Abstract
A newly discovered Bacteroides conjugative transposon (CTn), CTnBST, integrates more site specifically than two other well-studied CTns, the Bacteroides CTn CTnDOT and the enterococcal CTn Tn916. Moreover, the integrase of CTnBST, IntBST, had the C-terminal 6-amino-acid signature that is associated with the catalytic regions of members of the tyrosine recombinase family, most of which integrate site specifically. Also, in most of these integrases, all of the conserved amino acids are required for integration. In the case of IntBST, however, we found that changing three of the six conserved amino acids in the signature, one of which was the presumed catalytic tyrosine, resulted in a 1,000-fold decrease in integration frequency. Changes in the other amino acids had little or no effect. Thus, although the CTnBST integrase still seems to be a member of the tyrosine recombinase family, it clearly differs to some extent from other members of the family in its catalytic site. We also determined the sequence requirements for CTnBST integration in the 18-bp region where the crossover occurs preferentially during integration. We found that CTnBST integrates in this preferred site about one-half of the time but can also use other sites. A consensus sequence was tentatively derived by comparison of a few secondary sites: AATCTGNNAAAT. We report here that within the consensus region, no single base change affected the frequency of integration. However, 3 bp at one end of the consensus sequence (CTG) proved to be essential for integration into the preferred site. This sequence appeared to be at one end of a 7-bp crossover region, CTGNNAA. The other bases could vary without affecting either integration frequency or specificity. Thus, in contrast to well-studied site-specific recombinases which require homology throughout the crossover region, integration of CTnBST requires homology at one end of the crossover region but not at the other end.
Conjugative transposons (CTns) are integrated self-transmissible elements that are making an important contribution to the spread of antibiotic resistance genes in such bacterial groups as the human colonic Bacteroides spp. and the gram-positive bacteria. The best-studied CTns, the Bacteroides CTnDOT and the enterococcal Tn916, have in common that they do not integrate site specifically. Also, the model for excision that was originally proposed for Tn916 by Caparon and Scott (1) postulated a 6-bp region of heterology between the recombination sites, resulting in the formation of a heteroduplex. A similar heteroduplex was assumed to occur on either side of the integrating element. CTnDOT appeared to have a similar mechanism except that the region of heterology was 5 bp long.
Surprisingly, however, despite having a proposed mechanism of excision and integration that seemed to be very different from the mechanism of integration of site-specific tyrosine recombinases such as the phage lambda integrase, the integrases of Tn916 and CTnDOT had at least 5 amino acids of the 6-amino-acid C-terminal amino acid signature that has come to define the tyrosine recombinase family (12). In contrast to well-studied tyrosine recombinases, for which all of the six conserved amino acids are essential, only three of these amino acids in the CTnDOT were essential for function (9).
CTnBST is a newly discovered Bacteroides CTn. The sequence of CTnBST had no similarity to any part of the sequence of CTnDOT, raising the possibility that CTnBST was not a member of the CTnDOT family. Also, CTnBST differed from CTnDOT in that whereas the transfer frequency of CTnDOT is stimulated 100- to 1,000-fold by tetracycline, CTnBST exhibited constitutive transfer (4). The integrase of CTnBST (IntBST) also had the 6-amino-acid signature but appeared to differ from the other CTn integrases. CTnBST appears to be more like the other members of the tyrosine recombinase family, because it integrated much more site specifically (23). Target site selection is an important signature for each transposon, and it determines dissemination and the stability of the transposon. The preferred CTnBST integration site had 18 bp of sequence identity with a sequence that spanned the joined ends of the circular form of the CTn (ATAAATCTGGTAAATTTA). Strand exchanges that led to integration of the element presumably occurred within this region. CTnBST was also able to use other sites on the chromosome. A few secondary sites that were identified initially all shared a much smaller consensus region than the 18-bp site, AATCTGNNAAAT (23).
