Abstract
The function(s) of gram-positive wall teichoic acid is emerging with recent findings that it is an important virulence factor in the pathogen Staphylococcus aureus and that it is crucial to proper rod-shaped cell morphology of Bacillus subtilis. Despite its importance, our understanding of teichoic acid biosynthesis remains incomplete. The TagB protein has been implicated in the priming step of poly(glycerol phosphate) wall teichoic acid synthesis in B. subtilis. Work to date indicates that the TagB protein is localized to the membrane, where it adds a single glycerol phosphate residue to the nonreducing end of the undecaprenol-phosphate-linked N-acetylmannosamine-β(1,4)-N-acetylglucosamine-1-phosphate. Thus, membrane association is critical to TagB function. In this work we elucidate the mechanism of TagB membrane localization. We report the identification of a membrane targeting determinant at the amino terminus of TagB that is necessary and sufficient for membrane localization. The putative amphipathicity of this membrane targeting determinant was characterized and shown to be required for TagB function but not localization. This work shows for the first time that the amino terminus of TagB mediates membrane targeting and protein function.
Wall teichoic acids (WTAs) are phosphate-rich anionic extracellular polysaccharides found covalently bound to peptidoglycan in gram-positive bacteria. In the model gram-positive bacterium Bacillus subtilis 168, the major WTA is a poly(glycerol phosphate) polymer. Biosynthesis of WTA is carried out by enzymes encoded by the tag genes (19, 21, 32, 33). The inability to mutate specific tag genes suggested that WTA was indispensable for cell viability (1, 2, 5, 6, 28). Remarkably, while these genes were indispensable in single gene deletion experiments, the dispensability phenotype was contextual. Recent studies have revealed that the first step (tagO) of teichoic acid biosynthesis is dispensable in both B. subtilis and Staphylococcus aureus (12, 13, 37). Indeed, we demonstrated that the late-acting genes tagB, tagD, and tagF could be deleted in both B. subtilis and S. aureus only in the presence of an accompanying deletion in tagO, encoding the first enzyme in the teichoic acid biosynthetic pathway (12, 13). Nevertheless, mutants with mutations in the first step of teichoic acid synthesis (tagO) have shown that the polymer is vital for rod shape in B. subtilis (12) and for virulence in S. aureus infection (37).
Sequence-based homology of Tag proteins coupled with the known chemical structure of WTA has led to the proposal of a plausible biosynthetic model (18), whereby WTA synthesis is initiated on the cytoplasmic face of the membrane on an undecaprenol phosphate molecule. Polymer synthesis is carried out stepwise through addition of N-acetylglucosamine-1-phosphate, N-acetylmannosamine, and glycerol phosphate to the membrane-embedded prenol phosphate substrate. Glycerol phosphate addition, from the activated precursor CDP-glycerol, is proposed to be catalyzed by two enzymes, TagB and TagF, which share extensive sequence homology. We reported elsewhere that TagB catalyzed a reaction that was consistent with the addition of a single labeled glycerol phosphate to a membrane-bound acceptor (3). Furthermore, mutagenic analysis of the two proteins indicated a mechanistic similarity between these enzymes that implicated two conserved histidines as crucial catalytic residues (30). Subsequently TagB was shown to catalyze glycerol phosphate addition to an acceptor analogue of undecaprenol-phosphate-linked disaccharide (14). Thus, all evidence to date supports a role for TagB as a primase whose product is the substrate for TagF, the teichoic acid polymerase.
We also previously reported that TagB was localized to the cytoplasmic face of the B. subtilis membrane (3). Interestingly, we found that this association with the membrane was peripheral and not integral as predicted by sequence-based topology analysis. The same analysis indicated that a predicted amino-terminal transmembrane helix possessed an amphipathic character where polar but uncharged residues were oriented to the same face of the helix. In work reported here we characterize the role of the amino terminus of TagB in membrane association and function. We show by truncation analysis and fusion to a heterologous carrier protein that this region of TagB carries a membrane targeting determinant that is necessary and sufficient for efficient membrane association. In addition, we characterize the putative amphipathicity of this region and show that insertional mutagenesis, designed to disrupt the amphipathic helical face, has no effect on membrane localization but a profound impact on TagB function, suggesting that this region of the protein contains an unusual amphipathic helix. Taken together, our results suggest that the amino terminus of TagB mediates membrane targeting and protein function.
MATERIALS AND METHODS
Bacterial strains, reagents, and general methods.
