Abstract
The ability of Escherichia coli cells to produce type 1 pili depends upon the orientation of the fimA promoter. The orientation depends upon the ratios of the FimB and FimE recombinases. Here, we report that the two-component response regulator RcsB influences the piliation state by controlling fimB and fimE transcription.
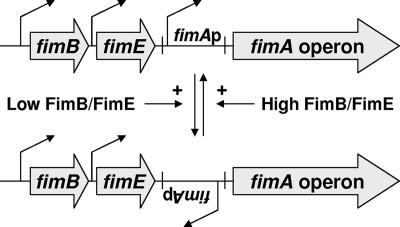
Type 1 pili are filamentous proteinaceous appendages produced by many members of the family Enterobacteriaceae (4) that play a major role in biofilm development and pathogenesis during the course of human infections (26). Escherichia coli cells can switch from a completely piliated state to a completely nonpiliated state (7). This ability depends on a process called phase variation (Fig. 1), in which a 314-bp invertible DNA element switches between two interconvertible orientations (1). When this element is in the “phase-on” orientation, the fimA promoter (fimAp) faces the fim operon and thus can drive its transcription. Since the fim operon includes genes for structural components and the machinery required for pilus assembly, cells in the “phase-on” orientation elaborate numerous type 1 pili. When the element is oriented in the “phase-off” state, the promoter faces the opposite direction, the promoter cannot drive fimA transcription, and the cells lack type 1 pili altogether. The invertible nature of the fimAp element depends upon two site-specific recombinases, FimB and FimE. Whereas FimE recombinase activity favors switching from “phase-on” to “phase-off,” FimB facilitates switching in both directions (16, 22, 23).
FIG. 1.
In E. coli, the abilities of cells to assemble type 1 pili depend upon the orientation of the fimA promoter. The orientation depends primarily on the ratio of the activities of FimB and FimE. A high activity favors the “phase-on” orientation (top); a low activity favors the “phase-off” orientation (bottom).
We previously reported that the fim operon is regulated by acetyl phosphate (35), a central metabolite that functions as a global signal (12, 34) by donating its phosphoryl group to a subset of response regulators (RRs) of the family of two-component signal transduction (2CST) pathways (8, 15a, 19). The most fundamental of 2CST pathways consists of an RR and a sensor kinase (SK). The SK autophosphorylates a conserved histidinyl residue, using ATP as its phosphoryl donor. The phospho-SK then serves as the phosphoryl donor to the RR, which autophosphorylates a conserved aspartyl residue (6, 29, 33). For a subset of RRs, the central metabolite acetyl phosphate can serve as an alternative phosphoryl donor (reviewed in reference 34). As its name implies, the RR is typically associated with a response domain, often one that permits binding to DNA. Thus, many RRs function as transcription factors (6, 29, 33).
The phosphorelay, a more complex version of the 2CST pathway, contains two additional domains. As in the fundamental 2CST pathway, ATP donates a phosphoryl group to the SK, which then donates it to an RR. In the phosphorelay, a histidine phosphotransferase transfers the phosphoryl group from the first RR to a second one (reviewed in references 2, 14, and 24). The core of the Rcs phosphorelay is composed of three proteins: RcsC (a hybrid SK-RR), RcsD (a histidine phosphotransferase also known as YojN), and RcsB (the terminal RR) (reviewed in references 10 and 20). RcsB can bind DNA either as a homodimer (3) or as a heterodimer in association with the accessory protein RcsA (31, 32). The stability of RcsA is controlled by the proteases Lon (30) and ClpYQ (18). Another accessory protein, the outer membrane lipoprotein RcsF, serves to activate the kinase activity of RcsC (20).
The Rcs phosphorelay is estimated to regulate some 5% of the Escherichia coli genome (reviewed in references 21 and 25). Most of these genes encode functions associated with the cell envelope. For instance, the Rcs phosphorelay activates the genes required for the biosynthesis of colanic acid, an extracellular polysaccharide required for biofilm development (13). It also activates the expression of several multiple-stress effectors that localize to the periplasm (3, 5), while repressing genes required for the biogenesis of flagella (11).