We show here that four of the six conserved amino acids that identify IntBST as a member of the tyrosine recombinase family are essential for integration. We also examine in more detail the sequence requirements for integration of CTnBST. We found that three base pairs (CTG), which are located on one side of the consensus region, are important for site selection. We deduced that one of the crossover events occurs adjacent to the C of the CTG sequence and that the second crossover event occurs 7 bp from this location.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteroides strains were grown anaerobically at 37°C in prereduced Trypticase-yeast extract-glucose broth (5) or on agar plates incubated in BBL GasPak jars. Escherichia coli strains were grown aerobically at 37°C in Luria-Bertani medium broth or on agar plates. The following antibiotic concentrations were used in this study: ampicillin, 100 μg/ml; chloramphenicol, 20 μg/ml; erythromycin, 10 μg/ml; and rifampin, 10 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant phenotypea | Source or descriptionb |
|---|---|---|
| E. coli strains | ||
| S17-1 | RecA Tpr TraRP4+ | Contains the transfer functions of RP4 integrated into the chromosome (19) |
| BW19851 | RecA TraRP4+ Tpr Pir+ | E. coli S17-1 with RK6 pir in uidA (10) |
| Bacteroides sp. strain BT4001 | Rifr | Spontaneous rifampin-resistant mutant of B. thetaiotaomicron 5482 |
| Plasmids | ||
| pEPE | (Ermr) Cmr | Suicide vector that is selectable in both E. coli and Bacteroides hosts (22) |
| pattBST410 | (Ermr) Cmr | 410-bp attBST PCR product cloned into pEPE |
| pattBST270 | (Ermr) Cmr | 270-bp attBST PCR product cloned into pEPE |
| pattBST365 | (Ermr) Cmr | 365-bp attBST PCR product cloned into pEPE |
| pattBST299 | (Ermr) Cmr | 299-bp attBST PCR product cloned into pEPE |
| pGEM-T | Apr | E. coli PCR cloning vector (Promega) |
| pLYL01 | (Tcr) Apr | Bacteroides shuttle vector (7) |
| pIntBST | (Tcr) Apr | 1.3-kb PCR product cloned into pLYL01; contains intBST and its promoter |
| pBJE2.1 | (Emr Int+) Apr | 2.1-kbp PCR product cloned into pGERM; contains the CTnBST joined ends and all of intBST (23) |
Phenotypes in parentheses are expressed only in Bacteroides, and phenotypes not in parentheses are expressed in E. coli. Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Emr, erythromycin resistance; Rifr, rifampin resistance; Strr, streptomycin resistance; Int+, ability to integrate.
The numbers in parentheses are references.
DNA manipulation and conjugation procedures.
Chromosomal DNA was prepared by a modification of a method described by Saito and Miura (16). Plasmids were isolated with a QIAGEN (Valencia, CA) Miniprep kit. Oligonucleotides were obtained from Integrated DNA Technologies (Coralville, IA). Restriction enzymes, T4 DNA ligase, and T4 DNA polymerase were purchased from Invitrogen (Carlsbad, CA), New England BioLabs (Beverly, MA), and Fisher Scientific. The manufacturers' instructions were followed for restriction digestion and ligation. Filter mating between E. coli and Bacteroides strains was performed and integration frequency was calculated as described previously (18, 20).
Site-directed mutagenesis of intBST.
Mutations in the CTnBST integrase were made using a Stratagene QuickChange mutagenesis kit. Primers carrying the specific mutations are shown in Table 2. The mini-BST plasmid pBJE2.1, which contains the intBST gene under control of its own promoter and the joined ends, was used as the template for the mutagenesis reaction (23). The mutagenized plasmid was transformed into E. coli S17-1 cells and isolated from the transformants. The intBST gene was sequenced to confirm the desired mutation and to exclude the presence of other mutations.
TABLE 2.
PCR primers used in this study
| Use and primer | Sequence (5′-3′) |
|---|---|
| Excision products | |
| BST6E4FJ | GTTGGTCAGCTCGATAATACG |
| BST6E4FJ2 | CAATCCGTTGACCAGGTTGA |
| BST12-1-2RJF | GTCCAAAGCTAGGCCAATTG |
| R1153 | GGTCGATATTGAGAAACAAACGA |
| F163 | AAACCATTTTTGTTCCTGATTTG |
| R388 | GAACCAATCAACTGAAAAGAAAGAA |
| Sequencing of attBST region | |
| R1130 | AGGTCGATATTGAGAAACAAACGAGA |
| Site-directed mutagenesis of intBST | |
| R237A-intBST | CTGCGGTCTGGCAATCAGTGATATAATCAAGTTGC |
| GC-R237A-intBST | GCAACTTGATTATATCACTGATTGCCAGACCGCAG |
| K264A-intBST | CGGTTTCCATGCAGGCAACCAAAGAACC |
| GC-K264A-intBST | GGTTCTTTGGTTGCCTGCATGGAAACCG |
| H322A-intBST | GCGTTTTACATTCGCTACGGCGCGGCA |
| GC-H322A-intBST | TGCCGCGCCGTAGCGAATGTAAAACGC |
| R325A-intBST | CATTCCATACGGCGGCTCACACGTTCGC |
| GC-R325A-intBST | GCGAACGTGTGAGCCGCCGTATGGAATG |
| H348A-intBST | CCAAGCTGCTCGGCGCTGCTGACGTGA |
| GC-H348A-intBST | TCACGTCAGCAGCGCCGAGCAGCTTGG |
| IntBST357Y-A | CCATGCTGACGTGAAAATGACACAGGTGGCCGCCAAAATCAC |
| GC-IntBST357Y-A | GATGATTTTGGCGGCCACCTGTGTCATTTTCACGTCACGATGG |
In vivo integration assay to determine the minimal attBST site.
