Abstract
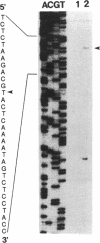
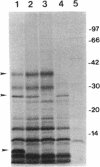
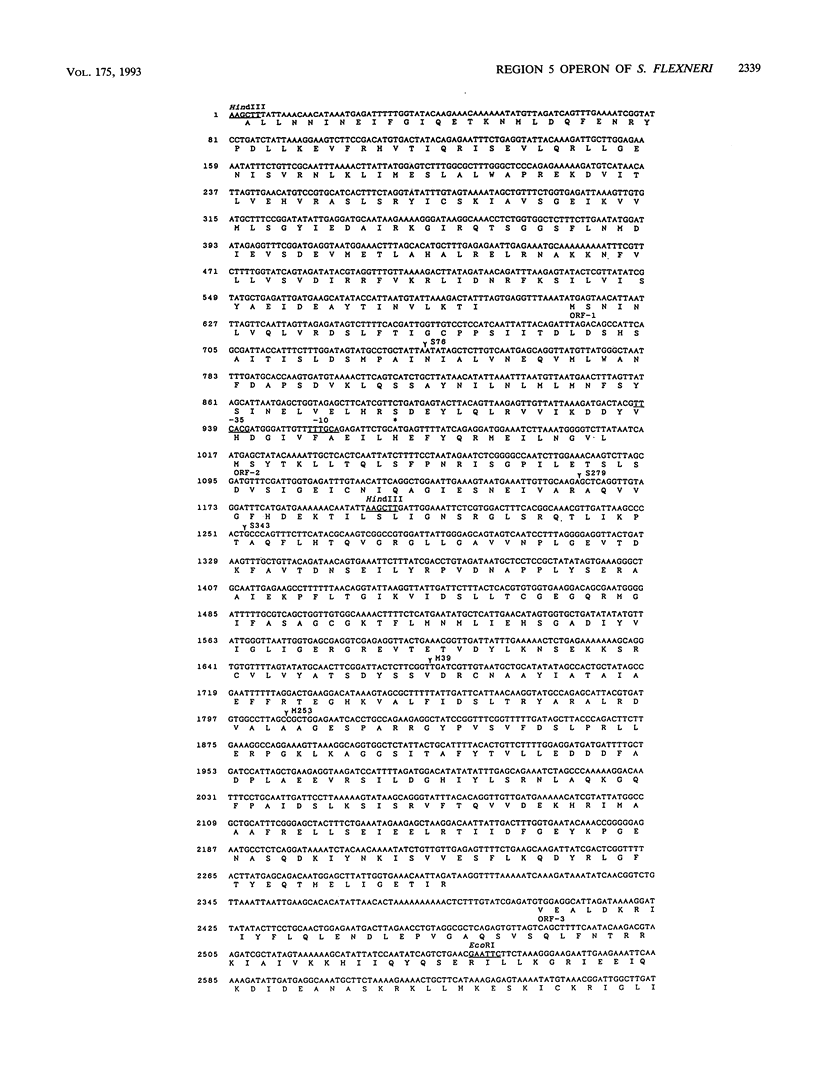
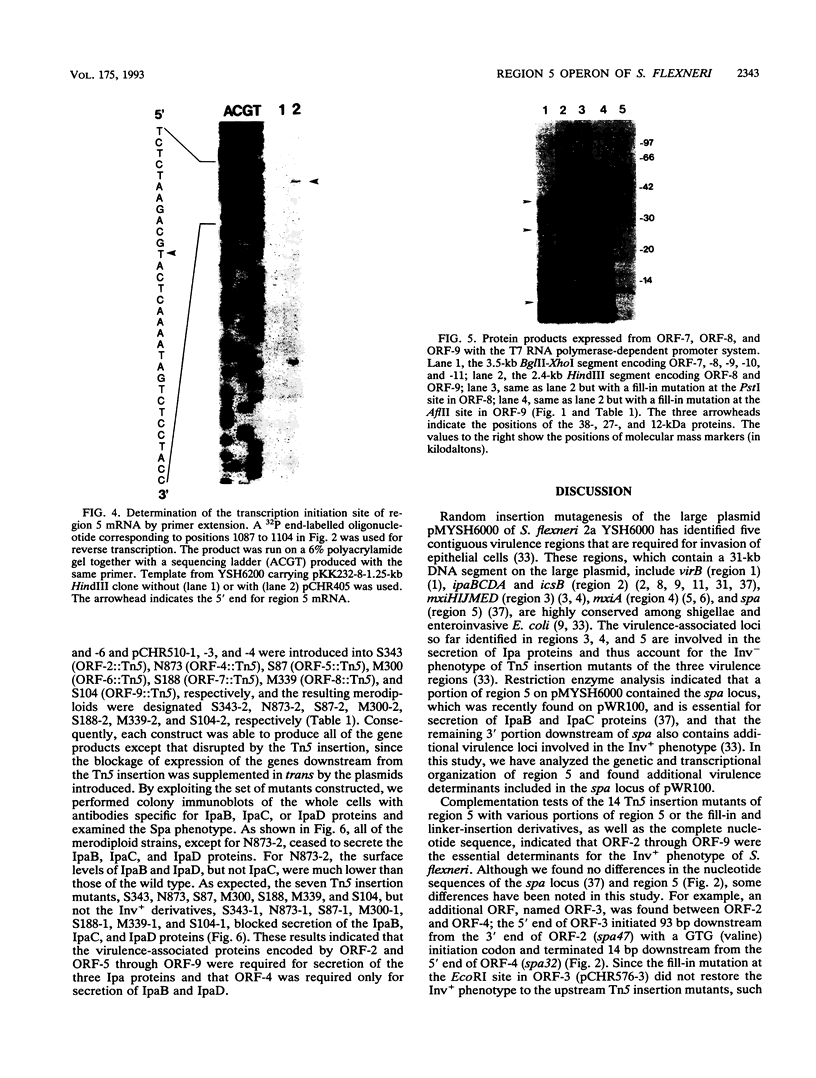
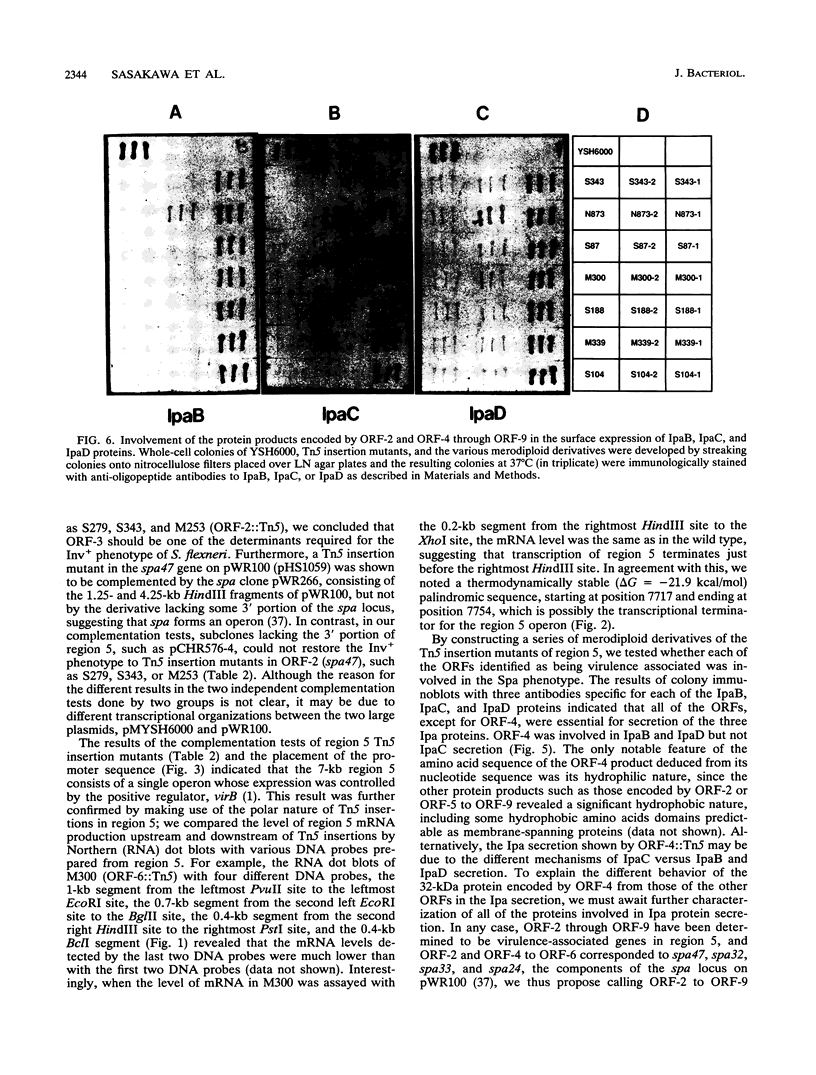
The 7-kb region 5 on the large 230-kb plasmid pMYSH6000 in Shigella flexneri 2a YSH6000 is one of the virulence-associated DNA segments required for the invasion of epithelial cells (C. Sasakawa, K. Kamata, T. Sakai, S. Makino, M. Yamada, N. Okada, and M. Yoshikawa, J. Bacteriol. 170:2480-2484, 1988). To elucidate the functional organization of region 5 and to determine the virulence-associated genes encoded by region 5, we performed insertion and deletion mutagenesis, DNA subcloning, and complete nucleotide sequencing of region 5 and found that region 5 contained 11 open reading frames (ORFs) named ORF-1 through ORF-11 which could be translated into proteins with molecular masses of 15.1, 47.5, 13.2, 33.0, 33.4, 24.2, 9.4, 28.5, 39.9, 9.1, and 10.4 kDa, respectively. Complementation tests of the 14 Tn5-induced noninvasive mutants of region 5 with the above plasmid constructs have indicated that region 5 consists of an operon and that ORF-2 through ORF-9, but not ORF-1, ORF-10, and ORF-11, are essential for invasion, and 7 of 8 ORFs (ORF-2 and ORF-4 through ORF-9) and presumably the remaining ORF (ORF-3) are required for secretion of the Ipa proteins. The transcriptional organization, as determined by a promoter-proving vector, S1 nuclease protection, and primer extension RNA sequencing analysis revealed that region 5 is transcribed from a promoter located 47 bp upstream of the 5' end of ORF-2 for the 47.5-kDa protein and that the promoter activity identified was regulated by the virB gene, the transcriptional activator on the 230-kb plasmid.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler B., Sasakawa C., Tobe T., Makino S., Komatsu K., Yoshikawa M. A dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol Microbiol. 1989 May;3(5):627–635. doi: 10.1111/j.1365-2958.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Allaoui A., Mounier J., Prévost M. C., Sansonetti P. J., Parsot C. icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol Microbiol. 