Abstract
In Escherichia coli K-12, components of the phosphoenolpyruvate-dependent phosphotransferase systems (PTSs) represent a signal transduction system involved in the global control of carbon catabolism through inducer exclusion mediated by phosphoenolpyruvate-dependent protein kinase enzyme IIACrr (EIIACrr) (= EIIAGlc) and catabolite repression mediated by the global regulator cyclic AMP (cAMP)-cAMP receptor protein (CRP). We measured in a systematic way the relation between cellular growth rates and the key parameters of catabolite repression, i.e., the phosphorylated EIIACrr (EIIACrr∼P) level and the cAMP level, using in vitro and in vivo assays. Different growth rates were obtained by using either various carbon sources or by growing the cells with limited concentrations of glucose, sucrose, and mannitol in continuous bioreactor experiments. The ratio of EIIACrr to EIIACrr∼P and the intracellular cAMP concentrations, deduced from the activity of a cAMP-CRP-dependent promoter, correlated well with specific growth rates between 0.3 h−1 and 0.7 h−1, corresponding to generation times of about 138 and 60 min, respectively. Below and above this range, these parameters were increasingly uncoupled from the growth rate, which perhaps indicates an increasing role executed by other global control systems, in particular the stringent-relaxed response system.
In Escherichia coli, the phosphoenolpyruvate (PEP)-dependent phosphotransferase systems (PTSs) represent important uptake systems for a number of carbohydrates which mediate transport and concomitant phosphorylation of their respective substrates (10, 44). In addition to their transport function, all components of the various PTSs of a cell form an important signal transduction system. The signal transduction properties of the PTS depend on the phosphorylation state of its proteins (26, 49). The PTSs usually consist of two general proteins, i.e., the PEP-dependent protein kinase enzyme I (EI), and the histidine-containing protein (HPr), and up to 20 different, substrate-specific enzymes II (EII). EII usually comprise two soluble domains EIIA and EIIB involved in phosphotransfer and the membrane-bound transporter domain EIIC (44). The major regulatory output signal of the PTS depends on the phosphorylation level of EIIACrr (according to its genetic nomenclature), also designated EIIAGlc due to its function as the EIIA domain for the glucose-specific PTS (9, 23, 52). EIIACrr inhibits the activity of a number of non-PTS transporters and enzymes (8, 32, 33, 35, 36), a process referred to as inducer exclusion. Furthermore, the phosphorylated form of EIIACrr (EIIACrr∼P) activates adenylate cyclase (1, 13, 41, 57), which in turn synthesizes cyclic AMP (cAMP) (59). The indicator molecule or alarmone cAMP is the coactivator of the important global transcription factor CRP (cAMP receptor protein). Together, they regulate in a process called cAMP-CRP-dependent catabolite repression efficient transcription of different genes involved in the synthesis of a large number of catabolic enzymes (4, 39, 43). The central role of EIIACrr∼P in the activation of adenylate cyclase is largely based on mutant analysis (13, 23, 33).
The phosphorylation state of the PTS and hence the intracellular cAMP concentrations are postulated to depend largely on two major factors: (i) the uptake rate of any PTS substrate which determines the dephosphorylation rate of EI (this kinase autophosphorylates in a reversible process with PEP to generate pyruvate [49]) and (ii) the ratio of PEP to pyruvate, two central intermediate metabolites in glycolysis and gluconeogenesis which directly influence the EI autophosphorylation reaction. This ratio, however, is especially difficult to measure in vivo, and there is little corresponding data available for cells growing under different conditions (18, 27). In one thorough study, starved cells were used (16, 17). The results indicated a correlation between the EIIACrr phosphorylation level and the PEP-to-pyruvate ratio, but it is not clear how these results reflected the conditions in growing cells. Furthermore, recent in vitro reconstitution experiments indicated the putative existence of additional factors which might also modulate adenylate cyclase activity (38, 41, 42, 47). Therefore, the determination of the phosphorylation level of EIIACrr was considered an alternative test for the intracellular PEP-to-pyruvate ratio during steady-state conditions. Metabolic reactions are very fast, and hence, the PTS phosphorylation levels as well as the PEP, pyruvate, and also intracellular cAMP concentrations should quickly reach a quasi-steady-state level during growth with nonlimiting concentrations of carbohydrates.
In this paper we systematically tested the correlation between growth rates, EIIACrr phosphorylation levels (meaning in this case the ratio of EIIACrr to EIIACrr∼P), extracellular cAMP concentrations, and the activity of a cAMP-CRP-dependent promoter in E. coli K-12 grown on different carbohydrates and with various carbohydrate concentrations but only for growth rates (μ) between 0.3 h−1 to 0.7 h−1. Above and below these growth rates, the correlation was less clear, which supports the idea that additional regulatory elements become relevant under these conditions.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The two strains used in this study were LJ110, an Fnr+ derivative of the E. coli K-12 mutant W3110 (22), and its genetically engineered derivative LJ210, which carries the scr genes for PTS-dependent transport and metabolism of sucrose integrated within its chromosome (2, 54).
Strains were grown in phosphate-buffered minimal medium (MM) as described by Tanaka et al. (58). For some bioreactor experiments, the ammonium concentration of the medium was increased to 90 mM to enable growth to higher cell densities. Carbohydrates were sterilized by filtration. If not indicated otherwise, they were added to 2 g/liter for experiments in shake flasks and to 5 g/liter for batch experiments in bioreactors. For the reporter gene assays, kanamycin was added to the cultures to 25 mg/liter. Biomass concentrations were determined by measuring the absorbance at 420 nm or at 560 nm in an Ultrospec3000 (Amersham Biosciences).
Strains were pregrown overnight in MM supplied with the same carbohydrate to be used in the experimental culture. The cultures were washed in fresh MM without the addition of carbohydrates. For experiments in shake flasks, the volumes in the flasks exceeding the culture volume at least five times, the washed cells were inoculated to 2.5 × 107 cells/ml. The cultures were incubated at 37°C under vigorous shaking (250 rpm) if not indicated otherwise. Growth was monitored by measurement of the absorbance at 420 nm. For batch experiments in bioreactors, preculture conditions were the same as for experiments in shake flasks. The cells were added to approximately 1 × 108 to 2 × 108 cells/ml. The cultures were continuously stirred under aerobic conditions (partial O2 pressure of >20% of saturation). For experiments with various carbohydrate concentrations, the reactor was set up with 3 liters of MM supplied with 0.1 g/liter of the respective carbohydrate. Cells were added to approximately 4 × 108 cells/ml, and 1 liter/h of MM supplied with 0.8 g/liter of the respective carbohydrate was continuously fed into the bioreactor while the same volume was withdrawn. Carbohydrate and extracellular cAMP concentrations were monitored by either directly taking supernatant from the reactor with the help of a filtration module in the reactor or by taking culture samples and removing cells quickly by centrifugation at low temperatures (4°C).
Measurements of metabolite concentrations and enzyme activities.