In the initial identification of the attachment site of CTnBST, the size of the joined-end segment was about 700 bp. To define more precisely the minimal attBST site that was essential for integration, different lengths of the attBST region were amplified using Pfu polymerase and cloned into the suicide vector pEPE (Table 1), which can replicate in E. coli pir+ strains but not in Bacteroides. The resulting plasmids were transformed into E. coli BW19851, and the strains were used as donors for filter mating (Fig. 1). The new vectors were designated pattBST410, pattBST270, pattBST299, and pattBST365 and were obtained using primer pairs F743/R1153, F163/R1153, F743/R333, and F743/R388 (Table 2), respectively.
FIG. 1.
In vivo integration assay for attBST derivatives. E. coli BW19851 with pattBST derivatives, containing various lengths of attB cloned in pEPE, was the donor B. thetaiotaomicron BT4001 contains pIntBST and was used as the recipient. The integration frequency was calculated by determining the number of transconjugants per recipient. The types of resistance in parentheses function in the Bacteroides recipient BT4001, and those outside parentheses function in the E. coli donor. CmR, chloramphenicol resistance; EmR, erythromycin resistance; TcR, tetracycline resistance; ApR, ampicillin resistance.
The 1.3-kb intBST PCR product, including all of the intBST open reading frame and its promoter, was cloned into pGEM-T Easy, sequenced, digested by SphI and SstI, and ligated into a Bacteroides shuttle vector, pLYL01 (Table 1). The vector was designated pIntBST and transformed into E. coli S17-1 cells. Filter mating was performed to transfer the construct from E. coli S17-1 to BT4001. BT4001 containing pIntBST was then used as the recipient to test integration of the pattBST vectors from the E. coli BW19851 donors.
Analysis of the integration specificity of CTnBST.
Two approaches were used to test whether a mini-element integrated into the preferred target site in the Bacteroides thetaiotaomicron chromosome. The mini-element was constructed as described previously (21). The mini-element contains a copy of intBST and attBST in a suicide vector so the transfer can be detected using the vector's antibiotic marker. The recipient does not have any CTnBST sequence in the chromosome. The first approach used was a Southern blot assay. Chromosomal DNA from independent colonies was isolated as described previously (16). DNA from each isolate was digested with HindIII, transferred to a membrane, and probed with a 423-bp PCR fragment (using primers F1418 and R1841 [Table 2]), which contains part of the intBST open reading frame. Thus, each integration event should have produced only one cross-hybridizing restriction fragment. A second approach was to use PCR to amplify the preferred site. Insertion into the preferred target site, attB1, was confirmed by PCR using primers to produce the right-end junction fragment (primers BST6E4FJ and BST12-1-2RJF [Table 2]). A lack of a PCR product indicated that there was integration into another site.
Cloning the end sequences of the integrated mini-elements.
A plasmid rescue technique was utilized to determine the DNA sequences at the locations of secondary target sites into which the mini-BST element had integrated. The procedure used for plasmid rescue experiments was performed as previously described (23). This method provided the sequence of one of the junctions of the integrated mini-element. The chromosomal DNA adjacent to the mini-element was sequenced using primer R1130 (Table 2). This procedure gave the DNA sequence on the left side, the end of the integrated element that did not contain the integrase. The sequence of the integration site (attB) was first deduced by comparing this sequence with the joined-end sequence of the circular form (attBST) and the genome sequence of B. thetaiotaomicron 5482 (accession number NC 004663) (24). The right-side sequence of the integrated element was amplified using CTnBST right-end primers and a primer designed from the chromosomal sequence. The resulting amplicons were sequenced.
DNA sequencing.
Sequencing reactions were preformed at the W. M. Keck Center for Comparative and Functional Genomics at the University of Illinois, Urbana. DNA and amino acid sequences were analyzed using the NCBI BLAST server.
RESULTS
Mutational analysis of the CTnBST integrase, IntBST.
IntBST (accession number EF067916) contains the conserved 6-amino-acid sequence RKHRHY in the C-terminal catalytic region, a characteristic of the tyrosine recombinases (12). A Clustal W analysis showed that IntBST was related to the well-characterized tyrosine recombinases, such as lambda integrase, Cre, XerD, and IntDOT (Fig. 2). Sequence diversity outside the C-terminal catalytic region is, however, a general trait of known tyrosine recombinases. To assess whether IntBST really belonged to the tyrosine recombinase family, site-directed mutagenesis was used to determine if the conserved residues in the catalytic C-terminal region were important for integration and site specificity. The residues were replaced by alanine (R237A, K264A, H322A, R325A, H348A, and Y357A), and the integration frequencies of the mutants were measured using an in vivo integration assay described by Wesslund et al. (23).
FIG. 2.
Phylogenetic tree of members of the tyrosine recombinase family obtained using Clustal W. IntBST is underlined.
As shown in Table 3, the strain carrying wild-type intBST was used as a positive control and had an integration frequency in the range from 10−2 to 10−3 transconjugant per recipient. For the negative control a 400-bp deletion of intBST in pDJE1.7 was employed, and the integration frequency was <10−9 transconjugant per recipient. The Y357A, R237A, and R325A mutations resulted in integration frequencies of <10−9 transconjugant per recipient. The K264A mutation resulted in a 100-fold decrease compared to the wild type. By contrast, the H322A and H348A mutants showed only a 10-fold decrease. These results showed that the Y357, R237, and R325 residues were important for in vivo integration, whereas the K264, H322, and H348 residues were much less important.
