Abstract
The plant pathogen Pseudomonas syringae may cope with osmotic stress on plants, in part, by importing osmoprotective compounds. In this study, we found that P. syringae pv. tomato strain DC3000 was distinct from most bacterial species in deriving greater osmoprotection from exogenous choline than from glycine betaine. This superior osmoprotection was correlated with a higher capacity for uptake of choline than for uptake of glycine betaine. Of four putative osmoregulatory ABC transporters in DC3000, one, designated OpuC, functioned as the primary or sole transporter for glycine betaine and as one of multiple transporters for choline under high osmolarity. Surprisingly, the homolog of the well-characterized ProU transporter from Escherichia coli and Salmonella enterica serovar Typhimurium did not function in osmoprotection. The P. syringae pv. tomato OpuC transporter was more closely related to the Bacillus subtilis and Listeria monocytogenes OpuC transporters than to known osmoprotectant transporters in gram-negative bacteria based on sequence similarity and genetic arrangement. The P. syringae pv. tomato OpuC transporter had a high affinity for glycine betaine, a low affinity for choline, and a broad substrate specificity that included acetylcholine, carnitine, and proline betaine. Tandem cystathionine-β-synthase (CBS) domains in the ATP-binding component of OpuC were required for transporter function. The presence of these CBS domains was correlated with osmoregulatory function among the putative transporters examined in DC3000 and was found to be predictive of functional osmoregulatory transporters in other pseudomonads. These results provide the first functional evaluation of an osmoprotectant transporter in a Pseudomonas species and demonstrate the usefulness of the CBS domains as predictors of osmoregulatory activity.
Prokaryotic ATP-binding cassette (ABC) transporters contrast with eukaryotic ABC transporters, which function mainly in export, by functioning mainly in the import of compounds that have bound to associated periplasmic proteins or lipoproteins. Genes encoding ABC transporters are the largest group of paralogous genes in bacterial genomes, particularly in bacteria associated with plants and soils (23, 43). For example, genomic sequences predict 119 transporters belonging to the ABC superfamily in the plant pathogen Pseudomonas syringae pv. tomato strain DC3000 and 200 in the plant symbiont Sinorhizobium meliloti strain 1021 but only 69 in Escherichia coli strain K-12 (43). The predominance of importers is illustrated by the prediction of 146 uptake systems but only 18 export systems in S. meliloti (43); this predominance may be due to their role in high-affinity acquisition of diverse nutrients (37).
Osmoregulatory ABC transporter systems contribute to bacterial adaptation to hyperosmolarity as well as to heat and chilling stresses (2, 4). The uptake of osmoprotectant compounds, i.e., compounds that can serve as or be converted to compatible solutes, can result in sufficient solute accumulation to provide osmotic homeostasis to cells. Since the ProU transporters of E. coli and Salmonella enterica were first identified as osmoregulatory ABC transporters more than 2 decades ago (11, 21), osmoregulatory ABC transporters have been identified primarily in gram-positive bacteria. These include OpuA, OpuB, and OpuC from Bacillus subtilis (30), Gbu and OpuC from Listeria monocytogenes (3), and OpuA (BusA) from Lactococcus lactis (52). The Erwinia chrysanthemi OusB transporter is among the few identified in a gram-negative species and is similar to ProU in sequence, substrate range, expression, and activation profile (14). S. meliloti ABC transporters that function in the uptake of osmoprotectant compounds have also been identified (1, 8, 19, 28), but the primary or sole function of each appears to be for catabolism rather than osmoprotection. Greater functional knowledge of osmoregulatory ABC transporters in gram-negative bacteria would improve our ability to identify such transporters among multiple candidates in a given organism, as has been attempted in studies examining structure-function relations within these transporters (45).
Prokaryotic ABC transporters typically consist of a periplasmic (or lipoprotein) substrate-binding protein, two integral membrane proteins (permeases), and two peripheral membrane proteins that bind and hydrolyze ATP. Recently, tandem cystathionine-β-synthase (CBS) domains in the ATPase component of the OpuA transporter of L. lactis, designated OpuAC, were shown to function in osmosensing by enabling OpuA activation by threshold levels of ionic osmolytes (7, 35). Based on this function, we propose that tandem CBS domains may be a predictive feature of functional osmoregulatory ABC transporters.
In this study, we examined the osmoregulatory ABC transporters in a member of the genus Pseudomonas. Pseudomonas species have been extensively studied due to their medical, agricultural, and environmental importance. For example, Pseudomonas aeruginosa and P. syringae serve as major models for elucidating the molecular mechanisms of animal and plant pathogenesis, whereas Pseudomonas putida serves as a model for bioremediation studies, Pseudomonas fluorescens as a model for biological control of phytopathogens, and Pseudomonas stutzeri as a model for understanding denitrification, natural transformation, and pollutant degradation processes. These various ecological functions are each influenced by the abilities of the bacteria to survive and maintain metabolic activity in their natural habitats despite often fluctuating and stressful environmental conditions, including high salinity and low water content. Here, we provide the first functional characterization of an osmoregulatory ABC transporter in a pseudomonad by using P. syringae, a widespread epiphyte that must cope with fluctuating osmotic conditions on and in aerial plant leaves (5, 25, 56), and show that tandem CBS domains are an effective predictor of function among putative osmoregulatory ABC transporters, at least in Pseudomonas species.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. Escherichia coli strains were grown at 37°C in LB medium (39) or M63 medium (46) supplemented with vitamin B1 (0.0005%). E. coli strains harboring the temperature-sensitive plasmid pKD46 were grown at 30°C. P. syringae strains were grown at 28°C in King's B medium (32), minimal medium MinA (39), or the low-osmoticum medium 1/2-21C (5, 22), which contained, per liter, 0.5 g of NH4Cl, 1.745 g of Na2HPO4 · 7H2O, 1.395 g of KH2PO4, 3 g of glucose, and 20 ml of Huntner's mineral solution (48) and was modified by the addition of 0.6 g of succinate to promote bacterial growth. In the absence of supplemental NaCl, 1/2-21C medium had an osmolality of 95 mosmol/kg H2O; the addition of NaCl to 0.22 M increased the osmolality to 490 mosmol/kg. Osmolality was determined using an osmometer (Osmomette A; Precision Systems Inc., Sudbury, MA). Antibiotics were used at the following concentrations (μg ml−1): ampicillin, 100; kanamycin, 50 for P. syringae or 20 for E. coli; rifampin, 100; and spectinomycin, 60.
TABLE 1.
Plasmids and strains used in this study
| Strain or plasmid | Description/relevant genotype | Reference or source |
|---|---|---|
| Escherichia coli | ||
| DH5α | Host for cloning | Invitrogen |
| MG1655 | Host for mutagenesis | 15 |
| MKH13 | ΔputPA101 ΔproP2 ΔproU::spc-608 ΔbetTIBA; Spr | 31 |
| Pseudomonas syringae pv. tomato | ||
| DC3000 | Wild type; Rfr | 41 |
| 0462::pKO | DC3000 with PSPTO_0462::pKnockout-Ω; Rfr Spr | This work |
| 3060::pKO | DC3000 with PSPTO_3060::pKnockout-Ω; Rfr Spr | This work |
| 4575::pKO | DC3000 with PSPTO_4575::pKnockout-Ω; Rfr Spr | This work |
| 5273::pKO | DC3000 with PSPTO_5273::pKnockout-Ω; Rfr Spr | This work |
| DC3000ΔopuCA | DC3000 with ΔPSPTO_4575; Rfr | This work |
| Plasmids | ||
| pKnockout-Ω | Suicide vector for rapid gene inactivation in P. syringae; Smr Spr | 55 |
| pKD13 | Template for kan cassette flanked by FLP recombination target sites; Apr Kmr | 15 |
| pKD46 | Encodes λ Red recombinase; repA101ts; Apr | 15 |
| pFlp2 | Encodes Flp recombinase, suicide vector in P. syringae; Apr | 26 |
| pRK2013 | RP4 transfer functions for mobilization; Kmr | 17 |
| pRK2073 | pRK2013 with Tn7 in the Kmr gene; Smr Spr | 6 |
| pKO4575-8 | pKnockout-Ω containing 9,018-bp PCR-amplified fragment with PSPTO_4575-PSPTO_4578 | This work |
| pME6041 | E. coli-Pseudomonas shuttle and cloning vector; oriVpVSIoriVp15AoriT; Kmr | 24 |
| pMEopuC | pME6041 containing a 9.6-kb genomic fragment with PSPTO_4575-PSPTO_4578 | This work |
Construction of knockout and deletion mutants of P. syringae pv. tomato strain DC3000 in putative osmoprotectant transporters.