The strains and plasmids and the oligonucleotides used in this study are listed in Tables 1 and 2, respectively. All chemicals, unless otherwise noted, were purchased from Sigma (Oakville, ON, Canada). General cloning methods for Escherichia coli and B. subtilis were used according to established protocols (11, 29). Reagents for molecular cloning were purchased from New England Biolabs (Beverly, MA). Cultures were grown on Luria-Bertani (LB) medium with antibiotic selection as follows, where appropriate: 50 μg/ml ampicillin, 10 μg/ml chloramphenicol (CHL), and 25 μg/ml kanamycin.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype/description | Reference or source |
|---|---|---|
| Strains | ||
| B. subtilis | ||
| EB6 | hisA1 argC4 metC3 | L5087 (6) |
| EB921 | hisA1 argC4 metC3 amyE::cat86 xylR PxylAtagBN30phoA | This work |
| EB925 | EB6 with pRBtagBΔN30gfp | This work |
| EB486 | pheA1 purA16 hisA35 trpC2 tag-1 | L5058 (22) |
| EB1240 | pheA1 purA16 hisA35 trpC2 tag-1 amyE::cat86 xylR PxylA | 30 |
| EB1652 | pheA1 purA16 hisA35 trpC2 tag-1 amyE::cat86 xylR PxylAtagBphoA | This work |
| EB1653 | pheA1 purA16 hisA35 trpC2 tag-1 amyE::cat86 xylR PxylAtagB+1phoA | This work |
| EB1654 | pheA1 purA16 hisA35 trpC2 tag-1 amyE::cat86 xylR PxylAtagB+2phoA | This work |
| EB1655 | pheA1 purA16 hisA35 trpC2 tag-1 amyE::cat86 xylR PxylAtagB+3phoA | This work |
| EB1656 | pheA1 purA16 hisA35 trpC2 tag-1 amyE::cat86 xylR PxylAtagB+4phoA | This work |
| EB1696 | pheA1 purA16 hisA35 trpC2 tag-1 amyE::cat86 xylR PxylAtagBN30+1phoA | This work |
| EB1697 | pheA1 purA16 hisA35 trpC2 tag-1 amyE::cat86 xylR PxylAtagBN30+2phoA | This work |
| EB1698 | pheA1 purA16 hisA35 trpC2 tag-1 amyE::cat86 xylR PxylAtagBN30+3phoA | This work |
| EB1699 | pheA1 purA16 hisA35 trpC2 tag-1 amyE::cat86 xylR PxylAtagBN30+4phoA | This work |
| E. coli | ||
| Novablue | endA1 hsdR17(rK-12− mK-12+) supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proA+B+lacIqZΔM15::Tn10 (Tcr)] | Novagen |
| MC1061 | [araD139] Δ(araA-leu)7697 Δ(codB-lacI)3 galK16 galE15 λ−mcrA mutant e14 mutant relA1 rpsL150(Strr) spoT1 mcrB mutant hsdR2 | 9 |
| Plasmids | ||
| pBStagB | pBluescript with wild-type tagB from B. subtilis | 3 |
| pSWEET-bgaB | B. subtilis xylose-inducible ectopic expression vector | 4 |
| pSWEET-tagBphoA | pSWEET with tagBphoA insert | This work |
| pSWEET-tagB+1phoA | pSWEET with tagB+1phoA insert | This work |
| pSWEET-tagB+2phoA | pSWEET with tagB+2phoA insert | This work |
| pSWEET-tagB+3phoA | pSWEET with tagB+3phoA insert | This work |
| pSWEET-tagB+4phoA | pSWEET with tagB+4phoA insert | This work |
| pSWEET-tagBN30+1phoA | pSWEET with tagBN30+1phoA insert | This work |
| pSWEET-tagBN30+2phoA | pSWEET with tagBN30+2phoA insert | This work |
| pSWEET-tagBN30+3phoA | pSWEET with tagBN30+3phoA insert | This work |
| pSWEET-tagBN30+4phoA | pSWEET with tagBN30+4phoA insert | This work |
| pRBtagBgfp | pRB374 with tagBgfp fusion insert | 3 |
| pRBtagBΔN30gfp | pRB374 with tagBΔN30gfp fusion insert | This work |
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence (5′ to 3′) |
|---|---|
| tagBΔN30for | CACCTTAATTAACATTAGTAAGGAGGTTTCTTTAATGACCCTCCTGATCTCATTCGAAGC |
| tagBN30rev | GGGGCTAGCTTTGCTTTCAGGTTTCAC |
| tagB+1Afor | GAGTTAATTAAGGAGGTTTCTTTATGAAAATAAGATCACTACTGGCGAATGCGTGCTATTTG |
| tagB+2ALfor | GAGTTAATTAAGGAGGTTTCTTTATGAAAATAAGATCACTACTGGCGAATGCGCTGTGCTATTTG |
| tagB+3ALAfor | GAGTTAATTAAGGAGGTTTCTTTATGAAAATAAGATCACTACTGGCGAATGCGCTGGCCTGCTATTTG |
| tagB+4ALALfor | GAGTTAATTAAGGAGGTTTCTTTATGAAAATAAGATCACTACTGGCGAATGCGCTGGCCCTCTGCTATTTG |
| tagBrev2 | CCTAGCTAGCGCTTATTAAATTTTCGATGA |
| tagBgfpRImutrev | GGGCTCGAGGCTTATTAAATTTTCGATGAAATTCAATAAATTTTGGCTTGAGTTCCCATCAG |
| phoAfor | CCTAGCTAGCCGGACACCAGAAATGCCTGT |
| phoArev | GGAAGGATCCTTATTTCAGCCCCAGAGCGG |
Construction of strains used in this study.
pRBtagBΔN30gfp was constructed by first PCR amplifying tagBΔN30 using tagBΔN30for and tagBgfpRImutrev primers. The resulting product was used to replace tagB in pRBtagBgfp. This clone was named pRBtagBΔN30gfp and transformed into B. subtilis 168 following passage through E. coli MC1061 (9). Transformants were selected for kanamycin resistance and verified by diagnostic digestion of plasmid isolated from the transformed cells.