TABLE 3.
In vivo integration frequency and target specificity of IntBST mutants compared to IntDOT
| IntBST | Mutation effects
|
Comparison with IntDOT and λ-Int
|
||||
|---|---|---|---|---|---|---|
| Integration frequencya | Target specificityb | Conserved residue | Relative frequencyc
|
|||
| IntBST | λ-Int | IntDOT | ||||
| Wild type | 10−3-10−2 | 7/15 | Wild type | 1 | 1 | 1 |
| R237A | <10−9 | NA | R/Sd | 0 | 0 | 1 |
| K264A | 10−5-10−4 | 9/10 | K | 0.01 | 0 | 0 |
| H322A | 10−4-10−3 | 7/12 | H | 0.1 | 0 | 1 |
| R325A | <10−9 | NA | R | 0 | 0 | 0 |
| H348A | 10−4-10−3 | 8/12 | H | 0.1 | 0 | 0.01 |
| Y357A | <10−9 | NA | Y | 0 | 0 | 0 |
| Negative control | <10−9 | NA | Negative control | 0 | 0 | 0 |
The donor was the IntBST mutant in pBJE2.1 in E. coli S17-1, while BT4001 was the recipient. The values are based on at least three independent matings.
Ratio of the number of colonies which have the preferred target insertion to the total number of colonies tested. NA, not available due to the lack of transconjugants.
Ratio between the mutant and the wild type; 0 = <10−9.
The first conserved amino acid in IntBST is R, while IntDOT has an S in the corresponding position (9).
The effects on target site selection of amino acid changes in IntBST were also determined. The insertions into the preferred target site, attB1, were detected as colony PCR products when primers that recognized the right junction of the integrated mini-element and predicted adjacent chromosomal junction sequences were used. For the wild-type mini-element, one-half of the integration events occurred in the preferred target site. The IntBST K264A, H322A, and 348A mutants integrated into attB1 at the same or higher frequencies than the wild type, showing that there was no loss of integration specificity.
Mutational analysis of the 18-bp attBST site.
Previously, a miniature form of CTnBST was constructed. This mini-BST plasmid contained the integrase gene, intBST, and over 700 bp of contiguous DNA that spanned the joined ends of the circular form, including the 18-bp sequence (23). To localize the attBST site and facilitate mutagenesis, we needed to reduce the size of attBST and separate the intBST gene from the attBST sequence. The minimal attBST site was determined using an in vivo integration assay that we developed, as shown in Fig. 1. The integrase gene, intBST, was cloned separately under control of its own promoter in the recipient strain. E. coli BW19851, which had a suicide vector, pEPE, containing different lengths of attBST, was used as the donor for filter mating. Figure 3 shows the deletion constructs cloned into the pEPE vector, and the results showed that in vivo integration required about 270 bp of attBST, including the 18-bp region.
FIG. 3.
Minimal attBST site determined by the in vivo integration assay. The filled rectangle represents the 18-bp common core sequence. intBST is at the right end of the sequence. The positive control, shown at the top, was pDJE2.1 containing about 700 bp of attBST, including the 18-bp sequence. The negative control, shown at the bottom, was the pEPE vector. The pattBST vectors were constructed as described in Materials and Methods, and filter mating was performed as shown in Fig. 1. The filled rectangle indicates the 18-bp sequence where crossover occurs.
In the same previous studies (23), alignment of the preferred target site, attB1, and several secondary sites gave a 12-bp consensus sequence. Within the region of identity for attBST and these attB sites, there were two conserved regions separated by two variable bases. The sequence of this region was AATCTGNNAAAT (where A, T, C, and G indicate identical bases and N indicates any base), but it was not clear whether the identical bases were required for integration. To assess the role of the conserved base pairs in integration, site-directed mutagenesis was used to obtain single-base and multiple-base mutations in the 18-bp region (Table 4). The mutant sites were identified by the position in the att site that was mutated. For example, when the A at position 1 in the CC region was mutated to C, the mutation was designated “A1C”. Multiple-base-pair mutations at positions 2 to 6 were designated Mut2-6. The integration frequencies were measured by the same in vivo integration assay used in the mutational analysis of IntBST (see Materials and Methods). Since changes in the conserved sequences might affect insertional specificity rather than integration frequency, the target specificity of the mutant attBST sites was assessed for each mutated attBST site using colony PCR to determine the number of times that the mutant site integrated in the preferred site.
TABLE 4.