The suicide vector pKnockout-Ω (55) was used to generate insertional mutations in the following DC3000 genes: PSPTO_0462, PSPTO_3060, PSPTO_4575, and PSPTO_5273. An internal fragment of each gene was amplified using the primers listed in Table S1 in the supplemental material and was subsequently cloned into XcmI-digested pKnockout-Ω. Insert orientation was determined by PCR using the primer Carol1 and the forward or reverse primer for each target gene (see Table S1 in the supplemental material). Single recombinants were selected after introducing each pKnockout-Ω construct into DC3000 via a triparental mating with pRK2013 (17), and insertions were confirmed by PCR. The resulting mutants were designated 0462::pKO, 3060::pKO, 4575::pKO, and 5273::pKO.
To generate a PSPTO_4575 deletion mutant, the PSPTO_4575-PSPTO_4578 locus, including 1,561 bp upstream of PSPTO_4578 and 4,034 bp downstream of PSPTO_4575, was amplified using AccuPrime Hi Fi polymerase (Invitrogen, Carlsbad, CA) and the primer set 4575L1/4575L2 (see Table S1 in the supplemental material). The 9-kb product was cloned into XcmI-digested pKnockout-Ω, forming pKO4575-8, which was then introduced into E. coli strain MG1655(pKD46) (Table 1) by electroporation. PCR amplification of pKD13 by use of the primer set 4575H1/4575H2 (see Table S1 in the supplemental material) resulted in a PCR product that contained a kan cassette surrounded by FLP recombination target (FRT) sites and by 36-bp regions that share sequence similarity with the termini of PSPTO_4575. This chimeric fragment was introduced into MG1655(pKD46, pKO4575-8) by electroporation, resulting in the replacement of PSPTO_4575 through lambda Red recombinase-mediated recombination (15). This plasmid containing the marked deletion was mobilized into DC3000 via triparental matings with pRK2073 (6). Deletion mutants were identified as Rfr Kmr Sps colonies and were confirmed by PCR. An unmarked deletion mutant was constructed by introducing pFlp2 (26).
To identify a genomic clone containing PSPTO_4575-PSPTO_4578, EcoRV-digested genomic DNA of DC3000 was ligated into pME6041 (24). This genomic library was introduced into the glycine betaine/choline transporter-deficient E. coli strain MKH13 (31) by electroporation, and a clone containing PSPTO_4575-PSPTO_4578 was identified based on restoration of growth on M63 medium amended with 0.6 M of NaCl and 1 mM of glycine betaine. The resulting strain was designated MKH13(pMEopuC).
Osmoprotection assay.
Bacterial growth in 1/2-21C or MinA medium amended with 0.3 M of NaCl and 1 mM of choline, glycine betaine, l-glutamate, acetylcholine, carnitine, dl-pipecolate, trehalose, taurine, sucrose, or l-proline was monitored spectrophotometrically to evaluate the osmoprotection conferred by each compound. Late-log-phase cells were inoculated to a density of 107 cells/ml, and growth was monitored either in test tubes based on optical density at 600 nm (OD600) or in microtiter plates based on measurements at both 630 nm and 450 nm to compensate for the optical interference of water condensation within the wells. The dual-wavelength measurements were converted into OD600 values by determining the OD600 and OD630/OD450 values for the same cultures and performing regression analysis to obtain the following relationship: y = 0.0344 + 0.477x + 6.621x2, where x was the OD630/OD450 measurement and y was the OD600 measurement. All cultures were incubated at 28°C with shaking.
Transport assays.
[methyl-14C]choline and [methyl-14C]proline (specific activity of 55 mCi/mmol) were obtained from American Radiolabeled Chemicals, Inc. (St. Louis, MO). [methyl-14C]glycine betaine was prepared by the oxidation of [methyl-14C]choline as described by Ko et al. (33). Proline betaine was prepared from proline as described by Musich and Rapoport (42) but was not purified beyond the filtration step. Cells were prepared by growing them in 1/2-21C or MinA medium to mid-log phase (OD600 of 0.3 to 0.5) and suspending washed cells in the same medium to an OD600 of 0.1 to 0.2 for glycine betaine uptake or to an OD600 of 1 for all other studies. Following introduction of NaCl, cells were incubated at 28°C for 2 to 3 h with shaking to allow the induction and expression of transporters.
The initial uptake rates were measured after adding a radiolabeled substrate to 0.5 ml of cells, shaking it for 2 min for glycine betaine uptake or 5 min for choline and proline uptake, and terminating uptake by centrifugation at 13,000 × g. The supernatant was immediately removed from each pellet, the cells were washed with 1 ml of medium that had the same osmolarity as the incubation medium, and the cells were suspended in 1 ml of ScintiVerse BD (Fisher Scientific, Fair Lawn, NJ). The radiolabel in the cells was determined using a liquid scintillation counter (Tri-Carb liquid scintillation analyzer, model 2100TR; Packard Instrument Co., Meriden, CT). Each sample was counted four times, and the average value was used as the reading.
For kinetic studies, the radiolabeled substrates were used at final concentrations of 1 to 100 μM for glycine betaine and choline (specific activity ranged from 6 to 600 μCi/mmol) and 1 to 2,000 μM for choline (specific activity ranged from to 0.6 to 1,199 μCi/mmol). For competition experiments, unlabeled compounds were used at final concentrations of 100 μM and 1 mM, whereas [methyl-14C]glycine betaine and [methyl-14C]choline were used at a final concentration of 10 μM (with specific activity of 0.55 mCi/mmol). The protein content of cell suspensions was determined using the Bio-Rad Bradford assay (Bio-Rad, Hercules, CA) following incubation of a subsample of cells in 1 M of NaOH at 95°C for 5 min. The data from the kinetic experiments were fit with the Michaelis-Menten equation, and the apparent affinity constant (Km) and maximal rate of uptake (Vmax) were determined.
Construction and analysis of PSPTO_4575 deletion derivatives.
C-terminal deletions of the PSPTO_4575 protein were constructed by amplifying PSPTO_4575 by PCR using one upstream primer, 5′-CTGGCCATCATCGCCGACCTG-3′, with each of eight downstream primers with sequence similarity to distinct endpoints within the PSPTO_4575 gene. The resulting fragments were cloned into the EcoRV site of pME6041 and were introduced into the PSPTO_4575 deletion mutant by electroporation. The abilities of the cloned genes to complement the PSPTO_4575 deletion were evaluated based on growth on MinA medium containing 0.5 M NaCl and 2 mM glycine betaine and choline.
Identification of osmoregulatory ABC transporter genes from other pseudomonads.
Genomic libraries of P. syringae pv. syringae B728a (34) and P. aeruginosa PAO1 (50) were constructed by ligating EcoRV- or PvuII-restricted genomic DNA into the EcoRV site of pME6041. The PSPTO_0462 and PSPTO_5269 genes were deleted in the PSPTO_4575 deletion mutant, described above, by using the lambda Red recombinase-mediated recombination system and the primers shown in Table S1 in the supplemental material, resulting in the construction of a DC3000 triple deletion mutant. The genomic libraries were transferred into this triple mutant by electroporation, and the transformants were plated on MinA media containing 0.5 M NaCl and either glycine betaine or choline (2 mM). The cloned fragments that permitted growth of the DC3000 triple mutant in the presence of either betaine or choline were sequenced.
CBS domain identification and homology search.
Amino acid sequences of the ATP-binding component of characterized and putative osmoregulatory ABC transporters were obtained from the GenBank database (http://www.ncbi.nlm.nih.gov). Alignments of these amino acid sequences were performed using ClustalW (http://www.ebi.ac.uk/clustalw/). The CBS domains were identified and evaluated using the Pfam HMM database (http://pfam.janelia.org/hmmsearch.shtml) and Motif Search (http://motif.genome.jp/).
RESULTS
Choline provides better osmoprotection than glycine betaine to DC3000.
