Abstract
The fermentation pattern of Lactococcus lactis with altered activities of the las enzymes was examined on maltose. The wild type converted 65% of the maltose to mixed acids. An increase in phosphofructokinase or lactate dehydrogenase expression shifted the fermentation towards homolactic fermentation, and with a high level of expression of the las operon the fermentation was homolactic.
In Lactococcus lactis sugars can be metabolized either by homolactic fermentation, producing lactate, or by mixed acid fermentation, producing formate, acetate, and ethanol. The former mode of fermentation is favored during growth on readily metabolizable sugars, such as glucose and lactose, whereas growth on slowly fermentable sugars, such as maltose (11) and galactose (17), usually results in mixed acid fermentation.
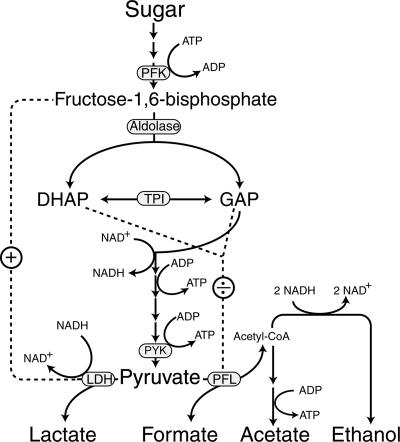
The mechanisms regulating this shift in metabolism are still unclear. It has been observed that under sugar starvation conditions the level of fructose-1,6-bisphosphate (FBP) is lowered (16), and since FBP is an allosteric activator of lactate dehydrogenase (LDH) (19), this could explain a shift in fermentation pattern (4, 16) (Fig. 1). The level of the triose phosphates dihydroxyacetone phosphate and glyceraldehyde-3-phosphate in L. lactis is also lowered during growth on slowly fermentable sugars (17). Since the triose phosphates are allosteric inhibitors of pyruvate formate lyase (PFL) (14), this may also be part of the explanation of the shift in fermentation mode (Fig. 1). Other investigators have suggested that the NADH/NAD+ ratio regulates the shift in metabolism. A correlation was found between this ratio and the glycolytic flux; a high ratio gave a high flux and vice versa (5). These investigators also determined that glyceraldehyde-3-phosphate dehydrogenase was activated and LDH was deactivated at low ratios, whereas the opposite was the case at high ratios. This type of regulation could, in combination with the above-mentioned effect of the triose phosphates, explain the shift in pyruvate metabolism. A more recent study indicated that both the level of FBP and the NADH/NAD+ ratio may be important, but with large variations between strains (18).
FIG. 1.
Simplified overview of glycolysis and its regulation by FBP and the triose phosphates dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP). Positive regulation by FBP of LDH (19) is indicated by a plus sign, and negative regulation by the triose phosphates of PFL (14) is indicated by a division sign. TPI, triose phosphate isomerase; Acetyl-CoA, acetyl coenzyme A.
In recent years the glucose metabolism in L. lactis has been studied extensively with respect to the glycolytic rate and product formation. One study focusing on the las operon, coding for phosphofructokinase (PFK), pyruvate kinase (PYK), and LDH (9), revealed that LDH has a strong negative effect on the mixed acid flux; the flux control coefficient was less than −1, meaning that a 10% reduction in LDH leads to a >10% increase in the mixed acid flux (1). Another study revealed that PYK exhibits strong positive control of formate and acetate production (flux control coefficient for PYK on the formate and acetate flux were both close to 1) (7). The positive control by PYK and the negative control by LDH almost cancelled out, resulting in very little effect of modulation of the entire operon on the fermentation pattern. In this paper we describe a control analysis of the three las enzymes in L. lactis for the glycolytic flux and product distribution during growth on maltose.
Fermentation pattern of L. lactis MG1363 during growth on glucose or maltose.
The fermentation pattern of L. lactis MG1363 (6) was studied in defined SALN (7) medium supplemented with glucose or maltose as the main energy source. When MG1363 was grown on glucose, 88% of the maltose ended up in lactate and only 4% ended up in mixed acid products. On maltose 65% of the maltose ended up in formate, acetate, and ethanol and only 25% of the maltose was fermented to lactate.
Activities of the las enzymes during growth on maltose or glucose.