While studying the impact exerted by acetyl phosphate upon the network of 2CST pathways (12), we obtained electron microscopic evidence that led us to hypothesize that RcsB functions as a positive regulator of type 1 pili. Here, we report attempts to test that hypothesis.
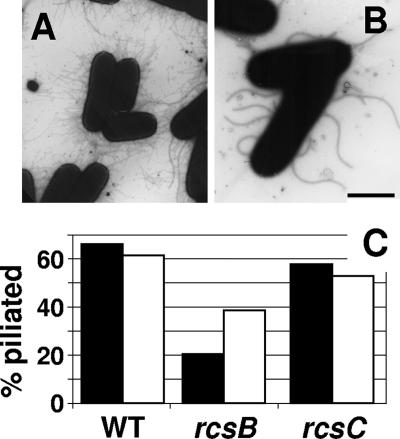
We grew cells at 37°C in tryptone broth (1% [wt/vol] tryptone, 0.5% [wt/vol] NaCl), harvested them during mid-exponential growth and shortly after entry into stationary phase, and monitored piliation by transmission electron microscopy as described previously (12). At both stages of growth, about 60% of wild-type (WT) cells (strain AJW678) (17) elaborated pili (Fig. 2A and C); isogenic rcsC mutant cells (strain AJW2144) (12) displayed similar behavior (Fig. 2C). In contrast, most isogenic rcsB mutant cells (strain AJW2143) (12) were nonpiliated (Fig. 2B). About 40% of the rcsB mutant cells elaborated pili after entry into stationary phase, while only 20% displayed pili during exponential growth (Fig. 2C).
FIG. 2.
RcsB enhances piliation. Cells were grown aerobically with 250-rpm agitation at 37°C in tryptone broth. Samples were negatively stained with 2% phosphotungstic acid (pH 7.0) and observed with a JEM-1200EXII electron microscope (JEOL, Tokyo, Japan). Micrographs were taken at an accelerating voltage of 80 kV. Transmission electron micrographs of WT cells (strain AJW678) (A) and rcsB mutant cells (strain AJW2143) (B) harvested at an optical density at 600 nm of 0.5 are shown. The thin and thick appendages are type 1 pili and flagella, respectively. The bar represents 2 μm. (C) Histogram showing percentages of piliated cells harvested at either 0.5 (solid bar) or 1.0 (open bar) optical density units at 600 nm. The sample sizes ranged from 83 to 178 cells.
The observation that the percentage of piliated cells was affected rather than the number of type 1 pili per cell or their length led us to hypothesize that RcsB influenced the orientation of the 314-bp fimAp invertible element. To test this hypothesis, we initially performed multiplex PCR amplifications on chromosomal DNA extracted from the rcsB mutant, the rcsC mutant, or their WT parent, using oligonucleotide primers specific for the “phase-on” and “phase-off” orientations of the invertible element (28) or, as a control, the E. coli ftsZ gene (27).
We previously reported that pH values and salt relevant to murine urine exerted substantial effects on phase variation (27). Therefore, we grew cells at 37°C to mid-exponential phase in LB (1% [wt/vol] peptone 140, 0.5% [wt/vol] yeast extracts, 1% [wt/vol] glycerol, 0.1 M sodium phosphate, 0.5% [wt/vol] NaCl) at either neutral pH (7.0) or acidic pH (5.5) and in either the presence of additional NaCl (final concentration, 490 mM [referred to as high salt]) or its absence (final concentration, 90 mM [referred to as low salt]).