The growth of P. syringae pv. tomato strain DC3000 in the low-osmolarity medium 1/2-21C was significantly delayed by NaCl concentrations as low as 0.1 M (Fig. 1), with an even more dramatic delay caused by 0.2 M. In the presence of NaCl concentrations of 0.4 M or higher, the final growth yield of DC3000 was reduced by at least 50% compared to that of the 0 M control. No growth was observed in the presence of NaCl concentrations of 0.7 M or higher, even after 7 days of incubation (data not shown).
FIG. 1.
Osmotolerance of P. syringae pv. tomato DC3000. Cells were grown in 1/2-21C medium amended with NaCl at the indicated concentrations. The initial concentrations were approximately 107 CFU/ml. Values shown are means ± standard errors of the mean (SEM) (n = 4).
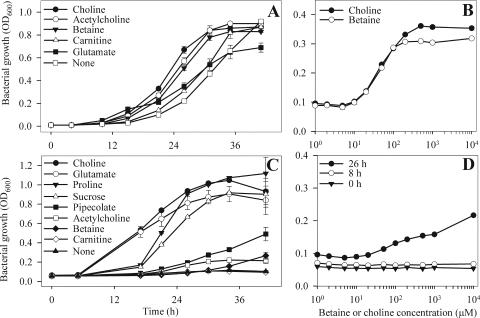
Glycine betaine and choline each provided strong osmoprotection (Fig. 2A). Surprisingly, choline provided consistently better protection than glycine betaine when provided at 1 mM (Fig. 2A). This contrasts with previous reports of other bacterial species for which glycine betaine provides better osmoprotection than choline (9, 33, 40). This superior osmoprotection by choline occurred at choline concentrations of ≥50 μM when examined after 8 h of growth (data not shown) and at concentrations of ≥100 μM when examined after 12 h (Fig. 2B). Acetylcholine provided good osmoprotection. Glutamate provided intermediate protection and growth to a lower final density than choline and glycine betaine, whereas carnitine supported delayed growth (Fig. 2A). No osmoprotection was observed in the presence of proline, ectoine, dl-pipecolate, mannitol, maltose, succinate, sucrose, trehalose, or taurine (data not shown). No significant bacterial growth occurred at NaCl concentrations of 0.7 M or higher, regardless of the presence of osmoprotectants or incubation time (data not shown).
FIG. 2.
Abilities of various exogenous compounds to serve as osmoprotectants or carbon sources for P. syringae pv. tomato DC3000. The OD600 values of DC3000 cultures are shown for growth in MinA medium amended with 0.3 M of NaCl and 1 mM of each of the indicated compounds (A), after 8 h of growth in MinA medium amended with NaCl at 0.4 M and glycine betaine and choline at the indicated concentrations (B), and for growth in glucose-free MinA medium amended with various compounds provided as a sole C source (10 mM) (C). The (D) OD600 values are shown for DC3000 ΔopuCA cultures after 0, 8, and 26 h in MinA medium amended with 0.4 M NaCl and glycine betaine at the indicated concentrations. Values are means ± SEM (n = 3).
The abilities of the potential osmoprotectants to serve as sole C sources were determined to better understand their use in osmoprotection. When provided at a final concentration of 10 mM, choline, glutamate, proline, and sucrose supported strong growth of DC3000, whereas dl-pipecolate, acetylcholine, and glycine betaine supported some growth (Fig. 2C). Glycine betaine supported significantly more growth when it was provided at a concentration of 20 mM (data not shown). Carnitine was unique among the osmoprotection-active compounds in its inability to serve as a sole C source for DC3000. These results indicate that DC3000 can transport and likely accumulate several osmoprotection-active compounds, despite its ability to catabolize them in the absence of osmotic stress, suggesting that DC3000 can regulate their catabolism under hyperosmotic conditions. No growth was observed on trehalose, taurine, or ectoine (data not shown), providing evidence against their transport or catabolism.
Superior osmoprotection by choline is correlated with a higher capacity for choline uptake by DC3000.
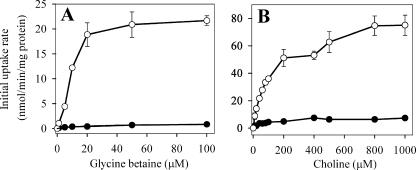
Because choline must be converted to glycine betaine to confer cellular osmoprotection (51), the superior growth of DC3000 with choline (≥100 μM) at high osmolarity suggested that more choline than betaine was transported at these concentrations. Uptake studies confirmed this prediction (Fig. 3). Specifically, the initial uptake rate for glycine betaine by salt-stressed DC3000 cells reached a plateau of approximately 22 nmol/min/mg protein at glycine betaine concentrations as low as 20 μM (Fig. 3A), whereas the initial uptake rate of choline was approximately twice this at a choline concentration of 100 μM and reached a plateau of approximately 80 nmol/min/mg protein with increasing choline concentrations (Fig. 3A). As expected, the initial uptake rates of both choline and glycine betaine were not significantly increased over the range of substrate concentrations tested in the absence of hyperosmotic stress. The uptake profiles for glycine betaine suggested the presence of at least one high-affinity, relatively low-capacity betaine transporter, whereas the uptake kinetics for choline suggested the presence of one or more low-affinity transporters with an overall high capacity for uptake.
FIG. 3.
Profiles for uptake of glycine betaine (A) and choline (B) by P. syringae pv. tomato DC3000 in 1/2-21C medium that was unamended (•) or amended with 0.2 M of NaCl (○). Log-phase cells were suspended in 1/2-21C medium containing [14C]glycine betaine or [14C]choline at various concentrations. Values are the means ± SEM (n = 4).
Only one of four putative transporters examined in DC3000 was functional as an osmoregulatory ABC transporter.
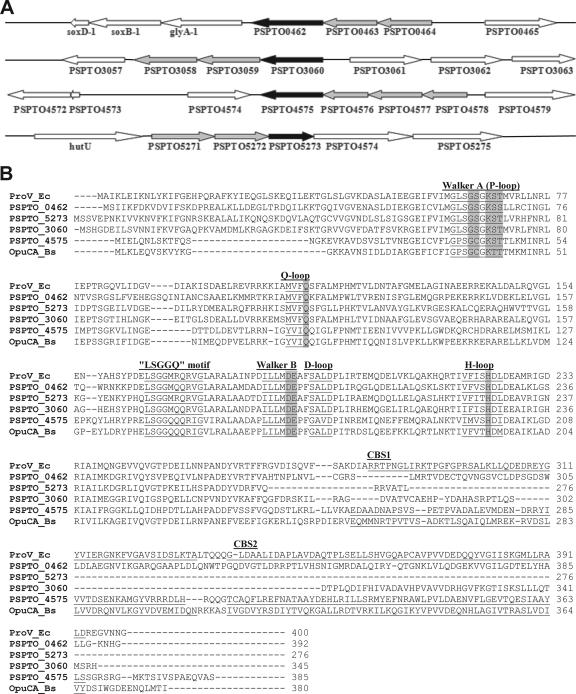
Using the Transporter Protein Analysis Database in 2004 (43), we identified four putative complete ABC transporter systems in DC3000 with glycine betaine or choline as a predicted substrate: PSPTO_0462-PSPTO_0464, PSPTO_3058-PSPTO_3060, and PSPTO_5271-PSPTO_5273 with glycine betaine and proline as predicted substrates and PSPTO_4575-PSPTO_4578 with glycine betaine, choline, and carnitine as predicted substrates (Fig. 4A). We also identified one putative secondary transporter for choline, PSPTO_5269 (not shown). Sequence alignment of the predicted substrate-binding components, ATPases, and permease components with known osmoregulatory transporters indicated that the highest level of conservation was among the ATPase components and the lowest level was among the substrate-binding components, consistent with previous studies (16). The predicted amino acid sequence of the ATPase PSPTO_4575 contained tandem CBS domains on the C terminus (Expect value [E] = 4 × 10−16), which are domains that have been associated with osmoregulatory function in L. lactis OpuA (7). The other three predicted ATPases lacked these domains (E ≥ 0.49).
FIG. 4.