To generate a recombinant construct with the first 30 residues of TagB fused to a passive carrier protein, the phoA gene (starting at the 64th nucleotide in order to omit the signal sequence) was amplified from E. coli genomic DNA using primers phoAfor and phoArev. The amplified product was digested with NheI and BamHI and cloned into a derivative of pBStagB (lacking the stop codon of tagB) digested with the same sites. The tagBphoA fragment was cloned into pSWEET-bgaB using PacI and BamHI restriction sites to replace bgaB, and the plasmid was named pSWEET-tagBphoA. Finally, coding sequence for the first 30 residues of TagB was amplified from pBStagB using the T7 forward and tagBN30rev1 primers, digested with PacI and NheI, and cloned into pSWEET-tagBphoA digested with the same enzymes, essentially replacing the tagB open reading frame (ORF) with one that coded for only the first 30 residues of TagB. This plasmid was renamed pSWEET-tagBN30phoA.
To generate TagB amino-terminal insertional variants with one, three, and four amino acid insertions, tagB was amplified using forward primers tagB+1Afor, tagB+3ALAfor, and tagB+4ALALfor, respectively, with the common reverse primer tagBgfpRImutrev. This reverse primer incorporates a silent mutation that disrupts the EcoRI site at the 3′ end of tagB. The amplified product was digested with PacI and XhoI restriction sites and cloned into pRBtagBgfp digested with the same sites. The tagB insertional variant ORFs were excised from these constructs using PacI and NheI sites (retaining an additional methionine residue at the carboxyl terminus of TagB) and cloned into pSWEET-tagBN30phoA digested with the same sites. These plasmids were named pSWEET-tagB+1phoA, pSWEET-tagB+3phoA, and pSWEET-tagB+4phoA. To generate the TagB double amino acid insertional variant, tagB was amplified using primers tagB+2ALfor and tagBrev2. The product was digested with PacI and NheI restriction enzymes and cloned into pSWEET-tagBN30phoA digested with the same enzymes. This plasmid was named pSWEET-tagB+2phoA.
To generate insertional variants in the TagBN30PhoA protein, the tagBN30phoA ORF was amplified using the insertional variant forward primers listed above with the phoArev1 reverse primer. The products were digested with PacI and BamHI and cloned into pSWEET-bgaB digested with the same enzymes. These clones were named pSWEET-tagBN30+1phoA, pSWEET-tagBN30+2phoA, pSWEET-tagBN30+3phoA, and pSWEET-tagBN30+4phoA. All clones were verified by sequence analysis and ectopically integrated into B. subtilis.
Immunodetection of TagB in B. subtilis.
Detection of TagB derivatives was performed as previously published (3). Briefly, strains were grown in LB medium at 30°C until late log phase. Cells were harvested, and pellets were resuspended in Bacillus lysis buffer and normalized to the same value for optical density at 600 nm. Cells were disrupted by passage through a French pressure cell, and the ensuing lysate was clarified by differential ultracentrifugation. Immunodetection was performed using commercial anti-PhoA polyclonal antiserum (Chemicon International, Temecula, CA), commercial BD Living Colors polyclonal antibody (BD Biosciences Canada, Mississauga, ON, Canada), anti-FtsY polyclonal antiserum, anti-TagD polyclonal antiserum, or anti-EzrA polyclonal antiserum. Donkey anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (BIO/CAN Scientific, Mississauga, ON, Canada) was used as secondary antibody. Immunoblots were detected using the Western Lightning Chemiluminescence Reagent kit (Perkin-Elmer, Woodbridge, ON, Canada) according to the manufacturer's specifications.
Fluorescence microscopy of B. subtilis.
Strains EB892 and EB925, which constitutively expressed TagBGFP and TagBΔN30GFP, respectively, were fixed as previously described (3). Fixed samples were visualized using a Leica Upright model DM6000 motorized microscope with a Semrock green fluorescent protein (GFP) filter cube, an Exfos X-Cite metal halide fluorescence light source, and a Leica HCX Plan Apo 63× oil 1.4-numerical-aperture objective. Images were captured using a Hamamatsu Orca AG monochrome camera and processed using Volocity Acquisition and Restoration software (Improvision).
Conditional complementation of tag-1 allele.
The tagB variants, cloned into the xylose-inducible pSWEET ectopic integration vector, were transformed into strain EB486. Transformants were confirmed by starch utilization assay (10) and PCR. The procedure was performed essentially as previously reported (30). Briefly, cultures of transformants containing the tag-1 allele and a complementing tagB mutant allele were grown in LB-CHL at 30°C until an optical density at 600 nm of ∼0.7 was reached. Samples were normalized for cell density and serially diluted by 10-fold. Two microliters of each diluted sample was spotted onto LB-CHL-xylose solid medium and grown at 47°C. To monitor expression of TagB constructs from the amyE locus, we took advantage of the carboxyl-terminal PhoA fusion. Strains EB1240 and EB1652 to 1656 were grown at the permissive temperature of 30°C in the presence of 2% xylose. Cultures were grown to mid-logarithmic phase, pelleted, and resuspended in Tris-EDTA buffer. Samples were treated with 1 mg/ml lysozyme for 15 minutes at 37°C and finally boiled in sodium dodecyl sulfate-polyacrylamide loading buffer. Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and immunoblotted with anti-PhoA antibody and anti-FtsY polyclonal antibody as a loading control.
RESULTS
Identification of a membrane targeting determinant at the amino terminus of TagB.