Site-directed mutagenesis of the 18-bp common core sequence in attBST
| attBST | Base at positiona:
|
Integration frequency | Site specificity | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||
| Wild type | A | T | A | A | A | T | C | T | G | G | T | A | A | A | T | T | T | A | 10−3-10−2 | 7/15 |
| A1C | C | 10−3-10−2 | 4/12 | |||||||||||||||||
| Mut2-6 | G | C | C | C | G | 10−3-10−2 | 8/12 | |||||||||||||
| C7G | G | 10−3-10−2 | 0/22 | |||||||||||||||||
| T8C | C | 10−4-10−3 | 0/22 | |||||||||||||||||
| G9C | C | 10−3-10−2 | 0/22 | |||||||||||||||||
| G10A | A | 10−3-10−2 | 6/12 | |||||||||||||||||
| Mut10-11 | C | C | 10−3-10−2 | 4/12 | ||||||||||||||||
| A12C | C | 10−3-10−2 | 8/12 | |||||||||||||||||
| A13C | C | 10−3-10−2 | 2/12 | |||||||||||||||||
| A14C | C | 10−3-10−2 | 9/12 | |||||||||||||||||
| T15G | G | 10−3-10−2 | 6/10 | |||||||||||||||||
| Mut13-18 | C | C | G | G | G | C | 10−6-10−5 | 5/10 | ||||||||||||
| Negativea | <10−9 | NAb | ||||||||||||||||||
The 1.7-kb truncated intBST was used as a negative control.
NA, not available.
Mut2-6 (bp 2 to 6) in the left side had no effect on the integration frequency or site selectivity. Mut13-18 resulted in a 100-fold decrease in integration frequency compared to the wild-type level, but site selectivity was not affected. Since the effects of multiple-base mutations can be due to mutation in the integration site, in the integrase binding site, or in both sites, single-base-pair mutations were constructed within the region. No effect on integration frequency was observed except for mutant G8C, which decreased the integration frequency by about 10-fold, a barely significant difference. In addition, mutant G8C lost target specificity for the preferred attB site. The results showed that CTnBST was able to recombine between attBST and attB in this 18-bp core region in the presence of the heterology, in contrast to lambda integrase, which requires complete homology between the sites of exchange for recombination.
Most mutant attBST sites integrated into the preferred target site with a frequency comparable to the wild-type level (50 to 75%). Mutants C7G and T8C had no effect on the integration frequency but, like mutant G9C, did not integrate into the preferred target site, attB1.
Target sites used by attBST mutants C7G, T8C, and G9C.
The mutational analysis of the 18-bp region revealed three base pairs (bp 7, 8, and 9) that played an important role in target site selection. Mutations in these base pairs abolished integration into the preferred site. To characterize the sites used for integration by the attBST mutants, we performed a Southern blot analysis and determined the sequences of the secondary sites chosen by the mutants. A plasmid rescue technique and PCR were used to obtain the sequences of the chromosomal sites as described in Materials and Methods.
Southern blot analysis of several independent insertions for each attBST confirmed the preliminary PCR finding that none of the mutants integrated into the preferred target site, attB1 (data not shown). All three attBST mutants integrated into many different sites in the chromosome and sometimes into multiple sites in the same recipient. Figure 4 shows the results for mutant G9C; three of the eight transconjugants contained multiple insertions (Fig. 4, lanes 1, 2, and 6).
FIG. 4.
Southern blot analysis of mini-BST insertions in BT4001 by G9C mutants. Mini-BST was transferred from E. coli S17-1 carrying pattBSTG(9)C to BT4001 by conjugation. DNA was extracted from eight independent isolates, digested with HindIII, and run on an agarose gel. The Southern blot of the gel was probed with a labeled 423-bp region of intBST which detected right-end junctions. The HindIII fragments of lambda were in lane λ, and the sizes of the bands (in kilobase pairs) are indicated on the left. The arrow indicates the expected band for the wild type.
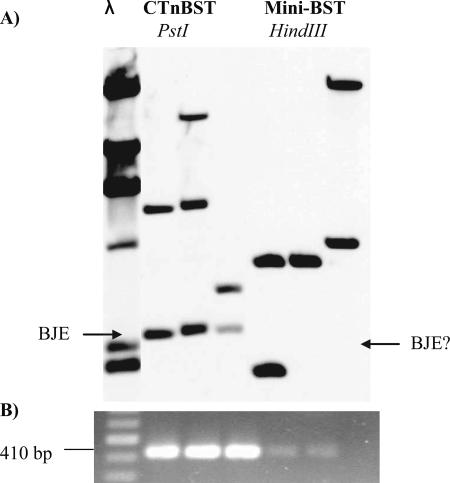
A previous study showed that although the mini-element could integrate, it could not excise, when excision was measured by Southern blotting. However, by using PCR as the assay for excision, we could detect a low level of excision if a high template DNA concentration was used. Figure 5 compares the excision levels of the mini-element and the intact CTnBST determined using PCR and Southern blotting. Clearly, genes outside intBST were required for a wild-type level of excision. This is not surprising since most CTns require at least one gene in addition to the integrase for excision. The fact that some excision was detected, however, allowed us to use PCR analysis to obtain the DNA sequence of the excised joined ends. The weak 410-bp PCR products were used as a template in a second PCR using primers F163 and R388 (Table 2). The excision products were sequenced, and the excision sequences together with the left- and right-end sequences of the integrated element were used to obtain insight into excision of the mutant attBST sites with changes in bp 7, 8, and 9.