The organization (A) and ATPase alignment (B) of four putative ABC transporter systems in P. syringae pv. tomato DC3000. Open reading frames that are predicted as the ABC transporter system are depicted in gray; the open reading frames that are predicted as the ATPase subunits are depicted in black. Alignment of 47 sequences of putative ABC transporters were performed using ClustalW (http://www.ebi.ac.uk/clustalw/); only the following sequences are shown: PSPTO_0462 (AAO54006), PSPTO_3060 (AAO56549), PSPTO_4575 (AAO58021), and PSPTO_5273 (AAO58699) as well as ProV from E. coli (ProV_Ec; AAA24427) and OpuCA from B. subtilis (OpuCA_Bs; O34992) for comparison. Conserved nucleotide-binding protein motifs, including the Walker A (or P-loop), Q-loop, Walker B, D-loop, and H-loop motifs, and the signature sequence for the nucleotide-binding protein of ABC transporters, the “LSGGQ” motif, are underlined and labeled. ATP-binding sites are shaded.
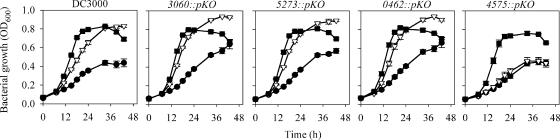
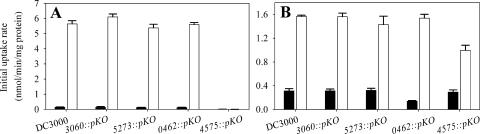
Mutants containing a pKnockout insertion in the ATPase gene of each of the four putative ABC transporters were constructed. Growth in 1/2-21C medium showed that the loss of these transporters did not affect DC3000 fitness in the absence of hyperosmolarity (data not shown) or under hyperosmotic conditions (0.3 M NaCl) in the absence of osmoprotectants (Fig. 5). Mutants inactivated in PSPTO_3060, PSPTO_5273, and PSPTO_0462 were not detectably altered in their growth when glycine betaine or choline was added. In contrast, the PSPTO_4575 mutant was dramatically reduced in growth in a glycine betaine-amended medium (Fig. 5), suggesting that a transporter system involving the PSPTO_4575 protein was the primary or sole betaine transporter in DC3000 under these conditions.
FIG. 5.
Growth of DC3000 and various mutants in 1/2-21C medium that lacked succinate and contained 0.3 M of NaCl and was unamended (•), amended with 1 mM of choline (▪), or amended with 1 mM of glycine betaine (▿). Mutants are designated by their PSPTO numbers. Values are the means ± SEM for three to six replicates. pKO, pKnockout.
The rates for uptake of [14C]glycine betaine and [14C]choline into DC3000 and the pKnockout mutants were compared in the presence and absence of 0.2 M of NaCl (Fig. 6). The mutants 3060::pKO, 5273::pKO, and 0462::pKO were similar to DC3000 in their uptake rates for both substrates under the hyperosmotic conditions. They were also similar under the nonhyperosmotic conditions, with the exception of mutant 0462::pKO, which was reduced in its choline uptake rate in the basal medium, suggesting a role in uptake for catabolism. 4575::pKO exhibited uptake rates that were reduced by at least 99% for glycine betaine and 35% for choline under the hyperosmotic conditions.
FIG. 6.
Uptake of radiolabeled glycine betaine (A) or choline (B) by P. syringae pv. tomato DC3000 and various mutants in 1/2-21C medium in the absence (solid bars) or presence (open bars) of 0.2 M of NaCl. Radiolabeled substrates were provided at a concentration of 5 μM. Values are the means ± SEM (n = 3).
In addition to an ATPase (PSPTO_4575), the PSPTO_4575-PSPTO_4578 genes were predicted to encode two permeases (PSPTO_4576 and PSPTO_4578) and one periplasmic binding protein (PSPTO_4577), with PSPTO_4578 as the first gene in the putative operon (Fig. 4A). Among the homologs of the ATPase that have been functionally characterized, the PSPTO_4575 protein shared the greatest sequence identity with the ATPase components of the gram-positive bacterial transporters OpuC of Listeria monocytogenes and OpuC and OpuB of Bacillus subtilis (OpuCA and OpuBA; 45 to 46% identity) (20, 29) and much less sequence similarity with the ATPase component of the gram-negative transporters ProU of E. coli (ProV; 36% identity) (38) and OusB of Erwinia chrysanthemi (OusBV; 35% identity) (14). To date, functional OpuC-type transporters have been characterized from gram-positive bacteria only. Loci encoding the OpuC transporters in L. monocytogenes and B. subtilis, as well as the OpuB transporter of B. subtilis, contain the same gene arrangement as PSPTO_4575-PSPTO_4578, including two permease genes, but occur in reverse order relative to the DC3000 locus (4, 30). Hereafter, we refer to the transporter encoded by the PSPTO_4575-PSPTO_4578 locus as the OpuC transporter and the ATPase component encoded by PSPTO_4575 as OpuCA, by convention with previous nomenclature (20, 29).
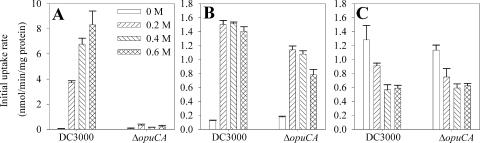
To minimize possible pleiotropic effects associated with an insertion mutation, a deletion mutant that lacked the complete opuCA gene was constructed. The effect of hyperosmolarity on the rate of uptake by DC3000 and the ΔopuCA mutant was evaluated for [14C]glycine betaine and [14C]choline as well as [14C]proline, which did not function as an osmoprotectant for this strain. The rate of glycine betaine uptake increased with increasing NaCl concentration for DC3000 (Fig. 7A) but was dramatically lower in the presence of 0.8 M or 1 M NaCl (data not shown). This was consistent with its inability to grow at NaCl concentrations greater than 0.7 M in the presence or absence of an osmoprotectant. The ΔopuCA mutant was reduced at least 95% in [14C]glycine betaine uptake at all NaCl concentrations (Fig. 7A) and exhibited a smaller but detectable reduction in [14C]choline uptake (Fig. 7B). The rate of [14C]proline uptake by DC3000 decreased under hyperosmotic conditions (Fig. 7C), consistent with the lack of osmoprotection by proline, and was not affected by the ΔopuCA mutation.
FIG. 7.
Effect of NaCl concentration on the uptake of radiolabeled glycine betaine (A), choline (B), or proline (C) by P. syringae pv. tomato DC3000 and the ΔopuCA mutant. Uptake was examined in 1/2-21C medium in the presence of 0, 0.2, 0.4, and 0.6 M of NaCl. Radiolabeled substrates were provided at a concentration of 5 μM. Values are the means ± SEM (n = 3).
Under hyperosmotic conditions, the ΔopuCA mutant exhibited low but detectable growth when glycine betaine was provided at concentrations of ≥50 μM, but not at concentrations of <50 μM (Fig. 2D), suggesting that this mutant has residual betaine uptake activity and therefore that OpuC is the primary, but not the sole, transporter for glycine betaine in DC3000. Similarly, the ΔopuCA mutant exhibited residual choline uptake activity (Fig. 7B) and better growth in the presence than in the absence of choline (Fig. 5), indicating that OpuC is one of multiple transporters for choline in DC3000.
OpuC is a high-affinity betaine transporter and a low-affinity choline transporter with relatively broad substrate specificity.
To evaluate the physical properties of OpuC, a fragment containing the PSPTO_4575-PSPTO_4578 locus was identified in a DC3000 genomic library that was generated using the broad-host-range vector pME6041. The resulting plasmid, pMEopuC, was introduced into E. coli strain MKH13, which lacked the PutP, ProP, and ProU transport systems and thus was unable to transport glycine betaine; this strain also lacked BetT and thus was unable to transport choline (31). Plasmid pMEopuC restored glycine betaine uptake activity to MKH13 based on an osmoprotection assay in which the pMEopuC- but not the pME6041-containing strain grew in M63 minimal medium amended with 0.5 M of NaCl and 1 mM of glycine betaine (data not shown). Choline did not provide osmoprotection to MKH13(pMEopuC), consistent with the fact that MKH13 lacks the betAB genes necessary to convert choline into its osmoprotective form, glycine betaine, and thus cannot derive osmoprotection from choline, regardless of its ability to transport it.