Measurements of extracellular carbohydrate concentrations were performed either enzymatically with the test kits from r-Biopharm GmbH (Germany) or on a Dionex DX-600 system (Dionex Corp.) equipped with an electrochemical detector and a Carbopac PA-100 column. Extracellular cAMP concentrations were measured with the cAMP enzyme immunoassay system (GE Healthcare) as recommended by the manufacturer. For these tests, the cells were precultured in the same medium and carbon source as used during the test. Cells washed free of cAMP as described above were inoculated to 5 × 107 cells per ml, the changes in cAMP concentrations were determined throughout a complete growth curve, and the cAMP concentration for a cell density of 5 × 108 cells per ml was obtained after interpolation. The β-galactosidase activities were determined essentially by the method of Pardee and Prestidge (37) and modified by Miller (31) and expressed in micromoles per milligram of protein and per minute. Determination of intracellular PEP and pyruvate concentrations were performed as described previously (53).
Analysis of the EIIACrr phosphorylation state.
The EIIACrr phosphorylation state was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting essentially as described previously (57). Deviating from the protocol, protein precipitation was carried out at −80°C overnight. Detection was performed with polyclonal EIIACrr antiserum from a rabbit. As secondary antibodies, goat anti-rabbit antibodies conjugated to horseradish peroxidase were used, and detection was performed with the SuperSignal West Femto Maximum sensitivity substrate (Pierce) and a cooled charge-coupled-device camera (INTAS, Germany). The sum of the EIIACrr-specific bands was set at 100%.
Reporter gene studies.
To analyze the activity of the cAMP-dependent scrYp and cAMP-independent scrKp promoters, fragments covering the promoter regions of both genes and approximately 200 bp upstream and downstream of the start codon including the known cAMP-CRP binding site of scrYp (55), were amplified by PCR, and each promoter fragment was cloned separately in front of the luxCDABE genes into pCS26 (3). The plasmids were transformed into E. coli LJ110, and the cells were grown in MM supplied with different carbohydrates. At various time points throughout a growth curve, samples were taken. The biomass concentrations of these samples were determined by measurement of the absorbance at 420 nm, and the relative luminescence units of a 100-μl sample was measured in a luminescence reader (Mithras; Berthold Technologies) (measurement time of 0.1 s). For analysis, the relative luminescence per 5 × 108 cells and the growth rates of the cultures were calculated. Average values for a specific experiment were taken from at least three measurements during the exponential phase of a culture.
RESULTS
Analysis of EIIACrr phosphorylation levels and of extracellular cAMP concentrations during growth on different carbohydrates.
To analyze the influence of growth rates on the EIIACrr phosphorylation level and on extracellular cAMP concentrations, growth was tested first in shake flasks. Various hexoses, pentoses, and organic acids, which feed into different parts of central metabolism, were used as single carbon sources, among them PTS and non-PTS substrates. Strain LJ110 or its sucrose-positive relative LJ210 were grown in standard minimal medium supplied with saturating amounts (2 g/liter) of the carbohydrate. Additionally, the EIIACrr phosphorylation levels were determined from experiments in bioreactors. As expected, the growth rates varied with different carbohydrates, but identically for both strains. Furthermore, no significant differences in growth rate and EIIACrr phosphorylation could be observed between both types of experiments. Consequently, they were summarized and presented together.
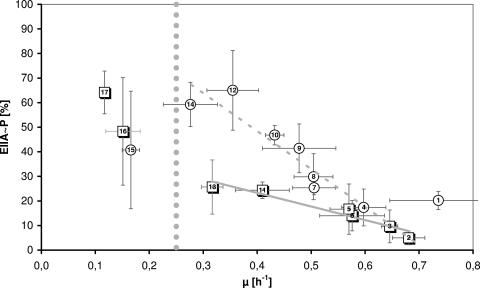
If, as hypothesized initially (13, 33), adenylate cyclase was activated only by EIIACrr∼P, then EIIACrr phosphorylation levels and cAMP concentrations should correlate closely. According to the data in Table 1 and Fig. 1, high growth rates seemed to correspond to low cAMP and low EIIACrr phosphorylation levels, and low growth rates seemed to correspond to high cAMP and high EIIACrr phosphorylation levels.
TABLE 1.
EIIACrr phosphorylation levels and extracellular cAMP concentrationsa
| Carbon source | No. in figuresb | μ (h−1) | EIIACrr∼P level (%) | Extracellular cAMP concn (nM) | PEP-to-pyruvate ratio |
|---|---|---|---|---|---|
| d-Glucose-6-phosphate | 1 | 0.74 ± 0.09 | 20 ± 4 | 55 ± 25 | 0.21 ± 0.29 |
| d-Glucose | 2 | 0.68 ± 0.03 | 5 ± 2 | 142 ± 24 | 0.15 ± 0.01 |
| Sucrose | 3 | 0.65 ± 0.02 | 10 ± 7 | 148 ± 18 | 0.45 ± 0.29 |
| Lactose | 4 | 0.60 ± 0.04 | 17 ± 7 | 137 ± 48 | 0.12 ± ND |
| N-Acetyl-d-glucosamine | 5 | 0.57 ± 0.03 | 17 ± 10 | 114 ± 19 | ND |
| d-Mannitol | 6 | 0.58 ± 0.06 | 14 ± 6 | 137 ± 23 | 0.22 ± ND |
| l-Arabinose | 7 | 0.51 ± 0.04 | 25 ± 2 | 111 ± 78 | ND |
| d-Gluconate | 8 | 0.50 ± 0.03 | 30 ± 9 | 99 ± 31 | 0.04 ± 0.06 |
| Maltose | 9 | 0.48 ± 0.07 | 41 ± 10 | 395 ± 140 | 0.27 ± ND |
| sn-Glycerol | 10 | 0.43 ± 0.02 | 47 ± 4 | 374 ± 125 | ND |
| d-Fructose | 11 | 0.41 ± 0.05 | 24 ± 3 | 400 ± 450 | 0.40 ± 0.29 |
| Succinate | 12 | 0.35 ± 0.05 | 65 ± 16 | 271 ± 133 | 1.07 ± ND |
| d-Glucitol | 13 | 0.32 ± 0.02 | 26 ± 10 | ND | ND |
| d-Galactose | 14 | 0.28 ± 0.06 | 59 ± 9 | 473 ± 96 | ND |
| Acetate | 15 | 0.17 ± 0.02 | 41 ± 24 | 1342 ± 1350 | 6.68 ± 1.95 |
| d-Mannose | 16 | 0.15 ± 0.03 | 48 ± 22 | 1447 ± 1142 | 1.4 ± 1.03 |
| d-Glucosamine | 17 | 0.12 ± 0.01 | 64 ± 9 | ND | ND |
The growth and test conditions from batch experiments with various carbon sources were as described in Materials and Methods using strains LJ110 and LJ210. Growth rates and phosphorylated EIIACrr∼P and extracellular cAMP concentrations measured at 5 × 108 cells per ml represent means ± standard deviations from at least two independent experiments. ND, not determined.
FIG. 1.