The activities of PYK and LDH have previously been shown to affect pyruvate metabolism in L. lactis (1, 7), and differences in fermentation patterns between glucose and maltose could thus be due to differences in the specific activities of these enzymes on maltose. The specific activities of the three las enzymes in wild-type strain MG1363 grown on maltose and on glucose were therefore measured and found to be almost identical (data not shown). Strains with either individually or simultaneously modulated las enzymes were then characterized. The strains were grown exponentially in defined SALN (7) medium supplemented with maltose (2 g/liter) as the energy source and analyzed to determine the growth rate, glycolytic flux, and fermentation products.
Effect of altered PFK activity on maltose fermentation mode.
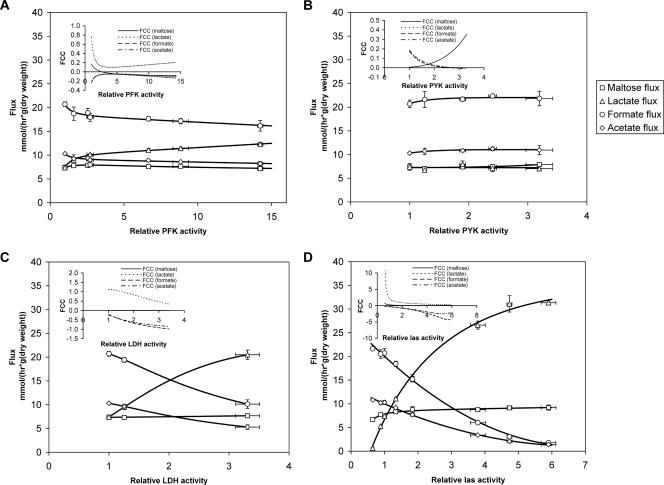
Strains containing an additional copy of pfk transcribed from a range of synthetic promoters have previously been constructed (12). Increased PFK activity had little effect on the growth rate except for the strain with the highest level of PFK, which grew slightly slower. The control of the growth rate by PFK was close to zero. A small but significant increase in the maltose flux was observed (8%), and the CPFKmaltose was calculated to be ∼0.17 at the wild-type level (Fig. 2A). The product fluxes were very sensitive to changes in PFK activity; the lactate production increased almost proportional to PFK activity, and the mixed acid fluxes decreased with increased PFK activity. CPFKlactate, CPFKformate, and CPFKacetate were calculated to be ∼0.76, ∼−0.23, and ∼−0.23, respectively, at the wild-type PFK level. High flux control by PFK for lactate production is in line with the early model for regulation of fluxes at the pyruvate node. Assuming that increased PFK activity results in increased levels of the downstream metabolites FBP (an allosteric activator of LDH [19]) and triose phosphates (allosteric inhibitors of PFL [14]), then a shift towards homolactic fermentation with increased PFK activity is indeed what should be expected (Fig. 1).
FIG. 2.
Metabolic fluxes for strains with modulated PFK, PYK, LDH, and las activities. The maltose, lactate, formate, and acetate fluxes are shown. The flux control coefficients (FCC) for the different fluxes are also shown. The standard deviations, indicated by error bars, are based on measurements for at least two individual cultures.
Effect of altered PYK activity on maltose fermentation.
Strains with modulated PYK activity have also previously been described for growth on glucose (7). When the strains were grown on maltose, changes in PYK activity had very little effect on the growth rate, and only small effects on the metabolic fluxes were observed, resulting in flux control coefficients close to zero for the maltose and lactate flux (Fig. 2B). The mixed acid fluxes increased slightly, resulting in CPFKformate and CPFKacetate of ∼0.17 and ∼0.18, respectively. The positive control of the mixed acid fluxes is in line with the previous work on glucose fermentation (7), even though the effect is much less pronounced in maltose medium.
Effect of altered LDH activity on maltose fermentation.
Strains containing an additional copy of the gene coding for LDH, ldh, transcribed from synthetic promoters were constructed. The strategy described by Solem and Jensen (12), where an additional copy of ldh transcribed from synthetic promoters was integrated into the TP901-1 attachment site by a pCS574 (13) derivative, was used. The following primers were used: LDH.fwd (5′-ACGACTAGTGGATCCATNNNNNAGTTTATTCTTGACANNNNNNNNNNNNNNTRRTAGAATANNGACAGGCCCTATTGTTGAA-3′) and LDH.rev (5′-CTCTACATGCATTGAAATTTTCTACTAACTC-3′). The PCR product was digested with SpeI/NsiI and ligated into pCS574 digested with XbaI/PstI. L. lactis LB436 (3) was transformed with the ligation mixture. Transformants containing an additional copy of ldh on the chromosome were isolated as blue colonies on GM17 plates (15) supplemented with tetracycline and 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid. Two strains with increased LDH activity were included in these experiments.