The kinetic properties of OpuC were identified using the heterologously expressed opuC locus, as has been done for similar transporters (10, 14). The apparent Km for glycine betaine uptake by OpuC was 3.7 ± 0.3 μM, with a Vmax of 34.3 ± 0.6 nmol/min/mg of protein (Fig. 8A), indicating that OpuC mediates high-affinity transport of glycine betaine. OpuC uptake of choline did not saturate in the substrate range from 1 to 50 μM (Fig. 8B, inset) but did over the range from 0 to 2,000 μM (Fig. 8B). The apparent Km for choline uptake by OpuC was 113 ± 13 μM, with a Vmax of 20 ± 0.7 nmol/min/mg of protein, indicating that OpuC mediates low-affinity transport of choline.
FIG. 8.
Kinetics of OpuC-mediated uptake of glycine betaine (A) and choline (B) in E. coli MKH13(pMEopuC). Log-phase cells grown in M63 medium amended with 0.5 M of NaCl were suspended in 50 mM of phosphate buffer (pH 7) containing 0.5 M of NaCl, 0.2% glucose, and [14C]glycine betaine or [14C]choline at various concentrations. The results are means ± SEM (n = 3).
In competition assays with MKH13(pMEopuC) for evaluating the substrate specificity of OpuC, [14C]glycine betaine uptake was inhibited 86 and 65% by the addition of unlabeled glycine betaine and proline betaine, respectively, when these competitors were added at a 10-fold-higher concentration than the radiolabeled glycine betaine and 97 and 94%, respectively, when they were added at a 100-fold excess (Table 2). Carnitine, choline, and acetylcholine had an intermediate effect on [14C]glycine betaine uptake, whereas phosphorylcholine, proline, ectoine, dl-pipecolate, trehalose, and glycine did not significantly affect [14C]glycine betaine uptake, even when provided at a 100-fold excess (Table 2). Glycine betaine, proline betaine, carnitine, choline, acetylcholine, and to a lesser extent phosphorylcholine also inhibited [14C]choline uptake when added at a 100-fold excess (Table 2). [14C]choline uptake appeared to be modestly inhibited by proline, ectoine, and dl-pipecolate, but this inhibition is consistent with our observation that [14C]choline transport was much more easily inhibited by other compounds than was glycine betaine transport. One possible explanation for this is that choline binding to the OpuC periplasmic binding component is weaker than glycine betaine binding, making choline uptake more easily inhibited by competitors.
TABLE 2.
Abilities of various compounds to inhibit the uptake of choline and glycine betaine by OpuC expressed in E. coli MKH13(pMEopuC)
| Competitor | % inhibition of uptake of indicated compound with unlabeled competitor provided at indicated final concna
|
||
|---|---|---|---|
| Glycine betaine
|
Choline (1 mM) | ||
| 100 μM | 1 mM | ||
| Glycine betaine | 86 | 97 | 99 |
| Proline betaine | 65 | 94 | 99 |
| Carnitine | 38 | 82 | 98 |
| Choline | 17 | 68 | 95 |
| Acetylcholine | 7 | 36 | 87 |
| Phosphorylcholine | 9 | 12 | 66 |
| Proline | −1 | −3 | 41 |
| Ectoine | 4 | −6 | 25 |
| dl-Pipecolate | −4 | 2 | 26 |
| Trehalose | 0 | −18 | −5 |
| Glycine | −4 | 13 | −20 |
Cells were grown in M63 medium amended with 0.5 M of NaCl, and uptake was realized with 10 μM of [14C]glycine betaine or 10 μM of [14C]choline. The results are expressed as percents inhibition of uptake and are means for three independent experiments with variations of less than 15%. The uptake rates in the absence of competitors were 1.5 nmol/min/mg protein for choline and 19 nmol/min/mg protein for glycine betaine.
Taken together, these results confirm that OpuC mediates the uptake of multiple substrates, including glycine betaine, choline, proline betaine, carnitine, and acetylcholine. Although phosphorylcholine served as an effective osmoprotectant for DC3000, we propose that phosphorylcholine itself was not a substrate for uptake but rather was converted to choline by a periplasmic phosphorylcholine phosphatase and thus was taken up as choline. Such a phosphatase has been identified as PchP in P. aeruginosa (36) and has been suggested by the presence of a homolog in DC3000 (the PSPTO_0436 protein). The absence of this enzyme in E. coli strains, including strain MKH13, may explain the lack or scarcity of competition by phosphorylcholine for betaine and choline uptake.
The presence of CBS domains correlated with osmoregulatory function by DC3000 OpuC and by transporters in other pseudomonads.
Small protein modules known as CBS domains are common in many proteins, including a variety of transporters such as the ATPase component of ABC transporters, and generally are present as tandem pairs. Similar to the ATPase of the L. lactis OpuA transporter, which is the only osmoregulatory ABC transporter for which the CBS domains have been functionally examined (7), the ATPase of the DC3000 OpuC transporter has a C-terminal tail adjacent to the tandem CBS domains, which are joined by a linker region (Fig. 9). To evaluate the requirement for these domains and the C-terminal tail in OpuC transporter function, we generated various C-terminal deletion constructs (Fig. 9). Multiple constructs were generated to address ambiguity in the assignment of individual amino acids to the CBS domains (27). Whereas the full-length opuCA construct restored growth of the ΔopuCA mutant under hyperosmotic stress conditions in the presence of glycine betaine or choline, only the deletion constructs that contained complete CBS1 and CBS2 domains, but not necessarily a C-terminal tail, promoted growth under similar conditions (Fig. 9). These data demonstrate that the C-terminal tail was not required for OpuC function and that the CBS2 domain, and possibly both CBS domains, may be critical to its function.
FIG. 9.
Complementation of a ΔopuCA deletion mutant of DC3000 by pME6041 containing opuCA or various deletion constructs that were predicted to encode truncated OpuCA derivatives. The ΔopuCA deletion mutant also contained deletions in PSPTO_5269, which encodes a putative secondary transporter for choline, and PSPTO_0462, which encodes a putative transporter for choline catabolism. Osmoprotection function was based on the ability to grow on MinA medium amended with NaCl (0.5 M) and glycine betaine and choline (2 mM each). The E values for the tandem CBS domains were obtained using the Pfam HMM database (http://pfam.janelia.org/hmmsearch.shtml).
The correlation between the presence of CBS domains and osmoregulatory function in the four DC3000 transporters examined prompted us to examine this correlation in transporters from other bacterial species. All of the transporters known to have osmoregulatory activity possess tandem CBS domains in their ATPase component, with E values of ≤4 × 10−5 for these domains, whereas those without these domains did not exhibit osmoregulatory activity (Table 3). The predicted E. coli transporter YehX is interesting in that it is closely related to the PSPTO_4575 protein (Fig. 10) and is induced by osmotic stress (12), but it does not appear to function in osmoprotection, based on its presence in the glycine betaine/choline transport-deficient mutant MKH13. The lack of tandem CBS domains at the YehX C terminus may explain its lack of osmoregulatory activity. Similarly, although the L. monocytogenes Lmo1421-associated transporter was predicted to transport choline based on its homology to B. subtilis OpuB (47), this transporter was recently found to lack osmoregulatory activity (2, 53). Other ATPases of characterized ABC transporters known to transport choline (ChoV) (19), ectoine (EhuA) (28), proline betaine (PrbV) (1), and histidine and glycine betaine (HutV/HisV) (8) were not activated by hyperosmolarity and lacked CBS domains; instead, these were induced by their substrates, suggesting a role in catabolism.
TABLE 3.