Correlation of EIIACrr phosphorylation state and growth rate during growth of E. coli LJ110 and LJ210 with various carbon sources. EIIACrr phosphorylation levels were determined by Western blotting as described in Materials and Methods. The data represent mean values from at least two independent cultures and from at least four samples taken during exponential growth of one culture. The numbers in the symbols refer to the carbon sources as indicated in Table 1 with error bars indicating standard deviations. Circles correspond to non-PTS substrates, while squares represent PTS substrates. The gray line connecting the data points for PTS substrates represents a trend line considering all PTS data points. The dashed gray line represents a trend line considering all non-PTS substrates with exceptions glucose-6-phosphate and acetate. The trend lines show almost linear correlations between EIIACrr phosphorylation levels and growth rate. The obtained R2s were 0.93 for the PTS and 0.86 for the non-PTS trend line. The gray dots at μ = 0.25 h−1 are drawn to point out the two areas mentioned in the Discussion.
Considering EIIACrr phosphorylation levels, for relatively high growth rates, no clear distinction between PTS substrates and non-PTS substrates could be seen. Thus, about 20% of EIIACrr remained phosphorylated during growth on the PTS substrates N-acetylglucosamine and mannitol and a similar percentage during fast growth on the non-PTS substrates lactose, l-arabinose, and gluconate.
Furthermore, a closer analysis revealed that the close correlation between growth rates, EIIACrr phosphorylation levels, and cAMP concentrations was valid only for cells growing with specific growth rates (μ) between 0.3 h−1 and 0.7 h−1 (corresponding to about 140- to 60-min generation time). This was in contrast to cells growing very slowly (μ < 0.3 h−1), in particular those growing on acetate, d-mannose, and d-glucosamine. One major difference with these slow-growing cells was not only the EIIACrr phosphorylation levels but also the extracellular cAMP concentrations deviated strongly from experiment to experiment, although both were sampled from the same cultures, and the deviations in growth rates were minor (see the error bars in Fig. 1 and Table 1). It is not clear whether this represents a systematic behavior of starved cells or whether this high variability was caused by experimental procedures, though these were highly standardized as described in Materials and Methods. Poor correlation could perhaps indicate that under these growth conditions, extracellular cAMP concentrations do not correspond directly to intracellular cAMP concentrations, e.g., because additional factors modulate cAMP excretion from the cell and subsequent uptake into the cell, degradation of cAMP, or cAMP production, respectively (4, 12).
Deviations of another type could be seen during growth on d-fructose, d-glucitol, and d-glucose-6-phosphate. (i) On fructose and on glucitol, the phosphorylation levels of EIIACrr∼P (24%) were too low, and the extracellular cAMP level during growth on fructose was too high compared to those of other carbohydrates in cells growing at similar growth rates. Growth on fructose, and in particular ΔptsH mutants in which fructose-specific protein (FPr) replaces the missing histidine-containing protein (HPr), have already been reported to cause enhanced cAMP production (6, 26). Moreover, in contrast to other PTS substrates, e.g., N-acetylglucosamine and mannitol, which allow high growth rates, growth on fructose and on glucitol is slow and the influence of the transport reactions on the EIIACrr phosphorylation state might become visible. Different characteristic curves of the correlation of growth rate and the level of EIIACrr∼P have also been predicted by a mathematical model that we have set up (2). This model predicts that the difference in both curves becomes more pronounced at lower growth rates (A. Kremling, unpublished results). These predictions are supported by the data presented in Fig. 1. (ii) Dephosphorylation was at its maximum (90 to 95%) during growth on sucrose (μ = 0.65 h−1) and, in particular, on glucose (μ = 0.68 h−1), but not on glucose-6-phosphate (μ = 0.74 h−1; 80% dephosphorylation), the fastest growth substrate tested here. Glucose-6-phosphate, the only phosphorylated carbon source tested here, stimulates a EIICBAGlc-dependent glucose/glucose-6-phosphate exchange (51). When present at high intracellular concentrations, it causes back phosphorylation of EIICBGlc by glucose-6-phosphate, which would consequently result in an elevated level of EIIACrr∼P.
Determination of the activity of a cAMP-CRP-dependent promoter compared to a cAMP-CRP-independent promoter.
Accurate and fast measurements of intracellular cAMP concentrations are difficult and further complicated by the high extracellular cAMP concentrations which amount to 95% of the total cAMP (12, 40). Intracellular cAMP determinations always require extensive washing. Such methods are impossible to validate, as no standards for intracellular cAMP exist and the influences of washing on cAMP levels are poorly understood. To minimize the problems, “in vivo” measurements were performed by using the cAMP-dependent scrYp promoter and the cAMP-independent scrKp promoter of the scr regulon from pUR400 (54). Both promoters were fused independently and in the absence of the specific repressor gene scrR and independently, to the luxCDABE genes of the low-copy-number vector pCS26, as described in Materials and Methods. The usage of the lux reporter genes, either behind a cAMP-dependent or cAMP-independent promoter, should allow accurate measurements of the activity of the cAMP-CRP complex. This in turn should closely correlate with the active intracellular cAMP concentrations. Consequently, constitutive expression of the scrKp promoter represents the overall capacity of the transcriptional and translational machinery of the cells. On the other hand, transcription from scrYp is very low in the absence of cAMP, and transcription should increase strictly correlated to increasing intracellular cAMP levels (54). Also, because both promoter activities were measured with the same reporter genes, from the same vectors, and in the same host strain, changes in the activity of the cAMP-independent promoter scrKp can be used to correct for changes in scrYp activity due to altered growth rates and to changes in plasmid copy numbers.
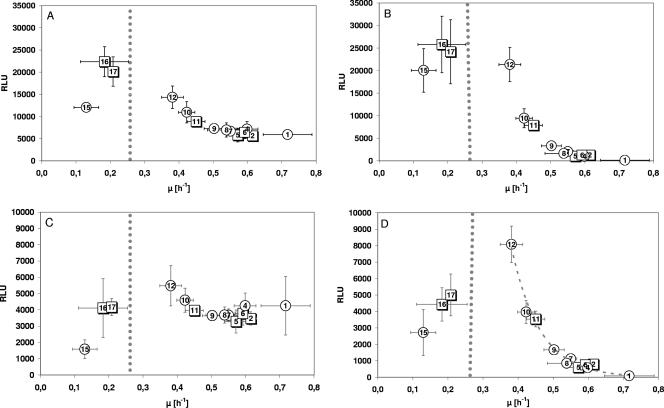
Cells of strain LJ110 carrying either of both constructs were grown in parallel on minimal medium with different carbohydrates. The relative luminescence units were determined throughout batch experiments in shake flasks, and all measurements were carried out in parallel to limit further day-to-day variations. Analysis of the units measured during the exponential growth phase revealed activity variations with changing growth rates, but the changes differed in a characteristic way for the two promoters, being more pronounced for scrYp than for scrKp (Fig. 2). In addition, while the scrKp promoter showed considerable activity at all growth rates, the scrYp activity was only marginal at high growth rates.