FIG. 5.
Southern blot and PCR results for CTnBST and mini-BST excision. Three strains containing CTnBST (CTnBST lanes) and three strains containing mini-BST (Mini-BST lanes) were tested to detect excision either by Southern blotting (A) or by PCR (B). The Southern blot of the gel was probed with a right-end fragment containing intBST. The location of the 2.4-kb PstI fragment from the excised CTnBST containing the joined ends is indicated on the left (BJE), and the location of the expected HindIII fragment of the excised mini-BST is indicated on the right (BJE?). The results for PCR amplification of BJE of the strains shown in panel A are shown immediately below the Southern blot in panel B. The size of the BJE amplicon is 410 bp.
To understand the integration and excision mechanism of IntBST, sequence results for three or four transconjugants for each of the three site specificity mutants are shown in Fig. 6. Transconjugants with single mini-element integration events, as shown in the Southern blot, were selected to identify the target sites to prevent confusion in sequencing due to multiple copies of the element. Alignment of the attB target sites with the three corresponding mutated attBST sites revealed that bp 7, 8, and 9 of the 18-bp region had to be identical in attBST and the target attB. Base pair changes in these three residues in the region had no influence on the integration frequency, but the target sites were selected to preserve the identity between attB and attBST. bp 5 and 6 appeared to be conserved, but identity between the two sites is not strictly required. Variations in the sequences at bp 10 and 11 were also observed, as previously described (23). Figure 6 also shows mismatch correction within bp 10 to 13. For example, as shown in Fig. 6A for attB2 sequences, a heteroduplex was produced during recombination in bp 11 (attBST has T and attB2 has G). The left-end and right-end sequences have T at position 11 due to mismatch correction which is observed in the crossover region in lambda and other conjugative transposons. Other examples are attB3 and attB4 in Fig. 6B, as well as attB2 and attB3 in Fig. 6C. These results indirectly confirmed that bp 10 to 13 were within the crossover region. The flanking sequences of the 7-bp crossover region were less conserved but had a high A+T content.
FIG. 6.
Sequences of attB sites used by mutant mini-elements and consensus sequences of the attB sites relative to attBST. The panels show the 18-bp sequences of an integrating mini-element and the corresponding 18-bp sequence of the chromosomal attB site. The sequences are shown with spaces between bp 6 and bp 7 and between bp 13 and bp 14 to emphasize the putative crossover between the attBST and the attBs. The 18-bp attBST sequence of CTnBST is shown at the top. The underlined bases in the attBST sites are the mutated bases. Immediately below each attBST sequence is the sequence of the site in the chromosome (attB) into which the mini-element integrated. Below each pair of attBST and attB sequences are the left and right junctions of the integrated mini-element. The left junction of each insertion was cloned by plasmid rescue and sequenced. The right junction was obtained by PCR using a primer designed from the identified chromosomal site and the right end of the mini-element. An alignment of attBST and the attB sites is included. The consensus sequence is shown at the bottom of each panel; residues in uppercase letters are conserved in all the attBST and attB sites, and residues in lowercase letters are conserved in a majority of the sequences. Panels A, B, and C show the integration sequences of three mutants. The vertical arrows indicate the base pair that underwent mismatch correction.
DISCUSSION
IntBST appears to belong to the tyrosine recombinase family, based on the conserved amino acid sequence signature. Mutational analysis of the conserved residues in CTnBST showed that Y357 was likely the catalytic tyrosine and R237 and R322 were important for the catalytic activity of the protein. Mutations in three other conserved amino acids (K264A, H322A, and H348A), however, had only modest effects on integration. The lysine and the two histidines are not completely conserved in the family, and the mutations in some recombinases, such as H322A of IntDOT, do not affect its recombination activity. In the case of lambda integrase, substitution of any of the six conserved in the integrase eliminates integration in vivo and in vitro. The fact that only three of six IntBST conserved residues had an essential role suggests that either the catalytic mechanism is different from that of the lambda integrase or other amino acids in the catalytic region perform the functions of the conserved amino acids found in the other tyrosine recombinases.
Since IntBST is a new member of the tyrosine recombinase family, CTnBST integration was characterized and compared with the known recombinase integration systems, such as lambda, CTnDOT, and NBU1. The integration differences among these conjugative transposons can help us understand their origin and evolution within different species. Moreover, the integrases provide a newly expanded view of the varieties of proteins in the tyrosine recombinase family. We carried out the first systematic mutational analysis of the crossover region of CTnBST. The minimal attBST site was about 270 bp long, a size comparable to that of the lambda attP site and att sites in many other tyrosine recombinase systems. Since the site within which the crossover occurs is much smaller (18 bp), the extra DNA is probably necessary for the formation of the intasome that catalyzes the integration reaction. This complex presumably includes the CTnBST integrase plus an unknown number of host factors.