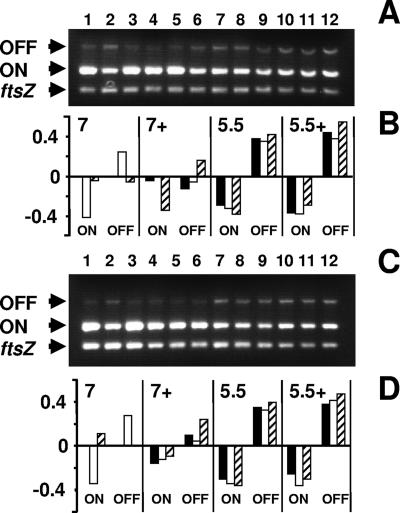
Overall, the PCR results followed those of our previous study (27): more WT cells grown at neutral pH (Fig. 3A, lanes 1 and 4, and B) than cells grown at acidic pH (Fig. 3A, lanes 7 and 10, and B) positioned their invertible fimAp element in the “phase-on” orientation. This analysis also showed that rcsB mutant cells grown under neutral-pH, low-salt conditions (Fig. 3A, lane 2) positioned their invertible element more in the “phase-off” orientation than did cells of either their WT parent (lane 1) or the isogenic rcsC mutant (lane 3) grown under the same conditions. Under neutral pH, high-salt conditions, the rcsC mutant (lane 6) appeared to position the fimAp promoter element less in the “phase-on” orientation than did either its WT parent (lane 4) or the rcsB mutant (lane 5). At acidic pH, regardless of salt, all strains produced similar “phase-off” and “phase-on” distributions (lanes 7 to 12).
FIG. 3.
Determination of the invertible element orientation by PCR. (A) Analysis was performed on chromosomal DNA isolated from WT cells (strain AJW678), an rcsB mutant (strain AJW2143), and an rcsC mutant (strain AJW2144). Cells were harvested during mid-exponential phase following aerobic growth with 250-rpm agitation at 37°C in pH 7.0 LB medium with either no added NaCl (low salt) or 400 mM added NaCl (+; high salt) or in pH 5.5 LB medium with either no added NaCl (low salt) or 400 mM NaCl (high salt). Multiplex PCRs were set up with INV and FIMA primers to amplify “phase-on”-oriented DNA (ON; 450-bp product) (28), FIME and INV primers to amplify “phase-off”-oriented DNA (OFF, 750-bp product) (28), and EcFtsZ 1 and 2 primers to amplify the ftsZ gene (302-bp product) (27). Each multiplex was run at least three separate times. The lanes were loaded as follows: lane 1, AJW678 (pH 7.0, low salt); lane 2, AJW2143 (pH 7.0, low salt); lane 3, AJW2144 (pH 7.0, low salt); lane 4, AJW678 (pH 7.0, high salt); lane 5, AJW2143 (pH 7.0, high salt); lane 6, AJW2144 (pH 7.0, high salt); lane 7, AJW678 (pH 5.5, low salt); lane 8, AJW2143 (pH 5.5, low salt); lane 9, AJW2144 (pH 5.5, low salt); lane 10, AJW678 (pH 5.5, high salt); lane 11, AJW2143 (pH 5.5, high salt); and lane 12, AJW2144 (pH 5.5, high salt). (B) Quantification of the data from panel A. Using ImageQuant software (Molecular Dynamics), the number of pixels for each band was quantified. For each lane, the intensities of the OFF and ON states were corrected to the intensity of the ftsZ band. The corrected values for both states were standardized to the respective WT band (lane 1). Since the resultant values were plotted as log10 numbers, the WT strain for both the OFF and ON states had a value of zero, while increased and decreased PCR products resulted in positive and negative values, respectively. Solid bars, strain AJW678 (WT); open bars, strain AJW2143 (rcsB mutant); hatched bars, strain AJW2144 (rcsC mutant). (C) Analysis was performed on chromosomal DNA isolated from WT cells (strain AJW678), an rcsB mutant (strain AJW2143), and an rcsB mutant complemented with the pHRcsB plasmid (strain AJW2307) without induction. Multiplex PCR amplifications were performed with the same primer pairs, using DNAs isolated from the strains grown as described for panel A. Each multiplex was run at least three separate times. Lane 1, AJW678 (pH 7.0, low salt); lane 2, AJW2143 (pH 7.0, low salt); lane 3, AJW2307 (pH 7.0, low salt); lane 4, AJW678 (pH 7.0, high salt); lane 5, AJW2143 (pH 7.0, high salt); lane 6, AJW2307 (pH 7.0, high salt); lane 7, AJW678 (pH 5.5, low salt); lane 8, AJW2143 (pH 5.5, low salt); lane 9, AJW2307 (pH 5.5, low salt); lane 10, AJW678 (pH 5.5, high salt); lane 11, AJW2143 (pH 5.5, high salt); and lane 12, AJW 2307 (pH 5.5, high salt). All PCR products were subjected to electrophoresis on 1.5% agarose gels. (D) Quantification of the data from panel C as described for panel B. Solid bars, strain AJW678 (WT); open bars, strain AJW2143 (rcsB mutant); hatched bars, strain AJW2307 (complemented rcsB mutant).