FIG. 2.
Correlation of the activities of the cAMP-independent scrKp and cAMP-dependent scrYp promoters to growth rates resulting from growth on different substrates. (A and B) Relative luminescence activities (in relative light units [RLU]) of E. coli LJ110 carrying either the constitutive and cAMP-independent scrKp promoter (A) or the constitutive and cAMP-dependent scrYp promoter (B) fused to luxCDABE genes of pCS26 during batch cultures with various carbon sources. (C and D) scrK and scrYp activities, respectively, after multiplication of the RLUs with the corresponding growth rate. This standardization is done to account for differences in dilution rate of the proteins that vary with growth rate. (D)Activity of the scrYp promoter after multiplication of the RLUs with growth rate. By using an exponential fit, we were able to obtain a R2 of 0.95, showing good correlation. The trend line was added to this plot as a dotted gray line. Values represent mean values from at least three independent experiments, and the error bars indicate standard deviations. The numbers in symbols correspond to carbon sources as in Table 1.
In growing cells, proteins are diluted constantly because of the increase in cell volume followed by cell division. Therefore, to correct for higher dilution rates of proteins in faster growing cultures, the activities of both promoters were expressed in relative luminescence units multiplied with the corresponding growth rate. Analysis of these corrected units revealed a rather constant basal activity of about 4,000 corrected units for the cAMP-independent promoter scrKp on all carbon sources, except for acetate (1,570 units) with its exceedingly slow growth rate (Fig. 2C). This indicated that the overall capacity of transcription and translation correlated with growth rate, except for very slow growth rates. In contrast, the corrected activities of the cAMP-dependent promoter varied drastically (≥100-fold [Fig. 2D]). As before, two distinct ranges could be detected in the experiments. For growth rates higher than 0.6 h−1, low intracellular cAMP concentrations were indicated by marginal activities of the cAMP-dependent scrYp promoter. This was expected in view of the low extracellular cAMP concentrations measured for these growth rates (Table 1). In contrast, decreasing growth rates correlated with higher scrYp activities, and intracellular cAMP concentrations peaked around 0.3 h−1 or 140-min generation time. At very slow growth rates (≤0.3 h−1), scrYp still showed significant activities, but here no clear correlation between growth rate and cAMP could be seen. The plot representing the corrected scrYp activity, i.e., intracellular cAMP (Fig. 2D), resembled the ratio of the EIIACrr phosphorylation level to growth rate (Fig. 1). Although the slope of the two curves differed for growth rates between ≥0.3 h−1 to ≤ 0.7 h−1, the plot showed the same ranges. This indicated that EIIACrr phosphorylation levels correlated, but not directly, with the intracellular cAMP concentrations.
Extracellular cAMP concentrations (Table 1) correlated less well, perhaps due to variable excretion or metabolism of cAMP. In addition, the reporter gene assays determined the activation of a promoter by the cAMP-CRP complex. This activation does not exclusively depend on the intracellular cAMP but also on the CRP concentrations (reference 10 and references therein). Data based solely on extracellular cAMP measurements thus do not necessarily mirror the true intracellular cAMP concentrations and must be considered with great caution.
Bioreactor experiments with various carbohydrate concentrations.
Up to now, we investigated the correlation of growth rate, EIIACrr phosphorylation level, and cAMP concentration when the growth rate of the cells was limited by the quality of the carbon source. An alternative method to vary the growth rate is to grow the cells under different or limiting concentrations of a specific substrate. Using batch cultures as well as a chemostat-like construction by means of dialysis bags, Notley-McRobb et al. (34) reported drastic changes in intra- and extracellular cAMP concentrations at external glucose concentrations around 300 μM for E. coli. According to the Km value of 3 to 10 μM for the Glc-PTS transport activity in whole cells (7, 23), a change in the phosphorylation state of EIIACrr at this high glucose concentration seemed unlikely. Unfortunately, the EIIACrr phosphorylation state was not determined in these experiments.
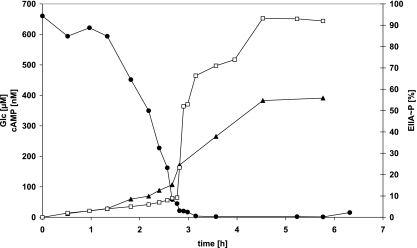
During the starting phase of continuous bioreactor experiments, the carbohydrate concentration drops until it becomes limiting, i.e., growth rates are mostly determined by the decreasing external carbon source concentrations. This decrease is much slower than it is in batch experiments, allowing for a better resolution of data in the low carbohydrate concentration ranges, in particular those related to changing growth rates, cAMP concentrations, and EIIACrr phosphorylation levels. Therefore, such an experiment was performed with glucose as the carbon source, and a typical time course is shown in Fig. 3. During the first 3 hours and with high glucose concentrations, the EIIACrr phosphorylation level remained low. The slow rise in phosphorylation level probably reflects adaptation to stronger aeration within the bioreactor. At about 2.7 h, when the extracellular glucose concentration had dropped to about 40 μM, the phosphorylation level changed within minutes from about 10 to 70%, finally reaching more than 90%. At about the same time, the extracellular cAMP concentrations began to increase considerably faster than before, which we interpret as due to higher cAMP production rates. Thus, the changes in the EIIACrr phosphorylation level and in the extracellular cAMP concentration occurred simultaneously, again indicating a correlation between both parameters. Growth rates could not be calculated precisely from the data because, due to the high dilution rate, changes in biomass were very small within the relevant time window. However, growth rates as calculated from the dilution rate were estimated to vary between 0.6 h−1 for growth under nonlimiting glucose concentrations and about 0.33 h−1 for growth under limiting glucose concentrations.
FIG. 3.
Time course of an experiment with various glucose concentrations. Show are the measurements from a continuous bioreactor experiment with E. coli LJ110 with glucose as the carbon source. Bioreactor setup and measurements were as described in Materials and Methods. cAMP samples were taken by taken samples from the bioreactor and by rapid centrifugation of these samples at 4°C. Open circles indicating the glucose concentration (in micromolar) are given on the leftmost y axis as well as extracellular cAMP concentrations given in nanomolar and represented by open triangles. The EIIACrr phosphorylation level represented by filled squares is plotted on the rightmost y axis.
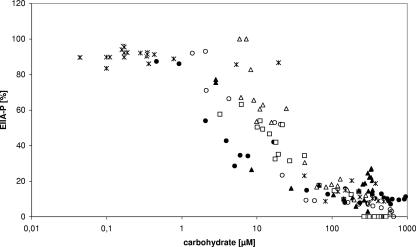
A set of continuous bioreactor experiments similar to the one shown in Fig. 3 was performed with glucose, sucrose, and mannitol (Fig. 4). These three PTS carbohydrates have similar Km values as determined in transport assays, i.e., 5 to 12 μM for glucose and the Glc-PTS (7, 23), 10 μM for sucrose and the Scr-PTS (55), and 2 to 11 μM for mannitol and the Mtl-PTS (15, 19, 25, 48), respectively. Therefore, they could be expected to give similar results. For each substrate, the phosphorylation levels of EIIACrr and the measured extracellular cAMP concentrations (data not shown) correspondingly and drastically began to increase when the external carbohydrate concentrations reached a level of 10 to 50 μM. As before, growth rates could be calculated only when based on dilution rates and decreasing carbohydrate concentrations. They were estimated to change from about 0.55 h−1 to 0.33 h−1 within the relevant time interval.
