Abstract
To mimic in vivo conditions during chlamydial infections, Chlamydia trachomatis serovar D and Chlamydia pneumoniae CWL029 were cultured in low-oxygen atmospheres containing 4% O2, with parallel controls cultured in atmospheric air. Both were enriched with 5% CO2. The results showed a dramatic increase in the growth of C. pneumoniae but not of C. trachomatis.
The chlamydial developmental cycle is biphasic, alternating between an infectious metabolically inactive elementary body (EB) specialized for extracellular survival and a noninfectious proliferating intracellular reticulate body (RB). During the course of an infection, the EB is endocytosed by a susceptible host cell into a host-derived vacuole, the chlamydial inclusion. After internalization, the EB develops into an RB, which proliferates by binary fission. Following several rounds of proliferation lasting 48 to 72 h, RBs transform into EBs and are released by the disruption of the host cell (for a review, see reference 9).
Chlamydia trachomatis is an obligate human pathogen causing ocular and genital infections. Chlamydia pneumoniae causes respiratory tract infections, often asymptomatic, but may cause bronchitis and pneumonia (5). Traditionally, both C. trachomatis and C. pneumoniae have been studied in vitro by infecting cell culture monolayers and incubating the infected cells in incubators in a humid atmosphere containing atmospheric air enriched with 5% CO2, resulting in an oxygen concentration of approximately 20%. However, the in vivo oxygen tension is much lower, generally in the range of 3 to 6% (Table 1), and as different tissues have different oxygen requirements, the in vivo oxygen tension may vary considerably from tissue to tissue. The oxygen requirements of Chlamydia have never been evaluated before, but it is known that the oxygen tension of host tissue is important for viral replication and the viral life cycle (3) and that many infecting microorganisms are microaerophilic. As Chlamydia proliferates in vivo where the oxygen tension varies between different tissues, it is plausible that Chlamydia is also affected by the oxygen tension and would experience enhanced growth in tissues with optimum oxygen tension.
TABLE 1.
Oxygen concentrations in air and in selected tissues
C. trachomatis and C. pneumoniae produced enlarged inclusions in 4% oxygen.
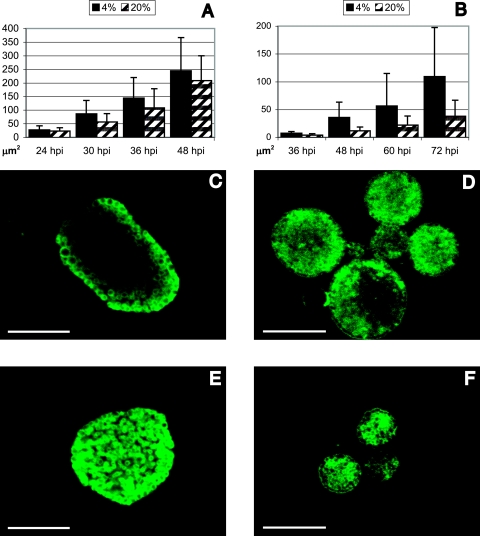
To determine the visible effect of low oxygen tension on chlamydial inclusions, infected HeLa cells were cultured on coverslips at 4% and 20% O2. HeLa 229 cells (ATCC, Rockville, MD) cultured in 24-well trays (TPP, Trasadingen, Switzerland) were infected with either C. trachomatis serovar D/UW-3/CX (ATCC) or C. pneumoniae CWL029 (ATCC) as previously described (12, 15) and cultured in the presence of cycloheximide in either 4% or 20% oxygen atmospheres. Low-oxygen atmospheres were achieved by placing trays with infected HeLa cells in an airtight custom-made box (40 by 30 by 16 cm [width by diameter by height]). An OX-500 Clark-type oxygen sensor (UniSense, Aarhus, Denmark) was placed inside the box to measure the oxygen concentration. The box was flushed with an air mixture containing 95% N2 and 5% CO2 until an oxygen concentration of 4% was reached. Infected cells cultured on coverslips were methanol fixed at 24, 30, 36, and 48 h postinfection (hpi) for C. trachomatis and at 36, 48, 60, and 72 hpi for C. pneumoniae. Immunofluorescence (IMF) microscopy was performed as described previously (2) by using either monoclonal antibody 32.3 (1) against major outer membrane protein (C. trachomatis), or polyclonal antibody 198 (8) against chlamydial outer membrane complex (COMC) (C. pneumoniae). As the chlamydial inclusions were not circular, the total area (μm2) of each inclusion was determined using Image Pro Plus 4.5.1.22 (Media Cybernetics, Silver Spring, MD) calibrated against a Leitz Wetzlar ruler (Leica) with 0.01-mm intervals. More than 100 inclusions at each time point in both oxygen tensions were measured. From 24 hpi to 48 hpi, the mean inclusion size of C. trachomatis increased from 28 μm2 to 245 μm2 at 4% oxygen versus 23 μm2 to 208 μm2 at 20% oxygen (Fig. 1A, C, and E). The greatest increase in inclusion size was observed at 30 hpi, where inclusions in cultures at 4% oxygen were on average 53% larger than inclusions in cultures at 20% oxygen. For C. pneumoniae, the inclusion size was measured from 36 hpi to 72 hpi, and the mean inclusion size increased from 7 μm2 to 109 μm2 at 4% oxygen versus 4 μm2 to 38 μm2 at 20% oxygen (Fig. 1B, D, and F). The greatest increase was found at 48 hpi, where the average inclusion size was 207% larger in cultures in 4% oxygen than in cultures in 20% oxygen. At all time points, both C. trachomatis and C. pneumoniae produced significantly larger inclusions at 4% oxygen (P < 0.05 by two-tailed Mann-Whitney test).