1992 Jun;6(12):1605–1616. doi: 10.1111/j.1365-2958.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Allaoui A., Sansonetti P. J., Parsot C. MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri lpa invasins. Mol Microbiol. 1993 Jan;7(1):59–68. doi: 10.1111/j.1365-2958.1993.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Allaoui A., Sansonetti P. J., Parsot C. MxiJ, a lipoprotein involved in secretion of Shigella Ipa invasins, is homologous to YscJ, a secretion factor of the Yersinia Yop proteins. J Bacteriol. 1992 Dec;174(23):7661–7669. doi: 10.1128/jb.174.23.7661-7669.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G. P., Hromockyj A. E., Coker C., Maurelli A. T. Two novel virulence loci, mxiA and mxiB, in Shigella flexneri 2a facilitate excretion of invasion plasmid antigens. Infect Immun. 1991 Jun;59(6):1997–2005. doi: 10.1128/iai.59.6.1997-2005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G. P., Maurelli A. T. mxiA of Shigella flexneri 2a, which facilitates export of invasion plasmid antigens, encodes a homolog of the low-calcium-response protein, LcrD, of Yersinia pestis. Infect Immun. 1992 Aug;60(8):3287–3295. doi: 10.1128/iai.60.8.3287-3295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerswald E. A., Ludwig G., Schaller H. Structural analysis of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):107–113. doi: 10.1101/sqb.1981.045.01.019. [DOI] [PubMed] [Google Scholar]
- Baudry B., Kaczorek M., Sansonetti P. J. Nucleotide sequence of the invasion plasmid antigen B and C genes (ipaB and ipaC) of Shigella flexneri. Microb Pathog. 1988 May;4(5):345–357. doi: 10.1016/0882-4010(88)90062-9. [DOI] [PubMed] [Google Scholar]
- Baudry B., Maurelli A. T., Clerc P., Sadoff J. C., Sansonetti P. J. Localization of plasmid loci necessary for the entry of Shigella flexneri into HeLa cells, and characterization of one locus encoding four immunogenic polypeptides. J Gen Microbiol. 1987 Dec;133(12):3403–3413. doi: 10.1099/00221287-133-12-3403. [DOI] [PubMed] [Google Scholar]