Comparison of characterized and putative osmoregulatory ATPases for their osmoregulatory function and the presence and nature of CBS domains and a C-terminal tail
| Organism | ATPase | Fnb | Result for indicated regiona
|
|||
|---|---|---|---|---|---|---|
| CBSc | CBS1d | CBS2d | C-terminal taild | |||
| E. coli K-12 | ProV | + | 4 × 10−5 | + (6:10, 56) | − (7:2, 52) | 0 (1:1, 7) |
| S. enterica serovar Typhimurium LT2 | ProV | + | 5 × 10−9 | + (7:10, 59) | − (7:2, 52) | 0 (1:1, 7) |
| E. chrysanthemi | OusBV | + | 3 × 10−11 | + (7:13, 59) | − (5:1, 52) | − (2:1, 7) |
| L. lactis | OpuAA | + | 1 × 10−13 | + (6:9, 56) | − (7:4, 52) | − (11:2, 17) |
| L. monocytogenes | GbuA | + | 5 × 10−18 | + (7:11, 56) | − (6:4, 52) | − (1:0, 8) |
| B. subtilis | OpuAA | + | 5 × 10−20 | + (6:11, 57) | − (8:4, 54) | − (5:1, 24) |
| B. subtilis | OpuCA | + | 5 × 10−25 | + (7:10, 55) | + (5:8, 53) | − (4:0, 14) |
| B. subtilis | OpuBA | + | 2 × 10−25 | + (7:10, 54) | + (8:9, 53) | − (3:1, 14) |
| L. monocytogenes | OpuCA | + | 3 × 10−26 | − (8:7, 55) | + (7:10, 53) | − (10:1, 31) |
| P. syringae DC3000 | PSPTO_4575 | + | 4 × 10−16 | − (12:7, 53) | − (8:4, 53) | + (1:3, 20) |
| P. syringae B728a | Psyr4249 | + | 4 × 10−16 | − (12:7, 53) | − (8:4, 53) | + (2:3, 23) |
| P. aeruginosa PAO1 | PA3891 | + | 1 × 10−19 | − (10:9, 53) | − (7:3, 53) | + (1:3, 20) |
| E. coli MC4100 | YehX | − | 0.42 | None | None | None |
| L. monocytogenes | Lmo1421 | − | 0.41 | None | None | None |
| S. meliloti 1021 | ChoV | − | 0.14 | None | None | None |
| S. meliloti 1021 | EhuA | − | >1.0 | None | None | None |
| S. meliloti 1021 | PrbV | − | >1.0 | None | None | None |
| S. meliloti 5000 | HisV/HutV | − | >1.0 | None | None | None |
| P. syringae DC3000 | PSPTO_5273 | − | >1.0 | None | None | None |
| P. syringae DC3000 | PSPTO_0462 | − | >1.0 | None | None | None |
| P. syringae DC3000 | PSPTO_3060 | − | 0.49 | None | None | None |
Values in parentheses are ratios of the anionic residue number/cationic residue number and the total numbers of amino acids in the indicated domain or tail.
The ability (+) or inability (−) to mediate the uptake of osmoprotectant compounds in response to increasing osmolality is indicated. Functional information was obtained from E. coli ProV (21), S. enterica serovar Typhimurium ProV (49), OusBV (14); L. lactis OpuAA (52); GbuA (3); B. subtilis OpuAA (31); B. subtilis OpuCA (30); OpuBA (30); L. monocytogenes OpuCA (4, 54); PSPTO_4575, Psyr4249, PA3891, and PP0868 (this study); YehX (lack of uptake in MKH13, which contains an intact yehX); Lmo1421 (3, 53); ChoV (19); EhuA (28); PrbV (1); HisV/HutV (8); and PSPTO_5273, PSPTO_0462, and PSPTO_3060 (this study).
E values for the tandem CBS domains were obtained by using the Pfam HMM database (http://pfam.janelia.org/hmmsearch.shtml).
The predicted net charge of the CBS1 domain, CBS2 domain, or C-terminal tail based on the number of cationic or anionic residues, with + indicating more cationic residues, − indicating more anionic residues, and 0 indicating equal numbers.
FIG. 10.
A neighbor-joining tree for the ATPase components of the glycine betaine and choline ABC transporters that have been functionally characterized in prokaryotes and those examined in this study. The osmoregulated E. coli YehX protein was included for comparison, although it is not functional (12). The numbers in parentheses indicate the number of genes in the locus encoding each transporter. The proteins that are shown include AAO56549 (P. syringae pv. tomato PSPTO_3060), AAC75724 (E. coli ProV), AAL21694 (Salmonella enterica serovar Typhimurium ProV), AAQ06630 (E. chrysanthemi OusBV), P4692 (B. subtilis OpuAA), CAC99092 (Listeria monocytogenes GbuA), AAF37878 (L. lactis OpuAA), AAO58021 (P. syringae pv. tomato PSPTO_4575), AAA60492 (E. coli YehX), Q45460 (B. subtilis OpuBA), O34992 (B. subtilis OpuCA), CAC99506 (L. monocytogenes OpuCA), CAC46836 (S. meliloti Prb), CAC48813 (S. meliloti EhuA, which transports ectoine and glycine betaine [28]), AAO54006 (P. syringae pv. tomato PSPTO_0462), CAC46980 (S. meliloti ChoV), AAO58699 (P. syringae pv. tomato PSPTO_5273), and CAC47281 (S. meliloti HisV, which transports histidine and glycine betaine [8]). Sequences were aligned using ClustalW (13), and a guide tree was constructed by the neighbor-joining method.
Based on the presence of CBS domains and the conserved ABC transporter domains shown in Fig. 4B, we predicted that the following ATPase components contribute to osmoregulatory transport in the Pseudomonas species for which genomic sequences are available: Psyr4249 in P. syringae pv. syringae B728a, PA3891 in P. aeruginosa PAO1, PP0868 in P. putida KT2440, PSPPH4276 in P. syringae pv. phaseolicola 1448A, PFL0868 in P. fluorescens Pf-5, and PSEEN1040 in Pseudomonas entomophila L48. Each of these is only one of several ABC transporters predicted to transport choline or glycine betaine by the Transporter Protein Analysis Database in 2004 (43). We tested this prediction for P. syringae pv. syringae B728a and P. aeruginosa PAO1 by screening genomic libraries of these strains for clones that conferred enhanced growth upon a DC3000 mutant that contained a ΔopuCA deletion as well as deletions in PSPTO_5269, which encodes a possible betaine/choline/carnitine transporter, and PSPTO_0462, which encodes a possible choline transporter for catabolic uptake (Fig. 6B). The sequence of the complementing clones indicated that transporters involving Psyr4249 from P. syringae pv. syringae strain B728a and PA3891 from P. aeruginosa PAO1 were functional, as predicted.
DISCUSSION
The uptake of exogenous compounds that confer osmoprotection may be important to the plant pathogen P. syringae in its natural, often water-limited habitat, aerial plant leaves. In this study, we began to characterize the transporters required for the uptake of osmoprotectant compounds by P. syringae pv. tomato strain DC3000, and in doing so, we characterized the first osmoregulatory ABC transporter in a Pseudomonas species, the P. syringae pv. tomato OpuC transporter. Surprisingly, this transporter shows greater similarity to the osmoprotectant transporters that have been characterized in gram-positive bacteria than to those in gram-negative bacteria (Fig. 10), with the strongest similarity to the OpuC transporters of L. monocytogenes and B. subtilis and the OpuB transporter of B. subtilis (20, 30). In contrast, the P. syringae pv. tomato homolog of the well-characterized ProU transporter from E. coli and S. enterica serovar Typhimurium, encoded by PSPTO_3058-PSPTO_3060, did not function in osmoprotection. The P. syringae pv. tomato OpuC transporter was similar to the B. subtilis and L. monocytogenes OpuC transporters in having two distinct putative permeases (OpuCB and OpuCD) rather than one, like the E. coli ProU (38) and Erwinia chrysanthemi OusB (14) transporters (Fig. 10). P. syringae pv. tomato OpuC was also similar in exhibiting a broad substrate specificity and a relatively high transport capacity for glycine betaine (Vmax values of 34 and 65 nmol/min/mg of protein for P. syringae pv. tomato and B. subtilis OpuC, respectively) but had a lower affinity and transport capacity for choline (30). One or more additional transporters in DC3000 appear to contribute to choline uptake by this strain.
For the four P. syringae pv. tomato DC3000 transporters examined in this study, the presence of CBS domains was strongly correlated with osmoregulatory transport activity, suggesting that tandem CBS domains may be a predictor of osmoregulatory activity. This was supported by four lines of evidence. First, Biemans-Oldehinkel et al. (7) demonstrated that these domains are involved in osmosensing by L. lactis OpuA. Second, these domains were required for P. syringae pv. tomato OpuC-mediated uptake for osmoprotection. Third, without exception, the ATPase components of all of the prokaryotic osmoregulatory ABC transporters characterized to date possess full-length CBS domains. And fourth, we demonstrated osmoregulatory function for two transporters that were predicted to function based on the presence of CBS domains. Specifically, we identified Psyr4249 and PA3891 as components of the P. syringae pv. syringae strain B728a and P. aeruginosa strain PAO1 transporters that we predicted to function in osmoregulation. We verified the accuracy of this prediction by screening for genomic clones that could restore growth to a choline/betaine uptake-deficient DC3000 mutant under hyperosmotic conditions and showing that the complementing clones encoded Psyr4249 and PA3891. Previous studies that have presumed functionality for the transporter encoded by PSPTO_3058-PSPTO_3060 (45) illustrate the need for such predictive power.