FIG. 1.
Inclusion size at 4% and 20% oxygen. (A and B) Increase in inclusion size of C. trachomatis (A) and C. pneumoniae (B) when cultured at 4% and 20% oxygen. (C to F) Representative confocal images of C. trachomatis and C. pneumoniae inclusions. Shown are C. trachomatis at 4% (C) and 20% (E) oxygen and C. pneumoniae at 4% (D) and 20% (F) oxygen. Bars represent 10 μm.
C. pneumoniae showed increased growth in 4% oxygen.
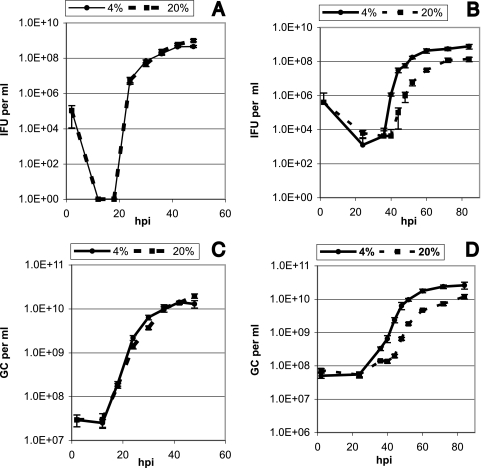
To investigate the growth dynamics of C. trachomatis and C. pneumoniae under low-oxygen conditions, the increase in inclusion-forming units (IFU) and genome copies (GC) was determined at specific time points during the developmental cycle when cultured in 4% and 20% oxygen (2, 12, 18, 24, 30, 36, 42, and 48 hpi for C. trachomatis and 2, 24, 36, 40, 44, 48, 52, 60, 72, and 84 hpi for C. pneumoniae). The increase in IFU was determined by IMF microscopy using aliquots of harvested samples for reinfection of host cell monolayers. Growth curves representing the temporal increase in IFU are shown in Fig. 2A and B. When the amounts of recovered IFU from C. trachomatis cultured at 4% and 20% oxygen were compared (Fig. 2A), at no point was there a significant difference between the two growth curves (P = 0.22 by two-tailed paired t test). Conversely, with C. pneumoniae (Fig. 2B), the amount of IFU recovered from cultures in 4% oxygen was significantly higher than the amount recovered from cultures in 20% oxygen (P < 0.01 by two-tailed paired t test). The difference in recovered IFU between cultures in 4% and 20% oxygen varied between a maximum of 300-fold at 44 hpi to 5-fold at 84 hpi. These results indicate that C. pneumoniae, but not C. trachomatis, experiences increased growth under physiological oxygen conditions. To verify that the increase in C. pneumoniae IFU was caused by an increased production of infectious chlamydial EBs and not by an increased infectivity of the individual EBs, we determined the increase in GC during the chlamydial developmental cycle. This was achieved using real-time LightCycler PCR performed on aliquots of the same samples used to determine IFU. The experimental procedures was performed as described previously (10), except that the primer and probe sequences for C. trachomatis were as follows: forward primer 5′-AGCAAGGGCACTATCAGGAC-3′, reverse primer 5′-ACGGAACCCTGCTTCTACATC-3′, fluorescein-labeled probe 5′-CCACGTGCTAGCGACTATGATTTGCCT-fluorescein-3′, and LightCycler Red640-labeled probe 5′-LightCycler Red640-GAAGCCCATATCCTACTCCACCTTTGCC-3′. Primers were obtained from DNA Technology, Aarhus, Denmark. Probes were obtained from TIB Molbiol, Berlin, Germany. The C. trachomatis PCR mixture contained 4 mM MgCl2, and the thermal cycling was performed as follows: activation of Hot Start DNA polymerase at 95°C for 10 min and cycling at 95°C for 15 s, 53°C for 8 s, and 72°C for 8 s repeated 45 times. The temperature transition rate was set at 20°C/s. The increase in GC is shown in Fig. 2C and D. There was no significant difference in GC when C. trachomatis cultured at 4% oxygen was compared with that at 20% oxygen (Fig. 2C) (P = 0.68 by two-tailed paired t test). With C. pneumoniae, we found a significant increase in the GC of the 4% cultures compared with that of the 20% cultures (Fig. 2D) (P < 0.05 by two-tailed paired t test). As the increase in GC correlates with the increase in IFU, it can be concluded that C. pneumoniae isolates experience increased growth when cultured at a 4% oxygen concentration. Interestingly, however, when the generation time during the exponential log phase was calculated, we found that the doubling rate was practically the same in cultures grown in 4% and 20% oxygen (2.4 h in 4% oxygen and 2.5 h in 20% oxygen). As a significantly higher number of chlamydial bodies was produced at 4% oxygen, this suggests that primarily the C. pneumoniae lag phase is affected by the lowered oxygen concentration.