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse J. M., Stover C. K., Oaks E. V., Venkatesan M., Kopecko D. J. Molecular cloning of invasion plasmid antigen (ipa) genes from Shigella flexneri: analysis of ipa gene products and genetic mapping. J Bacteriol. 1987 Jun;169(6):2561–2569. doi: 10.1128/jb.169.6.2561-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Ginocchio C., Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992 Jul;174(13):4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- High N., Mounier J., Prévost M. C., Sansonetti P. J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992 May;11(5):1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromockyj A. E., Maurelli A. T. Identification of Shigella invasion genes by isolation of temperature-regulated inv::lacZ operon fusions. Infect Immun. 1989 Oct;57(10):2963–2970. doi: 10.1128/iai.57.10.2963-2970.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Kamata K., Kurata T., Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986 Aug 15;46(4):551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- Michiels T., Vanooteghem J. C., Lambert de Rouvroit C., China B., Gustin A., Boudry P., Cornelis G. R. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J Bacteriol. 1991 Aug;173(16):4994–5009. doi: 10.1128/jb.173.16.4994-5009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. A., Buysse J. M., Oaks E. V. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect Immun. 1988 Nov;56(11):2933–2941. doi: 10.1128/iai.56.11.2933-2941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S., Tomura H., Matsuda K., Kagawa Y. Gene structure of the human mitochondrial adenosine triphosphate synthase beta subunit. J Biol Chem. 1988 Aug 15;263(23):11257–11262. [PubMed] [Google Scholar]
- Plano G. V., Barve S. S., Straley S. C. LcrD, a membrane-bound regulator of the Yersinia pestis low-calcium response. J Bacteriol. 1991 Nov;173(22):7293–7303. doi: 10.1128/jb.173.22.7293-7303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., d'Enfert C., Reyss I., Kornacker M. G. Genetics of extracellular protein secretion by gram-negative bacteria. Annu Rev Genet. 1990;24:67–90. doi: 10.1146/annurev.ge.24.120190.000435. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan G., Zhao J. L., Newton A. The cell cycle-regulated flagellar gene flbF of Caulobacter crescentus is homologous to a virulence locus (lcrD) of Yersinia pestis. J Bacteriol. 