To confirm the rcsB finding, we complemented the rcsB mutant with a plasmid containing a His-tagged WT rcsB gene (pHRcsB) (5), harvested cells during mid-exponential growth, and compared the complemented strain to the WT strain and the rcsB mutant parent (Fig. 3C and D). At the neutral-pH, low-salt condition, the complemented rcsB mutant (lane 3) oriented its invertible element more like the WT strain (lane 1) than the rcsB mutant (lane 2). Similar results were obtained when cells were harvested following entry into stationary phase (data not shown).
Taken together, these results support the hypothesis that RcsB regulates piliation under neutral-pH, low-salt conditions by influencing the orientation of the fimAp invertible element. These results also suggest that RcsC may influence the positioning of the invertible element under high-salt growth conditions. Finally, they show that neither RcsB nor RcsC exerts much influence at acidic pH and that some other factor must be involved.
To determine how RcsB may regulate the orientation of the invertible element, we asked whether RcsB and/or RcsC influences the transcription of fimB, fimE, or both recombinase genes. The rcsB mutant, the rcsC mutant, and their WT parent were transformed with single-copy plasmids pJB5A and pJLE4-3, which express the transcriptional fusions fimB-lacZYA and fimE-lacZYA, respectively (27). Because pH and salt have been shown to influence the transcriptional states of both fimB and fimE (27), we performed these reporter studies under the growth conditions used for the PCR analyses.
When cells were grown at acidic pH in the presence of either the low or high salt, transcription of either fimB or fimE was largely unaffected by the status of the Rcs phosphorelay (Table 1). This is consistent with the lack of any significant effect upon the populations of invertible elements (Fig. 3). In contrast, when cells were grown at neutral pH, the states of certain Rcs phosphorelay components influenced transcription. Furthermore, the critical Rcs component and the promoter affected depended upon the salt. For example, at the low salt, rcsB mutant cells transcribed fimB at significantly reduced levels relative to their WT parent and the rcsC mutant (Table 1). This effect appears to be specific because expression of the His-tagged WT rcsB allele from a compatible plasmid (pHRcsB) restored transcription to WT levels. In contrast, growth in high-salt medium resulted in a distinctly different pattern. Under this condition, relative to WT cells, both the rcsB and rcsC mutants exhibited elevated fimE transcription. Here, both the RcsB and RcsC effects appeared to be specific because expression from a compatible plasmid either with the His-tagged WT rcsB allele in the rcsB mutant (Table 1) or with the WT rcsC allele (pSG980) (9) in the rcsC mutant restored fimE transcription to WT levels (data not shown). In contrast, the vector controls had no effect (data not shown).
TABLE 1.
Effects of pH and salt on fimB-lacZ and fimE-lacZ fusions in WT E. coli compared to effects on isogenic rcsB and rcsC mutants
| Fusion and strain | Expression level (Miller units) under indicated growth conditiona
|
|||
|---|---|---|---|---|
| pH 7.0 | pH 7.0 (+) | pH 5.5 | pH 5.5 (+) | |
| fimB-lacZ fusion | ||||
| WT | 345 ± 30 | 259 ± 36 | 271 ± 20 | 219 ± 23 |
| rcsB | 201 ± 19b | 229 ± 27 | 274 ± 62 | 182 ± 48 |
| rcsB/pRcsBc | 329 ± 21 | 315 ± 13 | 297 ± 11 | 231 ± 11 |
| rcsC | 305 ± 34 | 235 ± 36 | 284 ± 51 | 238 ± 57 |
| fimE-lacZ fusion | ||||
| WT | 335 ± 26 | 318 ± 54 | 252 ± 35 | 237 ± 26 |
| rcsB | 332 ± 34 | 463 ± 68 | 260 ± 54 | 221 ± 60 |
| rcsB/pRcsB | 339 ± 32 | 377 ± 70 | 231 ± 55 | 245 ± 13 |
| rcsC | 365 ± 21 | 437 ± 53 | 260 ± 32 | 253 ± 48 |
Values indicate fimB and fimE promoter expression in terms of β-galactosidase activity and are means ± standard deviations from at least three independent experiments. Cells were grown in LB at pH 7.0 or pH 5.5 with the low or high (+) salt and harvested during mid-exponential phase.