While LDH had essentially no effect on the maltose flux with a flux control coefficient close to zero, LDH was found to have strong control over the mixed acid fluxes (Fig. 2C), with a calculated CLDHlactate of ∼1.1. CLDHformate and CLDHacetate were calculated to be ∼−0.21 and ∼−0.23, respectively, at the wild-type LDH level. The high level of positive control of the lactate flux is at first surprising when the data are compared to earlier work (1), in which LDH was found to have zero control of the lactate branch at the wild-type enzyme level. However, the previous study was conducted with cells grown on glucose, with which the lactate flux is high compared to the mixed acid fluxes. Indeed, at an LDH activity of 30% when cells are grown on glucose, the flux control coefficient also approaches 1 in these cells (7).
Effect of altered expression of the entire las operon.
The strong effects described above for the individual las enzymes prompted us to test whether these effects were additive. Strains in which the entire las operon has been modulated were available from a previous study (12). By increasing the expression of the las operon, it was possible to obtain a maltose flux 23% higher than that in the wild-type cells (Fig. 2D). Clasmaltose was estimated to be ∼0.23, and the control values for the fermentation pattern were determined to be as follows: Claslactate, ∼2.1; Clasformate, ∼−0.32; and Clasacetate, ∼−0.31. In conclusion, increasing the expression of the las enzymes results in a more homolactic fermentation pattern, and the strain with highest las expression was found to be as homolactic on maltose as the wild-type strain was on glucose. The flux control coefficients for simultaneous modulation of the entire las operon were found to be close to the sum of the coefficients of individual las enzymes, which showed that the effects of the las enzymes on the fluxes were additive.
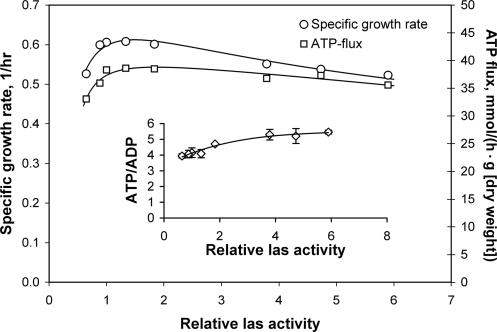
The effect on the specific growth rate of altered expression of the las operon deserves special attention. The specific growth rate was found to be maximum at the wild-type level (Fig. 3). At first sight, it is tempting to conclude that the cells become energy limited with higher las expression because of a lower ATP yield. However, the ATP flux can be calculated by assuming that one glucose moiety is either converted to two lactate molecules, yielding two ATP molecules per glucose unit, or to one formate molecule, one ethanol molecule, and one acetate molecule, yielding three ATP molecules per glucose molecule. Figure 3 shows that the wild type has the highest ATP production flux, but the relative decrease in the ATP flux with increased expression of the las operon is less than the relative decrease in the growth rate (Fig. 3), indicating that more ATP should become available for growth. Indeed, the intracellular [ATP]/[ADP] ratio increased slightly with increased las activity (Fig. 3), which indicates that the cells were not energy limited with high las expression. The observed lower growth rate and increased intracellular energy level may instead be the result of changes in other metabolite pools for anabolism. This effect could be reminiscent of our previous observation of glucose-grown cells with low PFK activity whose growth rate was strongly affected (2), but the exact mechanism involved in this phenomenon remains obscure. The high flux control coefficient for the las enzymes (>1) shows that significant negative control of the lactate flux must occur elsewhere in the metabolic network. A good candidate for an enzyme with negative control of the lactate flux is PFL, and an increased level of PFL may result in increased mixed acid fermentation at the expense of the lactate production, as previously described (10). It is also possible that glycolytic enzymes downstream of FBP and the triose phosphates may exert negative control by reducing these metabolites at increased activities. Finally, negative control of the lactate flux may also be found with anabolic reactions that consume the ATP generated from glycolysis. Indeed, a slight increase in formate production was observed when an additional ATP-consuming process was introduced into L. lactis grown on glucose (8).
FIG. 3.