We previously reported that TagB localized to the cytoplasmic face of the cell membrane via a peripheral interaction (3). The peripheral interaction was surprising given that sequence-based prediction programs universally identified a transmembrane helix between residues 6 and 25 of TagB. In the absence of any published data regarding the mechanism of TagB localization to the cell membrane, we began by determining the contribution of this sequence to membrane localization. We generated an amino-terminal truncation variant of TagB that deleted the first 30 residues of TagB, TagBΔN30. However, we were unable to detect this variant in B. subtilis lysate by Western blotting. Given the ready detection of full-length TagB from the same expression system, we hypothesized that this construct was unstable. In an attempt to increase the stability of this truncation variant we generated an in-frame carboxyl-terminal fusion to the GFP. Following expression of this fusion protein in B. subtilis, we were able to readily detect the fusion protein by immunoblotting, suggesting that the addition of GFP to the carboxyl terminus of this TagB truncation variant increased the stability of the variant protein.
Lysate from strain EB925 was separated into soluble and membrane fractions and subjected to immunodetection using commercial anti-GFP antibody. Compared to the nontruncated fusion protein (3), we observed a profound redistribution of TagBΔN30GFP localization with the majority of the truncated protein found in the soluble lysate (Fig. 1A). To control for our fractionation procedure, we analyzed the localization of TagD, the soluble glycerol-3-phosphate cytidylyltransferase (23), and EzrA, a membrane-bound cell division protein (20). TagD was predominantly distributed in the soluble lysate, whereas EzrA was predominantly distributed in the membrane fraction, verifying that membranes were separated from soluble lysate under our differential ultracentrifugation conditions. The increased distribution of TagBΔN30GFP in the soluble lysate was consistent with fluorescence microscopy examination of strain EB925. We note that in contrast to the peripheral fluorescence signal, normally observed with TagBGFP, the TagBΔN30GFP-expressing strain had a diffuse fluorescence signal throughout the cell (see Fig. S1 in the supplemental material). These data suggest that the amino terminus of TagB is necessary for efficient membrane localization in B. subtilis.
FIG. 1.
Localization of TagBΔN30GFP and TagBN30PhoA in B. subtilis 168. Whole-cell lysates (Lys.) of EB925 (tagBΔN30gfp) (A) and EB921 (tagBN30phoA) (B) were fractionated into soluble lysate (Sol.) and membrane samples (Mem.) by differential ultracentrifugation. Samples were subjected to immunodetection analysis using anti-GFP and anti-PhoA antisera, respectively. Samples were also probed with anti-TagD and anti-EzrA antisera to assess the quality of fractionation.
Characterization of heterologous protein targeting by the amino terminus of TagB.
We next tested if the membrane targeting function of the TagB amino terminus was independent of the remainder of the TagB protein. In this regard we attempted to create hybrid proteins that fused the first 30 amino acids of TagB to a heterologous passive carrier protein. We first tried to create a fusion of these residues to GFP. Intriguingly, we were unable to generate this recombinant clone in the pRB374 vector in E. coli. It has previously been reported that the vegII promoter, found on this plasmid, is capable of transcription in both E. coli and B. subtilis (7), suggesting that the TagBN30GFP ORF was toxic to E. coli. As an alternative we employed a gram-positive specific xylose-inducible expression system that allowed ectopic integration into B. subtilis at the amyE locus (4). We created a fusion of residues 1 to 30 of TagB to the amino terminus of the mature form of E. coli alkaline phosphatase (PhoA), e.g., PhoA lacking the first 21 residues that comprise its signal sequence, under the transcriptional control of the xylA promoter. E. coli PhoA was chosen because we were able to detect expression of PhoA in B. subtilis by immunoblot analysis.
B. subtilis lysate from strains expressing TagBN30PhoA (EB921) or the unfused PhoA ORF was generated by lysis of cells grown in the presence of xylose. Differential ultracentrifugation was used to generate soluble lysate and membrane fractions from these strains. Immunodetection analyses using commercial anti-PhoA antibody showed that the unfused PhoA control (lacking its signal sequence) was localized in the soluble lysate (data not shown). In contrast, the TagBN30PhoA ORF was predominantly localized to the membrane fraction (Fig. 1B). This suggested that the TagB amino terminus is sufficient for membrane localization in B. subtilis.
Disruption of TagB amino-terminal amphipathicity.
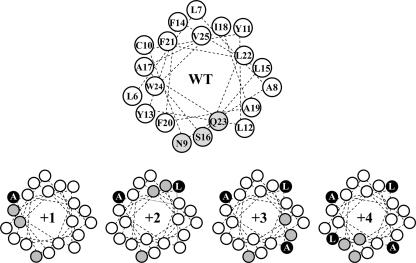
We previously noted that the putative transmembrane helix at the amino terminus of TagB possessed an unusual amphipathicity in which polar but uncharged residues were situated on one face of the helix when examined on a helical wheel (3). To examine the contribution of amphipathicity to TagB function and localization, we created insertional variants of TagB that were predicted by helical wheel analysis to disrupt the amphipathic face of the TagB helix (Fig. 2). We created insertional variants that introduced one, two, three, and four amino acids at position 10 of TagB. Insertion of up to three residues was predicted to disrupt the amphipathic face to various extents. In contrast, the insertion of four amino acids was predicted to restore amphipathicity to the putative helix, in essence adding a full turn to the helix. Given that localization of TagB to the membrane allows the enzyme to be situated in close proximity to its membrane-embedded substrate, we were interested in learning if amino-terminal amphipathicity was a mechanism for TagB localization or function.
FIG. 2.