Bold denotes that the differential expression is significant (P < 0.05) as determined by Student's t test.
rcsB/pRcsB is the rcsB mutant transformed with pHRcsB, which encodes a His-tagged WT rcsB allele under the control of the lac promoter. Induction, however, was unnecessary.
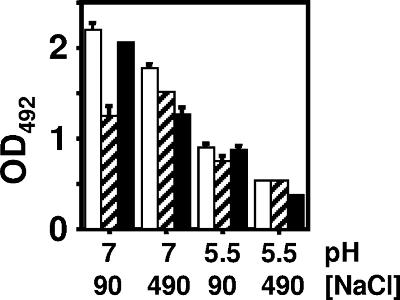
The PCR analysis (Fig. 3) and the fim-lacZ fusion data (Table 1) supported the hypothesis that RcsB can influence the orientation of the fimAp invertible element by controlling the transcription of the recombinase genes fimB and fimE. This RcsB-dependent behavior occurred only at neutral pH and depended upon RcsC only in the presence of the high salt. On the basis of these observations, we predicted that type 1 pilus expression would be affected in a condition-dependent manner by mutations in rcsB and rcsC. To test this prediction, enzyme immunoassays were performed according to the procedure of Hultgren et al. (15). As predicted, at neutral pH with the low salt, the rcsB mutant strain displayed significantly reduced type 1 pilus expression relative either to its WT parent or to the rcsC mutant (Fig. 4). At neutral pH with the high salt, however, both the rcsB and rcsC mutants displayed lower levels of type 1 pili than did their WT parent. In contrast, at acidic pH (regardless of salt), the status of rcsB or rcsC had, at most, a minor effect.
FIG. 4.
Enzyme immunoassay of strains. WT (AJW678; open columns), rcsB mutant (AJW2143; striped columns), and rcsC mutant (AJW2144; filled columns) cells were harvested in stationary phase after growth in pH 7.0 and 5.5 LB media with either 90 mM or 490 mM NaCl. Optical densities at 492 nm (OD492) were determined. The mean values ± standard deviations for two separate runs are indicated.
At neutral pH, therefore, it appears that RcsB helps mediate the inversion of the fimAp element either by increasing fimB transcription (low salt) or by decreasing fimE transcription (high salt). These results are consistent with our observation that significantly fewer rcsB mutant cells than cells of their WT parent expressed pili. Whether RcsB acts directly upon fim transcription awaits further experimentation; however, inspection of the sequence upstream of the fimB and fimE open reading frames reveals several sequences with some similarity to RcsB and RcsAB boxes (31, 32). Although RcsC appeared to have no discernible effect under neutral-pH, low-salt growth conditions, it appeared to influence the orientation of the invertible element under neutral-pH, high-salt conditions, presumably by decreasing fimE transcription. These results argue for the hypothesis that RcsB receives its phosphoryl groups from RcsC (its cognate SK) under neutral-pH, high-salt conditions but from an alternative donor (e.g., acetyl phosphate or a noncognate SK) under neutral-pH, low-salt conditions.
Our previous work showed that acidic growth conditions reduce type 1 pilus expression, while implicating the EnvZ/OmpR 2CST pathway as a fim inhibitor at least in the presence of the high salt (27). The current study supports the former conclusion and shows that it occurs in an RcsB-independent manner. Whether fim regulation under acidic growth conditions involves the EnvZ/OmpR pathway or some other acid tolerance gene product is currently under examination.