Specific growth rate, calculated ATP flux, and intracellular[ATP]/[ADP] ratio as a function of relative las activity. The standard deviations, indicated by error bars, are based on measurements for at least two individual cultures.
Acknowledgments
This work was supported by the Danish Dairy Research Foundation (Danish Dairy Board) and the Danish Research Agency.
Footnotes
Published ahead of print on 6 July 2007.
REFERENCES
- 1.Andersen, H. W., M. B. Pedersen, K. Hammer, and P. R. Jensen. 2001. Lactate dehydrogenase has no control on lactate production but has a strong negative control on formate production in Lactococcus lactis. Eur. J. Biochem. 268:6379-6389. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, H. W., C. Solem, K. Hammer, and P. R. Jensen. 2001. Twofold reduction of phosphofructokinase activity in Lactococcus lactis results in strong decreases in growth rate and in glycolytic flux. J. Bacteriol. 183:3458-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brøndsted, L., and K. Hammer. 1999. Use of the integration elements encoded by the temperate lactococcal bacteriophage TP901-1 to obtain chromosomal single-copy transcriptional fusions in Lactococcus lactis. Appl. Environ. Microbiol. 65:752-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crow, V. L., and G. G. Pritchard. 1977. Fructose 1,6-diphosphate-activated l-lactate dehydrogenase from Streptococcus lactis: kinetic properties and factors affecting activation. J. Bacteriol. 131:82-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrigues, C., P. Loubierre, N. D. Lindley, and M. Cocaign-Bousquet. 1997. Control of the shift from homolactic acid to mixed-acid fermentation in Lactococcus lactis: predominant role of the NADH/NAD+ ratio. J. Bacteriol. 179:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koebmann, B., C. Solem, and P. R. Jensen. 2005. Control analysis as a tool to understand the formation of the las operon in Lactococcus lactis. FEBS J. 272:2292-2303. [DOI] [PubMed] [Google Scholar]
- 8.Koebmann, B. J., C. Solem, M. B. Pedersen, D. Nilsson, and P. R. Jensen. 2002. Expression of the genes encoding F1-ATPase results in uncoupling of glycolysis from biomass production in Lactococcus lactis. Appl. Environ. Microbiol. 68:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llanos, R. M., C. J. Harris, A. J. Hillier, and B. E. Davidson. 1993. Identification of a novel operon in Lactococcus lactis encoding three enzymes for lactic acid synthesis: phosphofructokinase, pyruvate kinase, and lactate dehydrogenase. J. Bacteriol. 175:2541-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melchiorsen, C. R., K. V. Jokumsen, J. Villadsen, H. Israelsen, and J. Arnau. 2002. The level of pyruvate-formate lyase controls the shift from homolactic to mixed-acid product formation in Lactococcus lactis. Appl. Microbiol. Biotechnol. 58:338-344. [DOI] [PubMed] [Google Scholar]
- 11.Sjöberg, A., and B. Hahn-Hägerdal. 1989. β-Glucose-1-phosphate, a possible mediator for polysaccharide formation in maltose-assimilating Lactococcus lactis. Appl. Environ. Microbiol. 55:1549-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solem, C., and P. R. Jensen. 2002. Modulation of gene expression made easy. Appl. Environ. Microbiol. 68:2397-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solem, C., B. J. Koebmann, and P. R. Jensen. 2003. Glyceraldehyde-3-phosphate dehydrogenase has no control over glycolytic flux in Lactococcus lactis MG1363. J. Bacteriol. 185:1564-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi, S., K. Abbe, and T. Yamada. 1982. Purification of pyruvate formate-lyase from Streptococcus mutans and its regulatory properties. J. Bacteriol. 149:1034-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas, T. D., D. C. Ellwood, and M. C. Longyear. 1979. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J. Bacteriol. 138:109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas, T. D., K. W. Turner, and V. L. Crow. 1980. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J. Bacteriol. 144:672-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Niel, E. W. J., J. Palmfeldt, R. Martin, M. Paese, and B. Hahn-Hägerdal. 2004. Reappraisal of the regulation of lactococcal l-lactate dehydrogenase. Appl. Environ. Microbiol. 70:1843-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolin, M. J. 1964. Fructose-1,6-diphosphate requirement of streptococcal lactic dehydrogenases. Science 146:775-777. [DOI] [PubMed] [Google Scholar]