Predicted impact of insertional mutagenesis on helical amphipathicity of TagB. Residues 6 to 25 of TagB (WT) are shown in helical wheel analysis. The sequence of each residue is indicated, and polar residues are shaded gray. Helical wheel analyses of TagB variants containing insertions of up to four residues at position 10 are also depicted (denoted +1, +2, +3, and +4, respectively). The specific amino acids inserted at position 10 are indicated by black circles. Note the change in the distribution of polar residues (shaded gray) resulting from amino acid insertion such that the amphipathic face of the helix is disrupted by insertion of up to three residues but is restored by insertion of four residues.
In vivo activity of TagB insertional variants.
To examine TagB activity, we employed a previously characterized in vivo activity assay that is based upon the conditional complementation, by xylose-inducible TagB variants, of the temperature-sensitive tag-1 allele, identified as a TagBG188D mutation (30). An in vivo functional assay was employed because previous work has shown that this assay faithfully recapitulates the results of the in vitro functional assay of the TagF-like family of enzymes and, additionally, the TagB in vivo assay is much more robust than its in vitro counterpart (3, 30). We appended E. coli PhoA to the carboxyl termini of the TagB variants to allow verification of variant protein expression by immunodetection analysis. For these studies we examined the effect of insertion of up to four amino acids at position 10 of TagB. The xylose-inducible variant constructs were ectopically introduced at the amyE locus of strain EB486 and verified by PCR analysis. Strains harboring the tag-1 allele at the tag locus and either empty vector, wild-type tagB, or variants of tagB containing the alanine (single), alanine/leucine (double), alanine/leucine/alanine (triple), and alanine/leucine/alanine/leucine (quadruple) residue insertions integrated at the amyE locus were initially grown at the permissive temperature, normalized for growth, and grown at the restrictive temperature (to inactivate endogenous TagB) under conditions that induced variant protein expression.
As shown in Fig. 3A, the absence of a xylose-inducible copy of tagB yielded little growth at the nonpermissive temperature in the presence of xylose. We noted that the carboxyl-terminal fusion of PhoA to TagB did not affect TagB activity in the in vivo assay to an appreciable extent because the wild-type copy of tagB fused to phoA under the transcriptional control of PxylA (EB1652) gave rise to robust growth at the restrictive temperature in the presence of xylose. We did not observe any appreciable difference in growth between wild-type TagB (EB1652) and a variant of TagB that contained a single alanine insertion at position 10 (TagB+1PhoA; EB1653) where both strains grew to dilutions of 10−5. In contrast, insertion of either alanine/leucine (EB1654) or alanine/leucine/alanine (EB1655) at position 10 had a marked effect on TagB function. For both these protein variants growth was only 10-fold better than that of the negative control (EB1240) and approximately 2 to 3 orders of magnitude impaired with respect to the wild type (EB1652). Interestingly, the impairment of TagB function due to insertion of two or three amino acids at position 10 could be suppressed by the addition of four amino acids at the same position. The growth of this strain (EB1656) was indistinguishable from that of the positive control (EB1652).
FIG. 3.
In vivo functional analyses of TagBPhoA insertional variants. (A) TagB activity was monitored by complementation of the tag-1 allele at the restrictive temperature via xylose-inducible insertional variants of TagB. Strains EB1240 (empty vector; -ve), EB1652 (tagBphoA; wt), EB1653 (tagB+1phoA; +1), EB1654 (tagB+2phoA; +2), EB1655 (tagB+3phoA; +3), and EB1656 (tagB+4phoA; +4) were grown at the permissive temperature, diluted as indicated, spotted on LB-CHL-xylose solid medium, and grown at 47°C. (B) Immunodetection analysis of TagB variant expression from cell lysate using anti-PhoA antiserum. Immunodetection of FtsY levels was used as a loading control. The asterisk denotes a cross-reactive band.
To ensure that growth impairment in the TagB in vivo assay was not due to poor expression of TagB variants, whole-cell lysate of each strain (EB1240 and EB1652-EB1656) was prepared under inducing conditions and subjected to immunodetection analyses with commercial anti-PhoA antibody. As shown in Fig. 3B we could clearly detect TagB and TagB variant expression from the xylose-inducible promoter. This analysis suggests that all TagB variants were expressed to similar levels, implying that protein expression was not the cause of impaired activity in the in vivo activity assay. To ensure equal loading of cell lysates, we analyzed levels of FtsY protein.
Localization of TagB insertional variants.
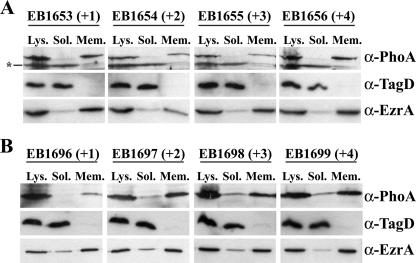
To ascertain whether the impaired in vivo activity of TagB+2PhoA and TagB+3PhoA variants was due to altered subcellular localization of TagB, cultures expressing the wild type and all four variant proteins were grown at the permissive temperature under inducing conditions. Whole-cell lysates from each culture were fractionated by differential ultracentrifugation into soluble lysate and membrane samples. The distribution of TagB within these fractions was examined by immunodetection using commercial PhoA antibody (Fig. 4A). Again, the distribution of soluble TagD and membrane-bound EzrA proteins was also examined to validate the fractionation procedure. These analyses verified separation of soluble lysate and membranes under these conditions as TagD exclusively localized in the soluble lysate and EzrA predominantly localized to the membrane. As shown in Fig. 4A, the insertional variants of TagB all appeared membrane localized, suggesting that altered subcellular distribution of the insertional variants could not account for the diminished in vivo activity of TagB+2PhoA and TagB+3PhoA.