FIG. 2.
Growth curves for C. trachomatis up to 48 hpi and C. pneumoniae up to 84 hpi when cultured at 4% and 20% oxygen. (A and C) Temporal increase in C. trachomatis IFU (A) and GC (C). (B and D) Temporal increase in C. pneumoniae IFU (B) and GC (D).
The C. pneumoniae lag phase is shortened at 4% oxygen.
During the lag phase, the chlamydial EBs are endocytosed to the cytoplasm, and primary differentiation for replication takes place. To further investigate how the C. pneumoniae lag phase differed depending on the oxygen concentration, the lag phase was investigated by incubating infected HeLa cells on coverslips at 4% and 20% oxygen. The cells were infected at a multiplicity of infection of 10, resulting in multiple inclusions in each cell. The infected cells were methanol fixed at 4, 8, 12, 16, 20, and 24 hpi, stained as described previously (2) using polyclonal antibody 198 against COMC, counterstained with Evans blue, and then investigated by confocal IMF microscopy (16). Confocal images obtained are shown in Fig. S1A to S1L in the supplemental material. At 4 hpi, EBs had attached and entered the host cell in cultures of both 4% and 20% oxygen (see Fig. S1A and S1B in the supplemental material) where multiple EBs had infected each host cell, and most aggregated in the perinuclear region. By 8 hpi, a few enlarged chlamydial bodies were observed at 4% oxygen (see Fig. S1C in the supplemental material), while enlarged bodies were not observed until 12 hpi at 20% oxygen (see Fig. S1F in the supplemental material). This suggests that some EB-to-RB transformation had begun by 8 hpi in 4% cultures but not until 12 hpi in 20% cultures. By 16 hpi, many RBs were visible in both 4% and 20% cultures (see Fig. S1G and S1H in the supplemental material); however, more RBs were present in cultures in 4% oxygen. At 20 hpi, some replicating RBs were observed in the 4% cultures (see Fig. S1I in the supplemental material), represented by clusters of two to three closely associated RBs. At this time, no multiplication was observed in the 20% cultures (see Fig. S1J in the supplemental material). By 24 hpi, several rounds of multiplication had occurred in the 4% oxygen cultures, as demonstrated by several distinct clusters of multiple RBs (see Fig. S1K in the supplemental material), whereas in the 20% cultures, no or only very limited levels of replication could be observed (see Fig. S1L in the supplemental material). This demonstrates that C. pneumoniae has a shortened lag phase when cultured at 4% oxygen.
Altogether, these data show for the first time a significant in vitro difference in the chlamydial developmental cycle caused by lowering the oxygen concentration to physiological levels. The finding that both C. trachomatis serovar D and C. pneumoniae CWL029 produced enlarged inclusions when cultured in 4% oxygen suggests that the chlamydial response to low oxygen availability is at least partially shared. However, as only C. pneumoniae and not C. trachomatis experienced a shortened lag phase and consequently increased growth, C. pneumoniae profits the most from a low oxygen concentration.