FIG. 4.
Growth experiments with changing carbohydrate concentrations. The figure shows measurements from continuous bioreactor experiments with strain LJ110 or LJ210 and with glucose, sucrose, or mannitol as the carbon source. The experimental setup and measurements were as described in Materials and Methods. The EIIACrr phosphorylation level is plotted semilogarithmically against the carbohydrate concentrations. Symbols: ▪, ○, and •, experiments with glucose as the carbon source; ▵ and ▴, experiments with sucrose as the carbon source; *, experiment with mannitol as the carbon source.
In summary, these data show a correlation between EIIACrr phosphorylation levels, cAMP production rates, and the extracellular carbohydrate concentrations which determine the growth rates. Using a mathematical model for the glucose-PTS which was able to reproduce the experiments (2), we calculated that an apparent Km value of 12 μM extracellular carbohydrate corresponds to a level of 50% phosphorylated EIIACrr. This was in general agreement with the known kinetics of the three PTSs. Although at a first glance the experimental setup of Notley-McRobb et al. (34) seems to allow growth experiments equivalent to our experiments in a continuous bioreactor, deviations in the experimental setup may account for the different results. Thus, at least for their dialysis cultures, it is not clear whether glucose and oxygen diffusion within the dialysis bag was sufficient. Furthermore, due to the known problems in the measurement of extracellular cAMP concentrations, it is also not clear how accurate cAMP concentrations could be determined under their experimental conditions. Comparing phosphorylation assays performed with cell extracts to growth and transport assays with whole cells, high deviations (10-fold) in the Km values have been measured for the glucose-PTS (50) and the three hexitol-PTSs (25). Yet it is difficult to envision, how these observations could explain the 30- to 90-fold deviation between the measured apparent Km value for the glucose-PTS and the onset of extracellular cAMP increase as observed by Notley-McRobb et al. Besides this discrepancy, both sets of results indicated a similar correlation between growth rates, the ratio of EIIACrr to EIIACrr∼P, and cAMP concentrations.
DISCUSSION
The data presented within this study demonstrate, to our knowledge for the first time, why the PTS is suited as a sensor system for the physiological state of the cell. The data show a strict correlation of growth rate, determined by the quality of the carbon source, and the EIIACrr phosphorylation state, at least for medium to high growth rates. We hypothesize that if growth is limited solely by the quality of the carbon source, i.e., by the cell's capacity to take up and metabolize the carbon source, then the PEP-to-pyruvate ratio in the cell is a direct measure of this growth rate. This PEP-to-pyruvate ratio is reflected by the phosphorylation state of EIIACrr, making this molecule an ideal candidate for sensing the physiological state of the cell. This information is subsequently transduced by the modulation of enzymatic activities, most importantly the activity of adenylate cyclase. In contrast to previous assumptions, we could show that the EIIACrr phosphorylation state not only represents a measure for the presence or absence of a PTS substrate but that the phosphotransferase system represents a universal sensor for the physiological state of the cell with respect to the carbohydrate metabolism. This is possible because the PTS phosphorylation state is directly linked to the PEP-to-pyruvate ratio and hence to the central metabolism.
In enteric bacteria, carbon and energy metabolism are controlled largely by two global regulatory mechanisms called cAMP-CRP-dependent catabolite repression and inducer exclusion. The combined phosphotransferase systems of a cell together constitute an expedient signal transduction system that senses intracellular changes in carbon catabolism and energy metabolism as changes in the phosphorylation levels of its components. All phosphoryl-transfer reactions within the PTS are reversible. Therefore, the major key parameters which determine the phosphorylation level of its components are the PEP-to-pyruvate ratio during growth on any carbon source, even a non-PTS carbon source, together with the uptake activity of the various PTSs during growth on PTS substrates. Furthermore, the EIIACrr phosphorylation level should reflect directly the PEP-to-pyruvate ratio in the cell regardless of the carbon source used for growth (49). Unfortunately, determinations of the true intracellular PEP and pyruvate concentrations are difficult to perform in growing cells. A network of reactions is coupled to the so-called pyruvate node, and the control of activity or of synthesis of the corresponding genes and enzymes has not yet been elucidated in detail. For an estimation of the true intracellular concentrations of PEP and pyruvate that may change in the millisecond range (28), a careful determination of all fluxes would be needed. At present, such an analysis can be performed only by using mutants (reference 20 and references therein) and mathematical models (18). We used measurement of the EIIACrr phosphorylation level as an alternative method. This measurement is based on the strict coupling of the PTS to the PEP-to-pyruvate ratio in the cell (49).
Correlation between growth rates and EIIACrr phosphorylation levels.
Our data show a good correlation of growth rates, as determined by the quality or quantity of the carbon source, and the EIIACrr phosphorylation states, at least for medium to high growth rates. Different growth rates were obtained first by using various carbohydrates. In such cells, growth was limited by the affinity and capacity of the uptake systems, as well as by the capacity of the first metabolic reactions. High growth rates clearly correlated with lower EIIACrr phosphorylation levels, indicating a lower PEP-to-pyruvate ratio, and vice versa, but only for cells grown with generation times of about 140 to 60 min, i.e., specific growth rates between 0.3 h−1 and 0.7 h−1.
Three apparent exceptions among the carbon sources were d-fructose, d-glucitol, and d-glucose-6-phosphate (substrates 11, 13, and 1, respectively, in Fig. 1). During growth on fructose, phosphorylation of EIIACrr was too low (∼20% determined versus ∼50% estimated) and extracellular cAMP levels were too high, compared to other carbon sources allowing similar growth rates. The low phosphorylation level of EIIACrr might be related to the involvement of the HPr-like protein FPr in the fructose-PTS. Thus, ΔptsH mutants, in which FPr replaces the missing HPr, also show enhanced cAMP production (6, 26). Furthermore, the repressor protein FruR (alternatively Cra) of the fru operon is involved as an activator in the regulation of the pps gene, encoding PEP synthase (14; our unpublished results), and of some other glycolytic and gluconeogenic genes in E. coli and Salmonella enterica serovar Typhimurium (5, 45, 46). Another possibility is that the low phosphorylation level of EIIACrr during growth on fructose simply results from dephosphorylation of the PTS during fructose transport. This is corroborated by the fact that with glucitol, the other PTS substrate, which allows medium growth rates, the same deviation was observed. Hence we postulate a distinction between PTS substrates and non-PTS carbon sources. This distinction was very weak for substrates allowing fast growth but became more pronounced for substrates resulting in medium growth rates. This is corroborated by modeling studies which predict that at high growth rates, the PTS phosphorylation activity should have a low impact on the phosphorylation level of EIIACrr, while at low growth rates this impact increases considerably (22). Although the comparison to glucitol and the analysis with the help of the model provide an explanation for the low phosphorylation state of EIIACrr during growth on fructose, they cannot explain the high levels of extracellular cAMP during growth on fructose that we and others have observed.