FIG. 4.
Localization analyses of TagBPhoA insertional variants. (A) Strains EB1653 (tagB+1phoA; +1), EB1654 (tagB+2phoA; +2), EB1655 (tagB+3phoA; +3), and EB1656 (tagB+4phoA; +4) were grown under inducing conditions, harvested, and lysed. Whole-cell lysate (Lys.) was fractionated into soluble lysate (Sol.) and membrane samples (Mem.) by differential ultracentrifugation. Samples were subjected to immunodetection with anti-PhoA antiserum. Samples were also probed with anti-TagD and anti-EzrA antisera to assess the quality of the fractionation. The asterisk denotes a cross-reactive band. (B) Samples from strains EB1696 (tagBN30+1phoA; +1), EB1697 (tagBN30+2phoA; +2), EB1698 (tagBN30+3phoA; +3), and EB1699 (tagBN30+4phoA; +4) were fractionated as outlined above and subjected to the same immunodetection analyses.
Although there did not appear to be an impact on the localization of the TagB insertional variants, we were mindful that a minor amount of TagB remained localized to the membrane in the absence of its first 30 residues (Fig. 1A). To determine if another membrane targeting determinant encoded in the remaining TagB sequence was responsible for the membrane localization of the insertional variants, the same insertions were generated in the TagBN30PhoA background. We reasoned that any impact on localization could be unambiguously attributed to insertion in the membrane targeting determinant since in the TagBN30PhoA construct only the first 30 residues of TagB directed membrane targeting (Fig. 1B). Strains carrying a xylose-inducible copy of TagBN30PhoA with insertions of one to four amino acids at position 10 (EB1696 to EB1699, respectively) were grown at the permissive temperature under inducing conditions. Lysates were generated and fractionated as indicated above. Immunodetection analysis using commercial anti-PhoA antibody again showed no appreciable difference in distribution of the TagBN30PhoA variants, with the bulk of the proteins localizing to the membrane (Fig. 4B). Again, the observed distributions of TagD and EzrA served to ensure adequate separation of membranes and soluble lysate. The membrane localization of the TagB insertional variants suggests that insertional disruption of the amino-terminal membrane targeting determinant does not impact on membrane targeting even though it results in a profound decrease of TagB function. This further suggests that the impact on TagB function is manifested at the level of biochemical activity.
DISCUSSION
Teichoic acid synthesis has been the subject of considerable study genetically (26) and biochemically. The latter encompasses vintage work with crude cell fractions (36) and more-recent studies with pure recombinant proteins (3, 14, 23, 24, 31, 33). While these studies have provided important insights into teichoic acid biogenesis, we still have a limited structure-function understanding of the enzymes directly involved in polymer synthesis. Indeed, biosynthesis models place teichoic acid synthesis at the cytoplasmic membrane, and yet how Tag proteins mediate this localization is unclear. In this work we have identified a membrane targeting determinant at the amino terminus of TagB. This region of the protein, encompassing the first 30 residues of TagB, is necessary and sufficient for membrane localization. We have also identified an unusual amphipathicity in this determinant, the disruption of which impacted on protein function but not localization.
The proposed TagB amino-terminal membrane targeting determinant identified in this work is often identified as a transmembrane helix using sequence-based prediction programs. On the basis of extraction data via chaotropic agent or alkali treatment, we previously concluded that TagB, in fact, interacted peripherally with the cell membrane (3). Furthermore, we predicted that this region of TagB, putatively encoding an α-helix, had an amphipathic character, in which polar but uncharged amino acids were distributed in a similar orientation about the helical face (3). This is reminiscent of the peripheral membrane proteins MinD and FtsA, which use amphipathic helices consisting of positively charged and hydrophobic amino acids to mediate interaction with the cytoplasmic face of the bacterial membrane (16, 25, 34). It is believed that the positively charged residues of the amphipathic helix interact electrostatically with phospholipid head groups to provide the initial recruitment to the membrane (17). In the case of MinD it is hypothesized that the hydrophobic residues of the amphipathic helix insert into the membrane directly upon ATP binding (38). However, the lack of charged residues in the putative helical region of this determinant (residues 6 to 25) suggests that TagB may use a mechanism different than either FtsA or MinD for membrane interaction. In this respect it is noteworthy that high ionic strength could only partially extract TagB from B. subtilis membranes (A. P. Bhavsar and E. D. Brown, unpublished observations). This suggests that hydrophobicity plays an important role in membrane localization, since high ionic strength augments hydrophobic interactions.
Our experimental results are the first to suggest that the amino terminus of TagB is folded into an amphipathic helix. Disruption of TagB function by the insertion of two and three amino acids at position 10, and the subsequent restoration of function upon insertion of four amino acids at the same position, supports this assertion given that α-helices contain 3.6 residues per turn. This further implies that the amino terminus of TagB contains not only membrane targeting information but also structural content that is crucial for TagB function. Intriguingly, we note that to date there is a complete lack of structural information for the TagF-like enzymes. Thus, while our data support the assertion that the amphipathicity of the TagB amino-terminal helix plays an important role in TagB function, we cannot rule out the possibility that the defect in TagB function is due to specific amino acid insertion.