Single-base-pair mutations in the common core sequence of CTnBST had no influence on integration frequency. This is different from what occurs in lambda and many other systems, where mutations in the common core sequence decrease the integration frequency dramatically. It is more similar to the common core sequence requirements of two other CTns, CTnDOT and Tn916, which tolerate changes in most of the bases (2, 6). Although changes in individual bases of the attBST site did not affect the integration frequency, some of these changes, in particular changes in 3 bp at the 5′ end of the 7-bp overlap region, did affect target site selection. A similar phenomenon has recently been observed in secondary site sequence requirements used by phage lambda (15). In the case of IntBST, however, integration in the secondary sites occurs for the mutant sites nearly as frequently as integration into the preferred wild-type site.
The requirement for sequence identity between the strand exchange sites in CTnBST is compatible with the strand-swapping model proposed for the lambda system (11). In this model, there are two symmetrical swaps of two or three nucleotides, connected by a central isomerization step between the four junction arms. Sequence homology is sensed during the annealing step prior to strand joining. Inhibition of the first swap by sequence heterology has a more severe effect on the overall reaction than inhibition of the second swap because the Holiday intermediate can be resolved by Int-independent mechanisms in vivo (11).
Recombination between wild-type attBST and attB sites could occur by the strand-swapping mechanism. However, recombinational events that created the crossovers shown in Fig. 6 are unlikely to occur by a strict strand-swapping mechanism because of mismatches in the sites. When the joined ends and the target sites have different sequences in the crossover region, mismatches form during recombination. If recombination occurs, the heterology is then resolved after recombination by DNA replication or mismatch repair. The mismatch correction that we observed suggests that both strands are exchanged during recombination. For example, the attB2 site shown in Fig. 6A has a G at position 11, while attBST has a T. Only a double-strand exchange can explain the appearance of T in the left- and right-end sequences. We found examples of mismatch correction at each of the four base pairs downstream of the conserved 3-bp region, suggesting that they are within the cleavage region (Fig. 6).
Figure 7 shows where cleavage could occur to explain the data shown in Fig. 6A to C. IntBST cleavage would occur between bp 6 and 7 on the top strand and between bp 13 and 14 on the bottom strand. The strand-swapping model requires that the 3 bp lie within the cleavage site. Strand exchange might occur first in the top strand, requiring strict homology, followed by exchange that does not require strict homology. The target site specificity may be determined by the ability to ligate after the top strand is cleaved and transferred to the partner site. The cleavage sites are adjacent to 5-bp inverted repeats in attBST, which might serve as the integrase binding sites. Lambda, CTnDOT, and NBU1 also have 7 bp between the cleavage sites within an inverted repeat (9, 13, 17).
FIG. 7.
Possible staggered cut sites for CTnBST integration. The 18-bp common core region for CTnBST and attB1 is shown. The vertical arrows indicate possible IntBST cleavage sites on the top and bottom strands. The underlined base pairs were important in target specificity. The italicized base pairs were shown to produce heterology which was resolved after integration. The inverted repeats are indicated by horizontal arrows.
This proposed CTnBST integration mechanism represents an intermediate between CTnDOT and lambda. The CTnBST integration model includes 3-bp invariant base pairs on the left side for the top strand cleavage first, while the next 4 bp on the right can be variable. CTnDOT also has a 2-bp conserved region on the left side, but the bottom-strand exchange tolerates heterology of the 5 bp on the right side. The lambda system requires homology of all 7 bp in the crossover region. This study expands the diversity of the integration mechanism mediated by a member of the tyrosine recombinase family. Further work is needed to establish how IntBST and other accessory proteins bind to attBST and to determine how these interactions are responsible for the requirement for the 3-bp identity between attBST and attB during integration and excision.
The much higher excision level of CTnBST than of the mini-BST plasmid pBJE2.1 suggests that excision genes are involved in mediating the high excision level observed for CTnBST by both Southern blotting and PCR analysis. IntBST alone, however, is able to mediate the low level of excision detected by PCR, suggesting that IntBST is able to perform strand exchange and DNA cleavage. This is similar to what occurs in vitro with lambda and Tn916 (8, 14). For Tn916, Int alone can promote excision in vivo in some hosts and is able to mediate a low level of excision under some conditions in vitro (14). IntDOT, however, has not been shown to mediate excision alone, and it requires multiple proteins for detectable excision (3).
Acknowledgments
This work was supported by grant AI/GM 22383 from the National Institutes of Health and by grant IDSS042703 from the Ellison Foundation.
Footnotes
Published ahead of print on 6 July 2007.