Supplementary Material
Acknowledgments
We are very grateful to Karin Sørensen for skilled laboratory practice. We also thank Lisbet Wellejus Pedersen for excellent linguistic assistance with this paper.
This study was supported by Danish Medical Research Council grants 22-03-0245 and 271-05-0488, the Aarhus Universitets Forskningsfond, and Network of Excellence, EuroPathoGenomics.
Footnotes
Published ahead of print on 13 July 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Birkelund, S., A. G. Lundemose, and G. Christiansen. 1988. Chemical cross-linking of Chlamydia trachomatis. Infect. Immun. 56:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clausen, J. D., G. Christiansen, H. U. Holst, and S. Birkelund. 1997. Chlamydia trachomatis utilizes the host cell microtubule network during early events of infection. Mol. Microbiol. 25:441-449. [DOI] [PubMed] [Google Scholar]
- 3.Ebbesen, P., and V. Zachar. 1998. Oxygen tension and virus replication. Acta Virol. 42:417-421. [PubMed] [Google Scholar]
- 4.Gorczynski, R. J., and B. R. Duling. 1978. Role of oxygen in arteriolar functional vasodilation in hamster striated muscle. Am. J. Physiol. 235:H505-H515. [DOI] [PubMed] [Google Scholar]
- 5.Hahn, D. L., A. A. Azenabor, W. L. Beatty, and G. I. Byrne. 2002. Chlamydia pneumoniae as a respiratory pathogen. Front. Biosci. 7:e66-76. [DOI] [PubMed] [Google Scholar]
- 6.Herold, U., H. Jakob, M. Kamler, R. Thiele, U. Tochtermann, J. Weinmann, J. Motsch, M. M. Gebhard, and S. Hagl. 1998. Interruption of bronchial circulation leads to a severe decrease in peribronchial oxygen tension in standard lung transplantation technique. Eur. J. Cardiothorac. Surg. 13:176-183. [DOI] [PubMed] [Google Scholar]
- 7.Hockel, M., K. Schlenger, C. Knoop, and P. Vaupel. 1991. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 51:6098-6102. [PubMed] [Google Scholar]
- 8.Knudsen, K., A. S. Madsen, P. Mygind, G. Christiansen, and S. Birkelund. 1999. Identification of two novel genes encoding 97- to 99-kilodalton outer membrane proteins of Chlamydia pneumoniae. Infect. Immun. 67:375-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulder, J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55:143-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mygind, T., S. Birkelund, E. Falk, and G. Christiansen. 2001. Evaluation of real-time quantitative PCR for identification and quantification of Chlamydia pneumoniae by comparison with immunohistochemistry. J. Microbiol. Methods 46:241-251. [DOI] [PubMed] [Google Scholar]
- 11.Ryan, J. M., and J. B. Hickam. 1952. The alveolar-arterial oxygen pressure gradient in anemia. J. Clin. Investig. 31:188-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw, A. C., K. Gevaert, H. Demol, B. Hoorelbeke, J. Vandekerckhove, M. R. Larsen, P. Roepstorff, A. Holm, G. Christiansen, and S. Birkelund. 2002. Comparative proteome analysis of Chlamydia trachomatis serovar A, D and L2. Proteomics 2:164-186. [DOI] [PubMed] [Google Scholar]
- 13.Sjostrom, P., L. Wiklund, and B. Odlind. 1994. Conjunctival oxygen tension is influenced by plasma and blood volume, and flow through the external carotid artery. Int. J. Clin. Monit. Comput. 11:99-103. [DOI] [PubMed] [Google Scholar]
- 14.Sommer, F., H. P. Caspers, K. Esders, T. Klotz, and U. Engelmann. 2001. Measurement of vaginal and minor labial oxygen tension for the evaluation of female sexual function. J. Urol. 165:1181-1184. [PubMed] [Google Scholar]
- 15.Vandahl, B. B., S. Birkelund, H. Demol, B. Hoorelbeke, G. Christiansen, J. Vandekerckhove, and K. Gevaert. 2001. Proteome analysis of the Chlamydia pneumoniae elementary body. Electrophoresis 22:1204-1223. [DOI] [PubMed] [Google Scholar]
- 16.Vandahl, B. B., A. Stensballe, P. Roepstorff, G. Christiansen, and S. Birkelund. 2005. Secretion of Cpn0796 from Chlamydia pneumoniae into the host cell cytoplasm by an autotransporter mechanism. Cell. Microbiol. 7:825-836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.