromoglycate
- TNC
Trigeminal Nucleus Caudalis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artico M, Cavallotti C. Catecholaminergic and acetylcholine esterase containing nerves of cranial and spinal dura mater in humans and rodents. Microsc Res Tech. 2001;53:212–20. doi: 10.1002/jemt.1085. [DOI] [PubMed] [Google Scholar]
- Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–42. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- Buzzi MG, Moskowitz MA. The antimigraine drug, sumatriptan (GR43175), selectively blocks neurogenic plasma extravasation from blood vessels in dura mater. Br J Pharmacol. 1990;99:202–206. doi: 10.1111/j.1476-5381.1990.tb14679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzi MG, Dimitriadou V, Theoharides TC, Moskowitz MA. 5-Hydroxytryptamine receptor agonists for the abortive treatment of vascular headaches block mast cell, endothelial and platelet activation within the rat dura mater after trigeminal stimulation. Brain Res. 1992;583:137–149. doi: 10.1016/s0006-8993(10)80017-4. [DOI] [PubMed] [Google Scholar]
- Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci. 2002;22:7737–45. doi: 10.1523/JNEUROSCI.22-17-07737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC. Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater mast cells. Neuroscience. 1991;44:97–112. doi: 10.1016/0306-4522(91)90253-k. [DOI] [PubMed] [Google Scholar]
- Dimlich RV, Keller JT, Strauss TA, Fritts MJ. Linear arrays of homogeneous mast cells in the dura mater of the rat. J Neurocytol. 1991;20:485–503. doi: 10.1007/BF01252276. [DOI] [PubMed] [Google Scholar]
- Hasselblatt M, Kohler J, Volles E, Ehrenreich H. Simultaneous monitoring of endothelin-1 and vasopressin plasma levels in migraine. Neuroreport. 1999;10:423–5. doi: 10.1097/00001756-199902050-00039. [DOI] [PubMed] [Google Scholar]
- Heatley RV, Denburg JA, Bayer N, Bienenstock J. Increased plasma histamine levels in migraine patients. Clin Allergy. 1982;12:145–9. doi: 10.1111/j.1365-2222.1982.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Hemmerich S, Sijpkens D, Pecht I. A novel cell-permeable cromoglycate derivative inhibits type I Fc epsilon receptor mediated Ca2+ influx and mediator secretion in rat mucosal mast cells. Biochemistry. 1991;30:1523–32. doi: 10.1021/bi00220a012. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Handwerker H, Miyachi Y, Schmelz M. Electrically evoked itch in humans. Pain. 2005;113:148–54. doi: 10.1016/j.pain.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Keller JT, Marfurt CF. Peptidergic and serotoninergic innervation of the rat dura mater. J Comp Neurol. 1991;309:515–34. doi: 10.1002/cne.903090408. [DOI] [PubMed] [Google Scholar]
- Koda H, Minagawa M, Si-Hong L, Mizumura K, Kumazawa T. H1-receptor-mediated excitation and facilitation of the heat response by histamine in canine visceral polymodal receptors studied in vitro. J Neurophysiol. 1996;76:1396–1404. doi: 10.1152/jn.1996.76.3.1396. [DOI] [PubMed] [Google Scholar]
- Koda H, Mizumura K. Sensitization to mechanical stimulation by inflammatory mediators and by mild burn in canine visceral nociceptors in vitro. J Neurophysiol. 2002;87:2043–51. doi: 10.1152/jn.00593.2001. [DOI] [PubMed] [Google Scholar]
- Levy D, Strassman AM. Distinct sensitizing effects of the cAMP-PKA second messenger cascade on rat dural mechanonociceptors. J Physiol (Lond) 2002a;538:483–493. doi: 10.1113/jphysiol.2001.013175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Strassman AM. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol. 2002b;88:3021–31. doi: 10.1152/jn.00029.2002. [DOI] [PubMed] [Google Scholar]
- Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A. 2004;101:4274–9. doi: 10.1073/pnas.0306147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DW, Diamond S, Scott D, Jones V. Prophylactic treatment of pediatric migraine. Headache. 2004;44:230–7. doi: 10.1111/j.1526-4610.2004.04052.x. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache. 2005;45(Suppl 1):S3–S13. doi: 10.1111/j.1526-4610.2005.4501001.x. [DOI] [PubMed] [Google Scholar]
- Malick A, Jakubowski M, Elmquist JK, Saper CB, Burstein R. A neurohistochemical blueprint for pain-induced loss of appetite. Proc Natl Acad Sci U S A. 2001;98:9930–5. doi: 10.1073/pnas.171616898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S, Saito K, Buzzi MG, Moskowitz MA. The development of neurogenic plasma extravasation in the rat dura mater does not depend upon the degranulation of mast cells. Brain Res. 1989;477:157–165. doi: 10.1016/0006-8993(89)91403-0. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Ottosson A, Edvinsson L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia. 1997;17:166–174. doi: 10.1046/j.1468-2982.1997.1703166.x. [DOI] [PubMed] [Google Scholar]