Although it is not yet understood how the amino terminus of TagB directs membrane targeting, e.g., via direct or indirect interactions, it is clear that this can be done in a manner that is independent of TagB activity, as exemplified by the identification of insertional variants that uncouple TagB localization and function. One plausible mechanism to reconcile these observations is that TagB might independently localize to the membrane but then be recruited into a multiprotein complex whose formation is requisite for glycerophosphotransfer to an undecaprenol-pyrophosphoryl-disaccharide moiety. It is interesting to note that the prospect of a teichoic acid synthesome has been previously suggested (21), although there is not yet any evidence to prove or disprove the hypothesis.
The concept of a multifunctional peptide sequence is not unique to TagB. Gram-negative lipoproteins contain an amino- terminal signal sequence that directs the nascent protein for secretion through the sec system, directs cleavage and acylation of the translocated protein, and directs final localization to either the outer leaflet of the inner membrane or the inner leaflet of the outer membrane (15). Mutations in this lipoprotein signal sequence impact on the proper localization of the lipoprotein, and this may lead to functional impairment of the protein. Similarly, it was recently demonstrated that the signal sequences of Streptococcus pyogenes PrtF and M6 proteins direct not only their secretion but also the spatial restriction of secretion (8). However, the bifunctional amino-terminal peptide sequence of TagB differs in that the information contained therein directs both localization and biochemical activity, with the latter separable from the former. This scenario is reminiscent of the 26-amino-acid B. subtilis sporulation protein SpoVM. This small membrane-binding protein is predicted to adopt an amphipathic α-helical fold, and most intriguingly, site-directed mutagenesis identified residues that disrupted SpoVM function without affecting its membrane targeting (35). Subsequent study localized these residues to the membrane interaction face of the amphipathic helix, the face opposite which a binding site for the sporulation protein SpoIVA was identified (27). Although the hydrophilic face of the SpoVM α-helix consists of six charged amino acids, in contrast to the polar but uncharged residues in the TagB amino terminus, the SpoVM studies suggest that an amphipathic helix can mediate both membrane binding and protein-protein interaction, which is an attractive model for the role of the amino terminus of TagB.
Supplementary Material
Acknowledgments
This work was funded by an operating grant (MOP-15496) and a Canada Research Chair in Chemical Biology from the Canadian Institutes of Health Research to E.D.B.
We acknowledge the generosity of the following: Petra Levin, who provided pPL51 and anti-EzrA antibody; David Andrews, who provided anti-FtsY antibody; and members of the laboratory, for helpful discussions.
Footnotes
Published ahead of print on 27 July 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Bhavsar, A. P., T. J. Beveridge, and E. D. Brown. 2001. Precise deletion of tagD and controlled depletion of its product, glycerol 3-phosphate cytidylyltransferase, leads to irregular morphology and lysis of Bacillus subtilis grown at physiological temperature. J. Bacteriol. 183:6688-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhavsar, A. P., L. K. Erdman, J. W. Schertzer, and E. D. Brown. 2004. Teichoic acid is an essential polymer in Bacillus subtilis that is functionally distinct from teichuronic acid. J. Bacteriol. 186:7865-7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhavsar, A. P., R. Truant, and E. D. Brown. 2005. The TagB protein in Bacillus subtilis 168 is an intracellular peripheral membrane protein that can incorporate glycerol phosphate onto a membrane-bound acceptor in vitro. J. Biol. Chem. 280:36691-36700. [DOI] [PubMed] [Google Scholar]
- 4.Bhavsar, A. P., X. Zhao, and E. D. Brown. 2001. Development and characterization of a xylose-dependent system for expression of cloned genes in Bacillus subtilis: conditional complementation of a teichoic acid mutant. Appl. Environ. Microbiol. 67:403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boylan, R. J., and N. H. Mendelson. 1969. Initial characterization of a temperature-sensitive Rod− mutant of Bacillus subtilis. J. Bacteriol. 100:1316-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briehl, M., H. M. Pooley, and D. Karamata. 1989. Mutants of Bacillus subtilis 168 thermosensitive for growth and wall teichoic acid biosynthesis. J. Gen. Microbiol. 135:1325-1334. [Google Scholar]
- 7.Bruckner, R. 1992. A series of shuttle vectors for Bacillus subtilis and Escherichia coli. Gene 122:187-192. [DOI] [PubMed] [Google Scholar]
- 8.Carlsson, F., M. Stalhammar-Carlemalm, K. Flardh, C. Sandin, E. Carlemalm, and G. Lindahl. 2006. Signal sequence directs localized secretion of bacterial surface proteins. Nature 442:943-946. [DOI] [PubMed] [Google Scholar]
- 9.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 10.Cutting, S. M., and P. B. Vander Horn. 1990. Genetic analysis, p. 175-209. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, New York, NY.
- 11.Cutting, S. M., and P. Youngman. 1994. Gene transfer in Gram-positive bacteria, p. 348-364. In R. G. E. Murray, N. R. Krieg, W. A. Wood, and P. Gerhardt (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, DC.