In summary, we propose that the fim locus is part of the RcsB regulon and that this global regulator inversely affects the transcription of fimB and fimE. Furthermore, this inverse regulation increases the probability of the “phase-on” orientation of the fimAp element and consequently the elaboration of type 1 pili. On the basis of the current study and our previous report (12), we can conclude that RcsB enhances the production of type 1 pili as well as the synthesis of the capsule, while inhibiting the biogenesis of flagella. Since these surface organelles play critical and/or essential roles in biofilm development and urinary tract infections, RcsB should now be considered a coordinator of these processes.
Acknowledgments
We thank Sylvia Reimann for strain construction.
This work was supported by NIH grant GM066130, awarded to A.J.W., grant RAI065432A, awarded to W.R.S., and a grant from the CREST project of JST to S.-I.A.
Footnotes
Published ahead of print on 20 July 2007.
REFERENCES
- 1.Abraham, S. N., J. P. Babu, C. S. Giampapa, D. L. Hasty, W. A. Simpson, and E. H. Beachey. 1985. Protection against Escherichia coli-induced urinary tract infections with hybridoma antibodies directed against type 1 fimbriae or complementary d-mannose receptors. Infect. Immun. 48:625-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleby, J. L., J. S. Parkinson, and R. B. Bourret. 1996. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell 86:845-848. [DOI] [PubMed] [Google Scholar]
- 3.Carballes, F., C. Bertrand, J. P. Bouche, and K. Cam. 1999. Regulation of Escherichia coli cell division genes ftsA and ftsZ by the two-component system rcsC-rcsB. Mol. Microbiol. 34:442-450. [DOI] [PubMed] [Google Scholar]
- 4.Clegg, S., and G. F. Gerlach. 1987. Enterobacterial fimbriae. J. Bacteriol. 169:934-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davalos-Garcia, M., A. Conter, I. Toesca, C. Gutierrez, and K. Cam. 2001. Regulation of osmC gene expression by the two-component system rcsB-rcsC in Escherichia coli. J. Bacteriol. 183:5870-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta, R., L. Qin, and M. Inouye. 1999. Histidine kinases: diversity of domain organization. Mol. Microbiol. 34:633-640. [DOI] [PubMed] [Google Scholar]
- 7.Eisenstein, B. I. 1981. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science 214:337-339. [DOI] [PubMed] [Google Scholar]
- 8.Feng, J., M. R. Atkinson, W. McCleary, J. B. Stock, B. L. Wanner, and A. J. Ninfa. 1992. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J. Bacteriol. 174:6061-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrieres, L., and D. J. Clarke. 2003. The RcsC sensor kinase is required for normal biofilm formation in Escherichia coli K-12 and controls the expression of a regulon in response to growth on a solid surface. Mol. Microbiol. 50:1665-1682. [DOI] [PubMed] [Google Scholar]
- 10.Francez-Charlot, A., J. Filee, M. P. Castanie-Cornet, and K. Cam. 2005. Regulation of flhDC by the His-Asp phosphorelay RcsCDB, p. 93-106. In B. M. Pruss (ed.), Global regulatory networks in enteric bacteria. Research Signpost, Kerala, India.
- 11.Francez-Charlot, A., B. Laugel, A. Van Gemert, N. Dubarry, F. Wiorowski, M.-P. Castanie-Cornet, C. Gutierrez, and K. Cam. 2003. RcsCDB His-Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol. Microbiol. 49:823-832. [DOI] [PubMed] [Google Scholar]
- 12.Fredericks, C. E., S. Shibata, S.-I. Aizawa, S. A. Reimann, and A. J. Wolfe. 2006. Acetyl phosphate-sensitive regulation of flagellar biogenesis and capsular biosynthesis depends on the Rcs phosphorelay. Mol. Microbiol. 61:734-747. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman, S., P. Trisler, and A. Torres-Cabassa. 1985. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J. Bacteriol. 162:1111-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoch, J. A., and T. J. Silhavy (ed.). 1995. Two-component signal transduction. American Society for Microbiology, Washington, DC.