- Palomaki VA, Laitinen JT. The basic secretagogue compound 48/80 activates G proteins indirectly via stimulation of phospholipase D-lysophosphatidic acid receptor axis and 5-HT1A receptors in rat brain sections. Br J Pharmacol. 2006;147:596–606. doi: 10.1038/sj.bjp.0706671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GWC. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–98. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato AB, Poole S, Ferreira SH, Cunha FQ. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol. 2000;387:111–118. doi: 10.1016/s0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- Rossi P, Fiermonte G, Pierelli F. Cinnarizine in migraine prophylaxis: efficacy, tolerability and predictive factors for therapeutic responsiveness. An open-label pilot trial. Funct Neurol. 2003;18:155–9. [PubMed] [Google Scholar]
- Rozniecki JJ, Dimitriadou V, Lambracht-Hall M, Pang X, Theoharides TC. Morphological and functional demonstration of rat dura mater mast cell-neuron interactions in vitro and in vivo. Brain Res. 1999;849:1–15. doi: 10.1016/s0006-8993(99)01855-7. [DOI] [PubMed] [Google Scholar]
- Rukwied R, Lischetzki G, McGlone F, Heyer G, Schmelz M. Mast cell mediators other than histamine induce pruritus in atopic dermatitis patients: a dermal microdialysis study. Br J Dermatol. 2000;142:1114–20. doi: 10.1046/j.1365-2133.2000.03535.x. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, Floridi A, Calabresi P. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46:200–7. doi: 10.1111/j.1526-4610.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino D, Tokunaga A, Tachibana T, Yoshiya S, Dai Y, Obata K, Yamanaka H, Kobayashi K, Noguchi K. The role of ERK signaling and the P2X receptor on mechanical pain evoked by movement of inflamed knee joint. Pain. 2006;123:193–2003. doi: 10.1016/j.pain.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Shefler I, Sagi-Eisenberg R. Gi-mediated activation of the Syk kinase by the receptor mimetic basic secretagogues of mast cells: role in mediating arachidonic acid/metabolites release. J Immunol. 2001;167:475–481. doi: 10.4049/jimmunol.167.1.475. [DOI] [PubMed] [Google Scholar]
- Sheftell F, Rapoport A, Weeks R, Walker B, Gammerman I, Baskin S. Montelukast in the prophylaxis of migraine: a potential role for leukotriene modifiers. Headache. 2000;40:158–163. doi: 10.1046/j.1526-4610.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Vos BP. Somatotopic and laminar organization of fos-like immunoreactivity in the medullary and upper cervical dorsal horn induced by noxious facial stimulation in the rat. J Comp Neurol. 1993;331:495–516. doi: 10.1002/cne.903310406. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Mineta Y, Vos BP. Distribution of fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J Neurosci. 1994;14:3725–3735. doi: 10.1523/JNEUROSCI.14-06-03725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Weissner W, Williams M, Ali S, Levy D. Axon diameters and intradural trajectories of the dural innervation in the rat. J Comp Neurol. 2004;473:364–76. doi: 10.1002/cne.20106. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Sieghart W, Greengard P, Douglas WW. Antiallergic drug cromolyn may inhibit histamine secretion by regulating phosphorylation of a mast cell protein. Science. 1980;207:80–2. doi: 10.1126/science.6153130. [DOI] [PubMed] [Google Scholar]
- Togha M, Ashrafian H, Tajik P. Open-label trial of cinnarizine in migraine prophylaxis. Headache. 2006;46:498–502. doi: 10.1111/j.1526-4610.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol. 2006;530:172–8. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Riccio MM, Weinreich D, Ellis JL, Myers AC. Neurophysiology of mast cell-nerve interactions in the airways. Int Arch Allergy Immunol. 1995;107:199–201. doi: 10.1159/000236976. [DOI] [PubMed] [Google Scholar]
- Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology. 2005;64:S9–15. doi: 10.1212/wnl.64.10_suppl_2.s9. [DOI] [PubMed] [Google Scholar]
- Wood JD. Histamine, mast cells, and the enteric nervous system in the irritable bowel syndrome, enteritis, and food allergies. Gut. 2006;55:445–7. doi: 10.1136/gut.2005.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]