1991 Nov;173(22):7283–7292. doi: 10.1128/jb.173.22.7283-7292.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Sasakawa C., Yoshikawa M. Expression of four virulence antigens of Shigella flexneri is positively regulated at the transcriptional level by the 30 kiloDalton virF protein. Mol Microbiol. 1988 Sep;2(5):589–597. doi: 10.1111/j.1365-2958.1988.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Adler B., Tobe T., Okada N., Nagai S., Komatsu K., Yoshikawa M. Functional organization and nucleotide sequence of virulence Region-2 on the large virulence plasmid in Shigella flexneri 2a. Mol Microbiol. 1989 Sep;3(9):1191–1201. doi: 10.1111/j.1365-2958.1989.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Berg D. E. IS50-mediated inverse transposition. Discrimination between the two ends of an IS element. J Mol Biol. 1982 Aug 5;159(2):257–271. doi: 10.1016/0022-2836(82)90495-8. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Makino S., Yamada M., Okada N., Yoshikawa M. Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J Bacteriol. 1988 Jun;170(6):2480–2484. doi: 10.1128/jb.170.6.2480-2484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T., Nagai S., Okada N., Adler B., Yoshikawa M., Sasakawa C. Temperature-regulated expression of invasion genes in Shigella flexneri is controlled through the transcriptional activation of the virB gene on the large plasmid. Mol Microbiol. 1991 Apr;5(4):887–893. doi: 10.1111/j.1365-2958.1991.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Kopecko D. J. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9317–9321. doi: 10.1073/pnas.85.23.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Oaks E. V. Surface presentation of Shigella flexneri invasion plasmid antigens requires the products of the spa locus. J Bacteriol. 1992 Mar;174(6):1990–2001. doi: 10.1128/jb.174.6.1990-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler A. P., Homma M., Irikura V. M., Macnab R. M. Salmonella typhimurium mutants defective in flagellar filament regrowth and sequence similarity of FliI to F0F1, vacuolar, and archaebacterial ATPase subunits. J Bacteriol. 1991 Jun;173(11):3564–3572. doi: 10.1128/jb.173.11.3564-3572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]