REFERENCES
- 1.Caparon, M. G., and J. R. Scott. 1989. Excision and insertion of the conjugal transposon Tn916 involves a novel recombination mechanism. Cell 59:1027-1034. [DOI] [PubMed] [Google Scholar]
- 2.Cheng, Q., B. J. Paszkiet, N. B. Shoemaker, J. F. Gardner, and A. A. Salyers. 2000. Integration and excision of a Bacteroides conjugative transposon, CTnDOT. J. Bacteriol. 182:4035-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng, Q., Y. Sutanto, N. B. Shoemaker, J. F. Gardner, and A. A. Salyers. 2001. Identification of genes required for the excision of CTnDOT, a Bacteroides conjugative transposon. Mol. Microbiol. 41:625-632. [DOI] [PubMed] [Google Scholar]
- 4.Gupta, A., H. Vlamakis, N. Shoemaker, and A. A. Salyers. 2003. A new Bacteroides conjugative transposon that carries an ermB gene. Appl. Environ. Microbiol. 69:6455-6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holdeman, L. V., and W. E. C. Moore. 1975. Anaerobe laboratory manual, 4th ed. Virginia Polytechnic Institute and State University, Blacksburg.
- 6.Jia, Y. H., and G. Churchward. 1999. Interactions of the integrase protein of the conjugative transposon Tn916 with its specific DNA binding sites. J. Bacteriol. 181:6114-6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li, L. Y., N. B. Shoemaker, and A. A. Salyers. 1993. Characterization of the mobilization region of a Bacteroides insertion element (NBU1) that is excised and transferred by Bacteroides conjugative transposons. J. Bacteriol. 175:6588-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu, F., and G. Churchward. 1995. Tn916 target DNA sequences bind the C-terminal domain of integrase protein with different affinities that correlate with transposon insertion frequency. J. Bacteriol. 177:1938-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malanowska, K., A. A. Salyers, and J. F. Gardner. 2006. Characterization of a conjugative transposon integrase, IntDOT. Mol. Microbiol. 60:1228-1240. [DOI] [PubMed] [Google Scholar]
- 10.Metcalf, W. W., W. Jiang, and B. L. Wanner. 1994. Use of the rep technique for allele replacement to construct new Escherichia coli hosts for maintenance of R6Kv origin plasmids at different copy numbers. Gene 138:1-7. [DOI] [PubMed] [Google Scholar]
- 11.Nunes-Duby, S. E., M. A. Azaro, and A. Landy. 1995. Swapping DNA strands and sensing homology without branch migration in lambda site specific recombination. Curr. Biol. 5:139-148. [DOI] [PubMed] [Google Scholar]
- 12.Nunes-Duby, S. E., H. J. Kwon, R. S. Tirumalai, T. Ellenberger, and A. Landy. 1998. Similarities and differences among 105 members of the Int family of site-specific recombinases. Nucleic Acids Res. 26:391-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajeev, L., A. A. Salyers, and J. F. Gardner. 2006. Characterization of the integrase of NBU1, a Bacteroides mobilizable transposon. Mol. Microbiol. 61:978-990. [DOI] [PubMed] [Google Scholar]
- 14.Rudy, C., K. L. Taylor, D. Hinerfeld, J. R. Scott, and G. Churchward. 1997. Excision of a conjugative transposon in vitro by the Int and Xis proteins of Tn916. Nucleic Acids Res. 25:4061-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutkai, E., L. Dorgai, R. Sirot, E. Yagil, and R. A. Weisberg. 2003. Analysis of insertion into secondary attachment sites by phage λ and by Int mutants with altered recombination specificity. J. Mol. Biol. 329:983-996. [DOI] [PubMed] [Google Scholar]
- 16.Saito, H., and K. I. Miura. 1963. Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochim. Biophys. Acta 72:619-629. [PubMed] [Google Scholar]
- 17.Schmidt, J. W., L. Rajeev, A. A. Salyers, and J. F. Gardner. 2006. NBU1 integrase: evidence for an altered recombination mechanism. Mol. Microbiol. 60:152-164. [DOI] [PubMed] [Google Scholar]
- 18.Shoemaker, N. B., C. Getty, J. F. Gardner, and A. A. Salyers. 1986. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J. Bacteriol. 165:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 20.Valentine, P. J., N. B. Shoemaker, and A. A. Salyers. 1988. Mobilization of Bacteroides plasmids by Bacteroides conjugal elements. J. Bacteriol. 170:1319-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Duyne, G. D. 2002. A structural view of tyrosine recombinase site-specific recombination, p. 93-117. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, DC.
- 22.Wang, J., N. B. Shoemaker, G. R. Wang, and A. A. Salyers. 2000. Characterization of a Bacteroides mobilizable transposon, NBU2, which carries a functional lincomycin resistance gene. J. Bacteriol. 182:3559-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wesslund, N., G. R. Wang, B. Song, N. B. Shoemaker, and A. A. Salyers,. 2007. Integration and excision of a newly discovered Bacteroides conjugative transposon, CTnBST. J. Bacteriol. 189:1072-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu, J., M. K. Bjursell, J. Himrod, S. Deng, L. K. Carmichael, H. C. Chiang, L. V. Hooper, and J. I. Gordon. 2003. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 299:2074-2076. [DOI] [PubMed] [Google Scholar]