- 12.D'Elia, M. A., K. E. Millar, T. J. Beveridge, and E. D. Brown. 2006. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J. Bacteriol. 188:8313-8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Elia, M. A., M. P. Pereira, Y. S. Chung, W. Zhao, A. Chau, T. J. Kenney, M. C. Sulavik, T. A. Black, and E. D. Brown. 2006. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J. Bacteriol. 188:4183-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsberg, C., Y. Zhang, Y. Yuan, and S. Walker. 2006. In vitro reconstitution of two essential steps in wall teichoic acid biosynthesis. ACS Chem. Biol. 1:25-28. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi, S., and H. C. Wu. 1990. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22:451-471. [DOI] [PubMed] [Google Scholar]
- 16.Hu, Z., and J. Lutkenhaus. 2003. A conserved sequence at the C-terminus of MinD is required for binding to the membrane and targeting MinC to the septum. Mol. Microbiol. 47:345-355. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, J. E., and R. B. Cornell. 1999. Amphitropic proteins: regulation by reversible membrane interactions. Mol. Membr. Biol. 16:217-235. [DOI] [PubMed] [Google Scholar]
- 18.Lazarevic, V., F. X. Abellan, S. B. Moller, D. Karamata, and C. Mauel. 2002. Comparison of ribitol and glycerol teichoic acid genes in Bacillus subtilis W23 and 168: identical function, similar divergent organization, but different regulation. Microbiology 148:815-824. [DOI] [PubMed] [Google Scholar]
- 19.Lazarevic, V., and D. Karamata. 1995. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol. Microbiol. 16:345-355. [DOI] [PubMed] [Google Scholar]
- 20.Levin, P. A., I. G. Kurtser, and A. D. Grossman. 1999. Identification and characterization of a negative regulator of FtsZ ring formation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 96:9642-9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauel, C., M. Young, and D. Karamata. 1991. Genes concerned with synthesis of poly(glycerol phosphate), the essential teichoic acid in Bacillus subtilis strain 168, are organized in two divergent transcription units. J. Gen. Microbiol. 137:929-941. [DOI] [PubMed] [Google Scholar]
- 22.Mauel, C., M. Young, A. Monsutti-Grecescu, S. A. Marriott, and D. Karamata. 1994. Analysis of Bacillus subtilis tag gene expression using transcriptional fusions. Microbiology 140:2279-2288. [DOI] [PubMed] [Google Scholar]
- 23.Park, Y. S., T. D. Sweitzer, J. E. Dixon, and C. Kent. 1993. Expression, purification, and characterization of CTP:glycerol-3-phosphate cytidylyltransferase from Bacillus subtilis. J. Biol. Chem. 268:16648-16654. [PubMed] [Google Scholar]
- 24.Pereira, M. P., and E. D. Brown. 2004. Bifunctional catalysis by CDP-ribitol synthase: convergent recruitment of reductase and cytidylyltransferase activities in Haemophilus influenzae and Staphylococcus aureus. Biochemistry 43:11802-11812. [DOI] [PubMed] [Google Scholar]
- 25.Pichoff, S., and J. Lutkenhaus. 2005. Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Mol. Microbiol. 55:1722-1734. [DOI] [PubMed] [Google Scholar]
- 26.Pooley, H. M., and D. Karamata. 1994. Teichoic acid synthesis in Bacillus subtilis: genetic organization and biological roles, p. 187-198. In J. M. Ghuysen and R. Hakenbeck (ed.), Bacterial cell wall. Elsevier, Amsterdam, The Netherlands.
- 27.Ramamurthi, K. S., K. R. Clapham, and R. Losick. 2006. Peptide anchoring spore coat assembly to the outer forespore membrane in Bacillus subtilis. Mol. Microbiol. 62:1547-1557. [DOI] [PubMed] [Google Scholar]
- 28.Rogers, H. J., M. McConnell, and I. D. Burdett. 1970. The isolation and characterization of mutants of Bacillus subtilis and Bacillus licheniformis with disturbed morphology and cell division. J. Gen. Microbiol. 61:155-171. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Schertzer, J. W., A. P. Bhavsar, and E. D. Brown. 2005. Two conserved histidine residues are critical to the function of the TagF-like family of enzymes. J. Biol. Chem. 280:36683-36690. [DOI] [PubMed] [Google Scholar]
- 31.Schertzer, J. W., and E. D. Brown. 2003. Purified, recombinant TagF protein from Bacillus subtilis 168 catalyzes the polymerization of glycerol phosphate onto a membrane acceptor in vitro. J. Biol. Chem. 278:18002-18007. [DOI] [PubMed] [Google Scholar]
- 32.Soldo, B., V. Lazarevic, and D. Karamata. 2002. tagO is involved in the synthesis of all anionic cell-wall polymers in Bacillus subtilis 168. Microbiology 148:2079-2087. [DOI] [PubMed] [Google Scholar]
- 33.Soldo, B., V. Lazarevic, H. M. Pooley, and D. Karamata. 2002. Characterization of a Bacillus subtilis thermosensitive teichoic acid-deficient mutant: gene mnaA (yvyH) encodes the UDP-N-acetylglucosamine 2-epimerase. J. Bacteriol. 184:4316-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szeto, T. H., S. L. Rowland, L. I. Rothfield, and G. F. King. 2002. Membrane localization of MinD is mediated by a C-terminal motif that is conserved across eubacteria, archaea, and chloroplasts. Proc. Natl. Acad. Sci. USA 99:15693-15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Ooij, C., and R. Losick. 2003. Subcellular localization of a small sporulation protein in Bacillus subtilis. J. Bacteriol. 185:1391-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward, J. B. 1981. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol. Rev. 45:211-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weidenmaier, C., J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond, and A. Peschel. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10:243-245. [DOI] [PubMed] [Google Scholar]
- 38.Zhou, H., and J. Lutkenhaus. 2003. Membrane binding by MinD involves insertion of hydrophobic residues within the C-terminal amphipathic helix into the bilayer. J. Bacteriol. 185:4326-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.