- 15.Hultgren, S. J., J. L. Duncan, A. J. Schaeffer, and S. K. Amundsen. 1990. Mannose-sensitive haemagglutination in the absence of piliation in Escherichia coli. Mol. Microbiol. 4:1311-1318. [DOI] [PubMed] [Google Scholar]
- 15a.Klein, A. H., A. Shulla, S. A. Reimann, D. H. Keating, and A. D. Wolfe. 2007. The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators. J. Bacteriol. 189:5574-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klemm, P. 1986. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 5:1389-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumari, S., C. M. Beatty, D. F. Browning, S. J. Busby, E. J. Simel, G. Hovel-Miner, and A. J. Wolfe. 2000. Regulation of acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 182:4173-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo, M. S., K. P. Chen, and W. F. Wu. 2004. Regulation of RcsA by the ClpYQ (HslUV) protease in Escherichia coli. Microbiology 150:437-446. [DOI] [PubMed] [Google Scholar]
- 19.Lukat, G. S., W. R. McCleary, A. M. Stock, and J. B. Stock. 1992. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc. Natl. Acad. Sci. USA 89:718-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majdalani, N., and S. Gottesman. 2005. The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 59:379-405. [DOI] [PubMed] [Google Scholar]
- 21.Majdalani, N., M. Heck, V. Stout, and S. Gottesman. 2005. Role of RcsF in signaling to the Rcs phosphorelay pathway in Escherichia coli. J. Bacteriol. 187:6770-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClain, M. S., I. C. Blomfield, K. J. Eberhardt, and B. I. Eisenstein. 1993. Inversion-independent phase variation of type 1 fimbriae in Escherichia coli. J. Bacteriol. 175:4335-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClain, M. S., I. C. Blomfield, and B. I. Eisenstein. 1991. Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J. Bacteriol. 173:5308-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizuno, T. 1998. His-Asp phosphotransfer signal transduction. J. Biochem. (Tokyo) 123:555-563. [DOI] [PubMed] [Google Scholar]
- 25.Prüss, B. M., C. Besemann, A. Denton, and A. J. Wolfe. 2006. A complex transcription network controls the early stages of biofilm development by Escherichia coli. J. Bacteriol. 188:3731-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schilling, J. D., M. A. Mulvey, and S. J. Hultgren. 2001. Structure and function of Escherichia coli type 1 pili: new insight into the pathogenesis of urinary tract infections. J. Infect. Dis. 183:S36-S40. [DOI] [PubMed] [Google Scholar]
- 27.Schwan, W. R., J. L. Lee, F. A. Lenard, B. T. Matthews, and M. T. Beck. 2002. Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic Escherichia coli. Infect. Immun. 70:1391-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwan, W. R., H. S. Seifert, and J. L. Duncan. 1992. Growth conditions mediate differential transcription of fim genes involved in phase variation of type 1 pili. J. Bacteriol. 174:2367-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 30.Stout, V., A. Torres-Cabassa, M. R. Maurizi, D. Gutnick, and S. Gottesman. 1991. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J. Bacteriol. 173:1738-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wehland, M., and F. Bernhard. 2000. The RcsAB box. Characterization of a new operator essential for the regulation of exopolysaccharide biosynthesis in enteric bacteria. J. Biol. Chem. 275:7013-7020. [DOI] [PubMed] [Google Scholar]
- 32.Wehland, M., C. Kiecker, D. L. Coplin, O. Kelm, W. Saenger, and F. Bernhard. 1999. Identification of an RcsA/RcsB recognition motif in the promoters of exopolysaccharide biosynthetic operons from Erwinia amylovora and Pantoea stewartii subspecies stewartii. J. Biol. Chem. 274:3300-3307. [DOI] [PubMed] [Google Scholar]
- 33.West, A. H., and A. M. Stock. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26:369-376. [DOI] [PubMed] [Google Scholar]
- 34.Wolfe, A. J. 2005. The acetate switch. Microbiol. Mol. Biol. Rev. 69:12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfe, A. J., D.-E. Chang, J. D. Walker, J. E. Seitz-Partridge, M. D. Vidaurri, C. F. Lange, B. M. Pruess, M. C. Henk, J. C. Larkin, and T. Conway. 2003. Evidence that acetyl phosphate functions as a global signal during biofilm development. Mol. Microbiol. 48:977-988. [DOI] [PubMed] [Google Scholar]