The third substrate that displayed a deviating behavior was glucose-6-phosphate. During fast growth on glucose-6-phosphate, the phosphorylation level of EIIACrr was higher than expected. It had already been reported that glucose-6-phosphate did not elicit catabolite repression, although cAMP levels were very low during growth with glucose-6-phosphate (11). It is tempting to speculate that glucose-6-phosphate interferes with the PTS phosphorylation level. Glucose-6-phosphate is the product of PTS-mediated glucose uptake. High intracellular levels of glucose-6-phosphate have been reported to inhibit PTS-mediated glucose uptake (21, 24). In addition, EIICBGlc/EIIACrr-mediated cross phosphorylation of glucose by glucose-6-phosphate has been reported (51). During growth on glucose-6-phosphate, high intracellular glucose-6-phosphate levels apparently allow rephosphorylation of the PTS, explaining the elevated phosphorylation levels of EIIACrr.
Compared to the batch experiments, the continuous bioreactor experiments allow a better resolution of data within the transition phase from fast growth on high substrate concentrations to lower growth rates caused by decreasing external substrate concentrations. We used three PTS substrates, i.e., d-glucose, sucrose, and d-mannitol, with similar transport Km values, of which the former two PTSs use EIIACrr as their phosphate donor. The corresponding results did show the same general trend as the previous experiments, i.e., higher growth rates correlated closely with decreased phosphorylation of EIIACrr. In particular, they did not show a significant increase in the EIIACrr phosphorylation level before the substrate concentrations decreased to concentrations in the range of the Km values (Fig. 3 and 4). Obviously, cells coordinate EIIACrr phosphorylation and cAMP levels with growth rates in a similar way, whether cell growth was limited because of the nature or amount of the carbon source used.
Correlation between growth rates and intracellular cAMP levels.
A second key parameter besides the PEP-to-pyruvate ratio in the control of carbon catabolism is the alarmone cAMP. This coactivator of the global transcription factor CRP is essential in controlling the synthesis of several hundred genes and catabolic enzymes. In a ΔcyaA mutant of E. coli, increasing growth rates on glucose could be obtained by adding increasing amounts of cAMP to such cells. Furthermore, different cAMP concentrations corresponded to the growth rate on diverse carbon sources (12). Unfortunately, we and others have been unable until now to test intracellular cAMP concentrations in growing cells directly, rapidly, and in a reliable way (30, 40). As shown in Table 1, extracellular cAMP concentrations have no simple relation to the intracellular, i.e., biologically active, cAMP amounts. Attempts to correlate intracellular cAMP levels with β-galactosidase activities transcribed from a constitutively expressed lacZp promoter (12) have not sufficiently taken into consideration indicator protein dilution, thus presenting an incomplete picture of the cell's physiology under various growth conditions. In an extension of such in vivo studies, we compared the activities from a cAMP-dependent promoter and a cAMP-independent promoter of the scr regulon. These constructs allowed correction for changes in promoter activities due to altered growth rates and concomitant protein dilution rates and to plasmid copy number effects. In agreement with the EIIACrr phosphorylation tests, these corrected data also indicated, first a major (central) phase, valid from medium to high growth rates (Fig. 2). Within this phase, the constitutively expressed and cAMP-independent promoter scrKp showed variations of less than twofold in its corrected promoter activities over the range of growth rates between 0.3 h−1 and 0.7 h−1. Because the corrected scrKp activities represent basically the general transcriptional and translational capacity of the cell, this capacity seems to correlate strictly with growth rates within this central range. The marked deviation during growth on acetate probably indicates the increasing starvation stress. This contrasted with the cAMP-dependent promoter scrYp, whose equally corrected, activities varied more than 100-fold within this central range of growth rates. Such drastic changes must obviously be attributed largely to changes in the intracellular cAMP concentrations and more precisely in the amount of active cAMP-CRP complexes. Similar to the EIIACrr phosphorylation level, the intracellular cAMP levels had a clear maximum around a μ of 0.3 h−1 (or 140-min generation time) (Fig. 2), and no difference between PTS and non-PTS substrates could be seen. Apparently, the physiologically relevant intracellular cAMP concentrations (Fig. 2) cannot be deduced easily from the extracellular cAMP concentrations (Table 1).
Conclusions.
To the best of our knowledge, we show here for the first time that below and above a specific growth rate of 0.3 h−1 and 0.7 h−1, the EIIACrr phosphorylation state and the activity of the cAMP-CRP-dependent promoter as represented by the scrYp activity became increasingly uncoupled from the growth rate. These deviations were not simply a consequence of the large inaccuracies in the corresponding tests. Rather, the results seem to indicate additional factors also modulating the PEP-to-pyruvate ratio, EIIACrr∼P levels, and cAMP production or the activity of cAMP-CRP-dependent promoters. For low growth rates, both promoter activities and the EIIACrr∼P level decreased roughly in parallel, while for high growth rates, the three key parameters became constant. Apparently, cAMP-dependent gene activation becomes less and less relevant during either very fast (μ ≥ 0.7 h−1), or very slow growth (μ ≤ 0.3 h−1), perhaps indicative of more global cellular changes in the corresponding cells. Thus, between specific growth rates of 0.4 h−1 to 0.8 h−1, the mean cell volume increases from 0.55 to 1.38 μm3, the number of ribosomes per cell doubles, and the ppGpp concentration decreases from 150 to 25 μM (29, 56). Such extreme physiological conditions which trigger stress responses seem to be increasingly controlled by other global antistress regulatory networks, e.g., RpoS in slow-growing and prolonged starving cells, and the “stringent-relaxed” control system which in fast-growing cells mainly determines growth rates. Similarly, other global regulatory systems can be expected to become active in cells growing under, e.g., phosphate or nitrogen limitation, and hence might override the regulation by cAMP-CRP. Finally, highly different EIIACrr phosphorylation levels during growth on poor growth substrates seem to indicate that under such extreme conditions PTS phosphorylation can be uncoupled from the PEP-to-pyruvate ratio, corroborating data pointing to additional factors which also control adenylate cyclase activity (34, 38).
Acknowledgments
We thank Andrea Focke, Helga Tietgens, and Marion Fleischer for excellent technical assistance.
K.J. was supported by the Deutsche Forschungsgemeinschaft through Sonderforschungsbereich 431, TP14.
Footnotes
Published ahead of print on 3 August 2007.