In addition to tandem CBS domains, the ATPases of the osmoregulatory ABC transporters also have a C-terminal tail. We observed that this C-terminal tail was not required for osmoregulatory transporter function (Fig. 9). In L. lactis OpuAC, this 18-amino-acid tail is anionic and functions in modulating OpuA activity in response to ionic strength and ionic lipids (35). Deletion of this anionic tail resulted in mutants that were capable of uptake but required higher cytoplasmic ion concentrations to activate uptake (35). Mahmood et al. (35) present a model in which L. lactis OpuC activation requires disruption of the electrostatic interactions between a CBS domain and the membrane, presumably mediated by a cationic CBS domain surface and anionic lipids in the membrane, with the charged tail influencing the ionic strength required to disrupt these interactions. In contrast to the anionic C-terminal tail of L. lactis OpuA, the Pseudomonas OpuC-type transporters all have a cationic C-terminal tail and exhibit distinct charges by their CBS regions (Table 3). Given that the C-terminal tails differ in length and charge among CBS domain-containing transporters of diverse bacteria (35), the conserved, cationic nature of the Pseudomonas species tails suggests the evolution of a Pseudomonas-specific approach for modulating the osmosensing activities of these transporters.
Our results do not exclude the possibility that the PSPTO_0462-PSPTO_0464, PSPTO_5271-PSPTO_5273, and PSPTO_3058-PSPTO_3060 loci encode transporters that function in uptake for catabolism. In fact, we now have evidence that PSPTO_0462-PSPTO_0464 contributes to the catabolic uptake of glycine betaine and choline (C. Chen and G. A. Beattie, unpublished data). This is consistent with the relative similarity of the PSPTO_0462 protein to the ChoV transporter of S. meliloti (Fig. 10), which functions in choline uptake for catabolism (19). The similarity of the PSPTO_5273 protein to S. meliloti HisV/HutV, which is involved in the uptake of histidine and glycine betaine under low osmolarity (8), suggests a possible role for PSPT5273 in catabolism, although this has not yet been examined. The similarity of the PSPTO_3060 protein to ProV of E. coli and S. enterica serovar Typhimurium, however, does not suggest a role in catabolism. Surprisingly, the PSPTO_3058-PSPTO_3060 locus is absent in the other Pseudomonas species strains for which complete genome sequence information is available, including two other P. syringae strains, suggesting that this locus is novel to DC3000 and possibly has been recently acquired.
In this study, we demonstrated that P. syringae pv. tomato DC3000 derived better osmoprotection from choline than from similar concentrations of glycine betaine when the compounds were provided at concentrations of 100 μM or higher; we have also observed this with two other P. syringae strains (data not shown). This contrasts with the relative levels of protection conferred by these compounds to P. aeruginosa and P. putida (18) and to most other bacterial genera examined. Superior osmoprotection by choline suggests that choline uptake is more efficient than glycine betaine uptake in DC3000 when these compounds are present at relatively high concentrations, particularly given that choline must be converted to glycine betaine to confer osmoprotection. Such efficiency may be reflected, in part, by the presence of a single primary transporter, OpuC, that transports glycine betaine under hyperosmotic conditions but at least two transporters that transport choline. The affinity of DC3000 for choline (Km of 118 μM) and glycine betaine (Km of 12 μM) also suggests that DC3000 is adapted to environments with low betaine and high choline concentrations. Interestingly, although the concentrations of glycine betaine and choline are not known in tomato and Arabidopsis thaliana, which are host plants for DC3000, previous studies suggest that plant-associated bacteria are likely to have greater access to choline than glycine betaine. In specific, glycine betaine accumulates in some plant species when they are drought or salinity stressed but is present in relatively few plant species in the absence of stress (44). In contrast, possibly due to its association with the synthesis and degradation of the major lipid component of plant membranes, phosphatidylcholine, choline is generally present at detectable levels in plant tissue (57). Choline may therefore be more widely available for uptake by plant-associated bacteria during periods of water limitation. Knowledge of the full complement of DC3000 osmoprotectant transporters, as has been initiated in this work, will allow us to test this hypothesis in future studies.
Supplementary Material
Acknowledgments
We thank Syste Henstra, Gary Smith, and Daniel Le Rudulier for sharing uptake and betaine synthesis protocols; Curtis Youngs for use of his osmometer; and Karl-Erich Jaeger for providing pKnockout sequence data.
This work was supported by National Science Foundation Award no. MCB-0524300.
Footnotes
Published ahead of print on 27 July 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alloing, G., I. Travers, B. Sagot, D. Le Rudulier, and L. Dupont. 2006. Proline betaine uptake in Sinorhizobium meliloti: characterization of Prb, an Opp-like ABC transporter regulated by both proline betaine and salinity stress. J. Bacteriol. 188:6308-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelidis, A. S., and G. M. Smith. 2003. Role of the glycine betaine and carnitine transporters in adaptation of Listeria monocytogenes to chill stress in a defined medium. Appl. Environ. Microbiol. 69:7492-7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelidis, A. S., and G. M. Smith. 2003. Three transporters mediate uptake of glycine betaine and carnitine by Listeria monocytogenes in response to hyperosmotic stress. Appl. Environ. Microbiol. 69:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelidis, A. S., L. T. Smith, L. M. Hoffman, and G. M. Smith. 2002. Identification of OpuC as a chill-activated and osmotically activated carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 68:2644-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axtell, C. A., and G. A. Beattie. 2002. Construction and characterization of a proU-gfp transcriptional fusion that measures water availability in a microbial habitat. Appl. Environ. Microbiol. 68:4604-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Better, M., and D. R. Helinski. 1983. Isolation and characterization of the recA gene of Rhizobium meliloti. J. Bacteriol. 155:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biemans-Oldehinkel, E., N. A. B. N. Mahmood, and B. Poolman. 2006. A sensor for intracellular ionic strength. Proc. Natl. Acad. Sci. USA 103:10624-10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boncompagni, E., L. Dupont, T. Mignot, M. Østeräs, A. Lambert, M.-C. Poggi, and D. Le Rudulier. 2000. Characterization of a Sinorhizobium meliloti ATP-binding cassette histidine transporter also involved in betaine and proline uptake. J. Bacteriol. 182:3717-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boncompagni, E., M. Østeräs, M.-C. Poggi, and D. Le Rudulier. 1999. Occurrence of choline and glycine betaine uptake and metabolism in the family rhizobiaceae and their roles in osmoprotection. Appl. Environ. Microbiol. 65:2072-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boscari, A., K. Mandon, L. Dupont, M.-C. Poggi, and D. Le Rudulier. 2002. BetS is a major glycine betaine/proline betaine transporter required for early osmotic adjustment in Sinorhizobium meliloti. J. Bacteriol. 184:2654-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cairney, J., I. R. Booth, and C. F. Higgins. 1985. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J. Bacteriol. 164:1224-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Checroun, C., and C. Gutierrez. 2004. σs-dependent regulation of yehZYXW, which encodes a putative osmoprotectant ABC transporter of Escherichia coli. FEMS Microbiol. Lett. 236:221-226. [DOI] [PubMed] [Google Scholar]
- 13.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31:3497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choquet, G., N. Jehan, C. Pissavin, C. Blanco, and M. Jebbar. 2005. OusB, a broad-specificity ABC-type transporter from Erwinia chrysanthemi, mediates uptake of glycine betaine and choline with a high affinity. Appl. Environ. Microbiol. 71:3389-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson, A. L., and J. Chen. 2004. ATP-binding cassette transporters in bacteria. Annu. Rev. Biochem. 73:241-268. [DOI] [PubMed] [Google Scholar]
- 17.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Souza-Ault, M. R., L. T. Smith, and G. M. Smith. 1993. Roles of N-acetylglutaminylglutamine amide and glycine betaine in adaptation of Pseudomonas aeruginosa to osmotic stress. Appl. Environ. Microbiol. 59:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupont, L., I. Garcia, M.-C. Poggi, G. Alloing, K. Mandon, and D. Le Rudulier. 2004. The Sinorhizobium meliloti ABC transporter Cho is highly specific for choline and expressed in bacteroids from Medicago sativa nodules. J. Bacteriol. 186:5988-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser, K. R., D. Harvie, P. J. Coote, and C. P. O'Byrne. 2000. Identification and characterization of an ATP binding cassette l-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4696-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gowrishankar, J. 1989. Nucleotide sequence of the osmoregulatory proU operon of Escherichia coli. J. Bacteriol. 171:1923-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halverson, L. J., and M. K. Firestone. 2000. Differential effects of permeating and nonpermeating solutes on the fatty acid composition of Pseudomonas putida. Appl. Environ. Microbiol. 66:2414-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harland, D. N., H. S. Garmory, K. A. Brown, and R. W. Titball. 2005. An association between ATP binding cassette systems, genome sizes and lifestyles of bacteria. Res. Microbiol. 156:434-442. [DOI] [PubMed] [Google Scholar]
- 24.Heeb, S., Y. Itoh, T. Nishijyo, U. Schnider, C. Keel, J. Wade, U. Walsh, F. O'Gara, and D. Haas. 2000. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in Gram-negative, plant-associated bacteria. Mol. Plant Microb. Interact. 13:232-237. [DOI] [PubMed] [Google Scholar]
- 25.Hirano, S. S., and C. D. Upper. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64:624-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 27.Ignoul, S., and J. Eggermont. 2005. CBS domains: structure, function, and pathology in human proteins. Am. J. Physiol. Cell Physiol. 289:C1369-C1378. [DOI] [PubMed] [Google Scholar]
- 28.Jebbar, M., L. Sohn-Bosser, E. Bremer, T. Bernard, and C. Blanco. 2005. Ectoine-induced proteins in Sinorhizobium meliloti include an ectoine ABC-type transporter involved in osmoprotection and ectoine catabolism. J. Bacteriol. 187:1293-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kappes, R. M., B. Kempf, and E. Bremer. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J. Bacteriol. 178:5071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kappes, R. M., B. Kempf, S. Kneip, J. Boch, J. Gade, J. Meier-Wagner, and E. Bremer. 1999. Two evolutionarily closely related ABC transporters mediate the uptake of choline for synthesis of the osmoprotectant glycine betaine in Bacillus subtilis. Mol. Microbiol. 32:203-216. [DOI] [PubMed] [Google Scholar]
- 31.Kempf, B., and E. Bremer. 1995. OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis. J. Biol. Chem. 270:16701-16713. [DOI] [PubMed] [Google Scholar]
- 32.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 33.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loper, J. E., and S. E. Lindow. 1987. Lack of evidence for in situ fluorescent pigment production by Pseudomonas syringae pv. syringae on bean leaf surfaces. Phytopathology 77:1449-1454. [Google Scholar]
- 35.Mahmood, N. A. B. N., E. Biemans-Oldehinkel, J. S. Patzlaff, G. K. Schuurman-Wolters, and B. Poolman. 2006. Ion specificity and ionic strength dependence of the osmoregulatory ABC transporter OpuA. J. Biol. Chem. 281:29830-29839. [DOI] [PubMed] [Google Scholar]
- 36.Massimelli, M. J., P. R. Beassoni, M. A. Forrellad, J. L. Barra, M. N. Garrido, C. E. Domenech, and A. T. Lisa. 2005. Identification, cloning, and expression of Pseudomonas aeruginosa phosphorylcholine phosphatase gene. Curr. Microbiol. 50:251-256. [DOI] [PubMed] [Google Scholar]
- 37.Mauchline, T. H., J. E. Fowler, A. K. East, A. L. Sartor, R. Zaheer, A. H. F. Hosie, P. S. Poole, and T. M. Finan. 2006. Mapping the Sinorhizobium meliloti 1021 solute-binding protein-dependent transportome. Proc. Natl. Acad. Sci. USA 103:17933-17938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.May, G., E. Faatz, M. Villarejo, and E. Bremer. 1986. Binding-protein dependent transport of glycine betaine and its osmotic regulation in Escherichia coli K12. Mol. Gen. Genet. 205:225-233. [DOI] [PubMed] [Google Scholar]
- 39.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 40.Miller, K. J., and J. M. Wood. 1996. Osmoadaptation by rhizosphere bacteria. Annu. Rev. Microbiol. 50:101-136. [DOI] [PubMed] [Google Scholar]
- 41.Moore, R. A., A. N. Starratt, S.-W. Ma, V. L. Morris, and D. A. Cuppels. 1989. Identification of a chromosomal region required for biosynthesis of the phytotoxin coronatine by Pseudomonas syringae pv. tomato. Can. J. Microbiol. 35:910-917. [Google Scholar]
- 42.Musich, J. A., and H. Rapoport. 1977. Reaction of O-methyl-N,N′-diisopropylisourea with amino acids and amines. J. Org. Chem. 42:139-141. [DOI] [PubMed] [Google Scholar]
- 43.Ren, Q., K. H. Kang, and I. T. Paulsen. 2004. TransportDB: a relational database of cellular membrane transport systems. Nucleic Acids Res. 32:D284-D288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhodes, D., and A. D. Hanson. 1993. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44:357-384. [Google Scholar]
- 45.Schiefner, A., J. Breed, L. Bösser, S. Kneip, J. Gade, G. Holtmann, K. Diederichs, W. Welte, and E. Bremer. 2004. Cation-π interactions as determinants for binding of the compatible solutes glycine betaine and proline betaine by the periplasmic ligand-binding protein ProX from Escherichia coli. J. Biol. Chem. 279:5588-5596. [DOI] [PubMed] [Google Scholar]
- 46.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 47.Sleator, R. D., C. G. M. Gahan, and C. Hill. 2003. A postgenomic appraisal of osmotolerance in Listeria monocytogenes. Appl. Environ. Microbiol. 69:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smibert, R. M., and N. R. Kreig. 1994. Phenotypic characterization, p. 607-654. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Kreig (ed.), Methods for general and molecular bacteriology. American Society of Microbiology, Washington, DC.
- 49.Stirling, D. A., C. S. J. Hulton, L. Waddell, S. F. Park, G. S. A. B. Stewart, I. R. Booth, and C. F. Higgins. 1989. Molecular characterization of the proU loci of Salmonella typhimurium and Escherichia coli encoding osmoregulated glycine betaine transport systems. Mol. Microbiol. 3:1025-1038. [DOI] [PubMed] [Google Scholar]
- 50.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K.-S. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. W. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 51.Styrvold, O. B., P. Falkenberg, B. Landfald, M. W. Eshoo, T. Bjørnsen, and A. R. Strøm. 1986. Selection, mapping, and characterization of osmoregulatory mutants of Escherichia coli blocked in the choline-glycine betaine pathway. J. Bacteriol. 165:856-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Heide, T., and B. Poolman. 2000. Osmoregulated ABC-transport system of Lactococcus lactis senses water stress via changes in the physical state of the membrane. Proc. Natl. Acad. Sci. USA 97:7102-7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wemekamp-Kamphuis, H. H., R. D. Sleator, J. A. Wouters, C. Hill, and T. Abee. 2004. Molecular and physiological analysis of the role of osmolyte transporters BetL, Gbu, and OpuC in growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 70:2912-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wemekamp-Kamphuis, H. H., J. A. Wouters, R. D. Sleator, C. G. M. Gahan, C. Hill, and T. Abee. 2002. Multiple deletions of the osmolyte transporters BetL, Gbu, and OpuC of Listeria monocytogenes affect virulence and growth at high osmolarity. Appl. Environ. Microbiol. 68:4710-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Windgassen, M., A. Urban, and K.-E. Jaeger. 2000. Rapid gene inactivation in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 193:201-205. [DOI] [PubMed] [Google Scholar]
- 56.Wright, C. A., and G. A. Beattie. 2004. Pseudomonas syringae pv. tomato cells encounter inhibitory levels of water stress during the hypersensitive response of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 101:3269-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeisel, S. H., M.-H. Mar, J. C. Howe, and J. M. Holden. 2003. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 133:1302-1307. (Erratum, 133:2918-2919.) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.