REFERENCES
- 1.Amin, N., and A. Peterkofsky. 1995. A dual mechanism for regulating cAMP levels in Escherichia coli. J. Biol. Chem. 270:11803-11805. [DOI] [PubMed] [Google Scholar]
- 2.Bettenbrock, K., S. Fischer, A. Kremling, K. Jahreis, T. Sauter, and E. Gilles. 2006. A quantitative approach to catabolite repression in Escherichia coli. J. Biol. Chem. 281:2578-2584. [DOI] [PubMed] [Google Scholar]
- 3.Bjarnason, J., C. M. Southward, and M. G. Surette. 2003. Genomic profiling of iron-responsive genes in Salmonella enterica serovar Typhimurium by high-throughput screening of a random promoter library. J. Bacteriol. 185:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botsford, J. L., and J. G. Harman. 1992. Cyclic AMP in procaryotes. Microbiol. Rev. 56:100-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin, A. M., D. A. Feldheim, and M. H. Saier, Jr. 1989. Altered transcriptional patterns affecting several metabolic pathways in strains of Salmonella typhimurium which overexpress the fructose regulon. J. Bacteriol. 171:2424-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crasnier-Mednansky, M., M. C. Park, W. K. Studley, and M. H. Saier, Jr. 1997. Cra-mediated regulation of Escherichia coli adenylate cyclase. Microbiology 143:785-792. [DOI] [PubMed] [Google Scholar]
- 7.Curtis, S. J., and W. Epstein. 1975. Phosphorylation of d-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J. Bacteriol. 122:1189-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boer, M., C. P. Broekhuizen, and P. W. Postma. 1986. Regulation of glycerol kinase by enzyme IIGlc of the phosphoenolpyruvate:carbohydrate phosphotransferase system. J. Bacteriol. 167:393-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Reuse, H., and A. Danchin. 1988. The ptsH, ptsI, and crr genes of Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: a complex operon with several modes of transcription. J. Bacteriol. 170:3827-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deutscher, J., C. Francke, and P. W. Postma. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70:939-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumay, V., A. Danchin, and M. Crasnier. 1996. Regulation of Escherichia coli adenylate cyclase activity during hexose phosphate transport. Microbiology 142:575-583. [DOI] [PubMed] [Google Scholar]
- 12.Epstein, W., L. B. Rothman-Denes, and J. Hesse. 1975. Adenosine 3:5-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc. Natl. Acad. Sci. USA 72:2300-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feucht, B. U., and M. H. Saier, Jr. 1980. Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J. Biol. Chem. 141:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geerse, R. H., J. Vanderpluijm, and P. W. Postma. 1989. The repressor of the PEP-fructose phosphotransferase system is required for the transcription of the pts gene of Escherichia coli. Mol. Gen. Genet. 218:348-352. [DOI] [PubMed] [Google Scholar]
- 15.Grenier, F. C., E. B. Waygood, and M. H. Saier, Jr. 1986. The bacterial phosphotransferase system: kinetic characterization of the glucose, mannitol, glucitol and N-acetylglucosamine systems. J. Cell. Biochem. 31:97-105. [DOI] [PubMed] [Google Scholar]
- 16.Hogema, B. M., J. C. Arents, R. Bader, K. Eijkemans, H. Yoshida, H. Takahashi, H. Alba, and P. W. Postma. 1998. Inducer exclusion in Escherichia coli by non-PTS substrates: the role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol. Microbiol. 30:487-498. [DOI] [PubMed] [Google Scholar]
- 17.Hogema, B. M., J. C. Arents, T. Inada, H. Aiba, K. vanDam, and P. W. Postma. 1997. Catabolite repression by glucose 6-phosphate, gluconate and lactose in Escherichia coli. Mol. Microbiol. 24:857-867. [DOI] [PubMed] [Google Scholar]
- 18.Holms, H. 1996. Flux analysis and control of the central metabolic pathways in Escherichia coli. FEMS Microbiol. Rev. 19:85-116. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson, G. R., C. A. Lee, J. E. Leonard, and M. H. Saier, Jr. 1983. Mannitol-specific enzyme II of the bacterial phosphotransferase system. I. Properties of the purified permease. J. Biol. Chem. 258:10748-10756. [PubMed] [Google Scholar]
- 20.Kao, K. C., L. M. Tran, and J. C. Liao. 2005. A global regulatory role of gluconeogenic genes in Escherichia coli revealed by transcriptome network analysis. J. Biol. Chem. 280:36079-36087. [DOI] [PubMed] [Google Scholar]
- 21.Kornberg, H. L. 1973. Nature and regulation of sugar uptake by Escherichia coli. Proc. Aust. Biochem. Soc. 6:4. [Google Scholar]
- 22.Kremling, A., K. Bettenbrock, B. Laube, K. Jahreis, J. Lengeler, and E. Gilles. 2001. The organization of metabolic reaction networks. III. Application for diauxic growth on glucose and lactose. Metab. Eng. 362-379. [DOI] [PubMed]
- 23.Lengeler, J., A. M. Auburger, R. Mayer, and A. Pecher. 1981. The phosphoenolpyruvate-dependent carbohydrate-phosphotransferase system enzymes II as chemoreceptors in chemotaxis of Escherichia coli K-12. Mol. Gen. Genet. 183:163-170. [DOI] [PubMed] [Google Scholar]
- 24.Lengeler, J., and H. Steinberger. 1978. Analysis of regulatory mechanisms controlling activity of hexitol transport-systems in Escherichia coli K-12. Mol. Gen. Genet. 167:75-82. [DOI] [PubMed] [Google Scholar]
- 25.Lengeler, J. W. 1975. Nature and properties of the hexitol transport systems in Escherichia coli. J. Bacteriol. 124:26-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lengeler, J. W., K. Bettenbrock, and R. Lux. 1994. Signal transduction through phosphotransferase systems, p. 182-188. In A.-M. Torriani-Gorini, E. Yagil, and S. Silver (ed.), Phosphate in microorganisms. Cellular and molecular biology. ASM Press, Washington, DC.
- 27.Lowry, O. H., J. Carter, J. B. Wood, and L. Glaser. 1971. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J. Biol. Chem. 246:6511-6521. [PubMed] [Google Scholar]
- 28.Lux, R., V. R. N. Munasinghe, F. Castellano, J. W. Lengeler, J. E. T. Corrie, and S. Khan. 1999. Elucidation of a PTS-carbohydrate chemotactic signal pathway in Escherichia coli using a time-resolved behavioral assay. Mol. Biol. Cell 10:1133-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marr, A. G. 1991. Growth rate of Escherichia coli. Microbiol. Rev. 55:316-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matin, A., and M. K. Matin. 1982. Cellular levels, excretion, and synthesis rates of cyclic AMP in Escherichia coli grown in continuous culture. J. Bacteriol. 149:801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 32.Misko, T., W. Mitchell, N. Meadow, and S. Roseman. 1987. Sugar transport by the bacterial phosphotransferase system. Reconstitution of inducer exclusion in Salmonella typhimurium membrane vesicles. J. Biol. Chem. 262:16261-16266. [PubMed] [Google Scholar]
- 33.Nelson, S., J. Wright, and P. Postma. 1983. The mechanism of inducer exclusion. Direct interaction between purified IIIGlc of the phosphoenolpyruvate-sugar phosphotransferase system and the lactose carrier of Escherichia coli. EMBO J. 2:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Notley-McRobb, L., A. Death, and T. Ferenci. 1997. The relationship between external glucose concentration and cAMP levels inside Escherichia coli: implications for models of phosphotransferase-mediated regulation of adenylate cyclase. Microbiology 143:1909-1918. [DOI] [PubMed] [Google Scholar]
- 35.Novotny, M. J., W. L Frederickson, E. B. Waygood, and M. H. Saier, Jr. 1985. Allosteric regulation of glycerol kinase by enzyme IIIGlc of the phosphotransferase system in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 162:810-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osumi, T., and M. H. Saier, Jr. 1982. Regulation of the lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose specific enzyme III to the lactose permease. Proc. Natl. Acad. Sci. USA 79:1457-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardee, A. B., and L. S. Prestidge. 1961. Initial kinetics of enzyme induction. Biochim. Biophys. Acta 49:77-88. [DOI] [PubMed] [Google Scholar]
- 38.Park, Y. H., B. R. Lee, Y. J. Seok, and A. Peterkofsky. 2006. In vitro reconstitution of catabolite repression in Escherichia coli. J. Biol. Chem. 281:6448-6454. [DOI] [PubMed] [Google Scholar]
- 39.Pastan, I., and R. L. Perlman. 1968. The role of the lac promoter locus in the regulation of beta-galactosidase synthesis by cyclic 3′,5′-adenosine monophosphate. Proc. Natl. Acad. Sci. USA 61:1336-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterkofsky, A., and C. Gazdar. 1971. Glucose and metabolism of adenosine 3′-5′-cyclic monophosphate in Escherichia coli. Proc. Natl. Acad. Sci. USA 68:2794-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterkofsky, A., Y. J. Seok, N. Amin, R. Thapar, S. Y. Lee, R. E. Klevit, E. B. Waygood, J. W. Anderson, J. Gruschus, H. Huq, and N. Gollop. 1995. The Escherichia coli adenylyl-cyclase complex: requirement of PTS proteins for stimulation by nucleotides. Biochemistry 34:8950-8959. [DOI] [PubMed] [Google Scholar]
- 42.Peterkofsky, A., I. Svenson, and N. Amin. 1989. Regulation of Escherichia coli adenylate cyclase activity by the phosphoenolpyruvate:sugar phosphotranferase system. FEMS Microbiol. Rev. 63:103-108. [DOI] [PubMed] [Google Scholar]
- 43.Postma, P. W., A. R. Broekhuizen, J. Schuitema, A. P. Vogler, and J. W. Lengeler. 1988. Carbohydrate transport and metabolism in Escherichia coli and Salmonella typhimurium: regulation by the PEP:carbohydrate phosphotransferase system, p. 43-52. In F. Palmieri and E. Quagliariello (ed.), Molecular basis of biomembrane transport. Elsevier Science Publishing, Amsterdam, The Netherlands.
- 44.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1996. Phosphoenolpyruvate:carbohydrate phosphotransferase systems, p. 1149-1174. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, DC. [Google Scholar]
- 45.Prost, J. F., D. Negre, C. Oudot, K. Murakami, A. Ishihama, A. J. Cozzone, and J. C. Cortay. 1999. Cra-dependent transcriptional activation of the icd gene of Escherichia coli. J. Bacteriol. 181:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramseier, T. M., D. Negre, J. C. Cortay, M. Scarabel, A. J. Cozzone, and M. H. Saier, Jr. 1993. In vitro binding of the pleiotropic transcriptional regulatory protein, FruR, to the fru, pps, ace, pts and icd operons of Escherichia coli and Salmonella typhimurium. J. Mol. Biol. 234:28-44. [DOI] [PubMed] [Google Scholar]
- 47.Reddy, P., N. Meadow, S. Roseman, and A. Peterkofsky. 1985. Reconstitution of regulatory properties of adenylate-cyclase in Escherichia coli extracts. Proc. Natl. Acad. Sci. USA 82:8300-8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roossien, F. F., M. Blaauw, and G. T. Robillard. 1984. Kinetics and subunit interaction of the mannitol-specific enzyme II of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system. Biochemistry 23:4934-4939. [DOI] [PubMed] [Google Scholar]
- 49.Roseman, S., and N. D. Meadow. 1990. Signal transduction by the bacterial phosphotransferase system: diauxie and the crr gene (J. Monod revisited). J. Biol. Chem. 265:2993-2996. [PubMed] [Google Scholar]
- 50.Ruijter, G. J. G., G. Vanmeurs, M. A. Verwey, P. W. Postma, and K. Vandam. 1992. Analysis of mutations that uncouple transport from phosphorylation in enzyme IIGlc of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system. J. Bacteriol. 174:2843-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saier, M. H., Jr., B. U. Feucht, and W. K. Mora. 1977. Sugar phosphate:sugar transphosphorylation and exchange group translocation catalyzed by the Enzyme II complexes of the bacterial phosphoenolpyruvate:sugar phosphotransferase system. J. Biol. Chem. 252:8899-8907. [PubMed] [Google Scholar]
- 52.Saier, M. H., Jr., and S. Roseman. 1976. Sugar transport. The crr mutation: its effect on repression of enzyme synthesis. J. Biol. Chem. 251:6598-6605. [PubMed] [Google Scholar]
- 53.Sauter, T., and E. D. Gilles. 2004. Modeling and experimental validation of the signal transduction via the Escherichia coli sucrose phosphotransferase system. J. Biotechnol. 110:181-199. [DOI] [PubMed] [Google Scholar]
- 54.Schmid, K., R. Ebner, J. Altenbuchner, R. Schmitt, and J. W. Lengeler. 1988. Plasmid-mediated sucrose metabolism in Escherichia coli K12: mapping of the scr genes of pUR400. Mol. Microbiol. 2:1-8. [DOI] [PubMed] [Google Scholar]
- 55.Schmid, K., R. Ebner, K. Jahreis, J. W. Lengeler, and F. Titgemeyer. 1991. A sugar-specific porin, ScrY, is involved in sucrose uptake in enteric bacteria A. Mol. Microbiol. 5:941-950. [DOI] [PubMed] [Google Scholar]
- 56.Shehata, T. E., and A. G. Marr. 1971. Effect of nutrient concentration on the growth of Escherichia coli. J. Bacteriol. 107:210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi, H., T. Inada, P. Postma, and H. Aiba. 1998. CRP down-regulates adenylate-cyclase activity by reducing the level of phosphorylated IIAGlc, the glucose-specific phosphotransferase protein, in Escherichia coli. Mol. Gen. Genet. 259:317-326. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka, S., S. A. Lerner, and E. C. C. Lin. 1967. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J. Bacteriol. 93:642-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang, J. K., and W. Epstein. 1983. Purification and characterization of adenylate cyclase from Escherichia coli K12. J. Biol. Chem. 258:3750-3758. [PubMed] [Google Scholar]