Abstract
Vibrio vulnificus, a marine bacterium capable of causing wound infection and septicemia, secretes a 45-kDa metalloprotease (vEP) with many biological activities. The precursor of vEP consists of four regions: a signal peptide, an N-terminal propeptide (nPP), a C-terminal propeptide, and the mature protease. Two forms of vEP—vEP-45, which contains the mature protease plus the C-terminal propeptide, and vEP-34, which contains only the mature protease—were expressed in Escherichia coli and purified. vEP-45 and vEP-34 had similar activities with azocasein as a substrate, but vEP-34 had reduced activity toward insoluble proteins. The nPP of vEP was expressed as a His tag fusion protein, and its effect on vEP activity was investigated. nPP inhibited the activities of both vEP-45 and vEP-34 but not that of thermolysin, a different but related zinc-dependent protease. The inhibition of vEP by nPP was further examined using vEP-34 as a representative enzyme. The inhibition could be completely reversed under conditions of low enzyme and propeptide concentrations and with prolonged incubation, which resulted from the degradation of nPP by vEP. However, even at high nPP and vEP concentrations, inhibition of vEP by nPP at high temperatures was not effective, resulting in the degradation of both nPP and vEP. These results demonstrate that the nPP of vEP could bind to vEP and inhibit its activity, resulting in the degradation of the propeptide.
Extracellular proteases are generally synthesized as inactive proenzymes consisting of a signal peptide, an N-terminal propeptide, and a mature region having catalytic activity. For some enzymes, the mature region also contains a C-terminal propeptide that usually undergoes autoprocessing following enzyme activation. The role of the signal peptide is to assist in the secretion of the enzyme across the cytoplasmic membrane. The propeptide is thought to act as a molecular chaperone by assisting with the proper folding of the protease during maturation (4, 13, 18, 24-26, 28). It can also function as an inhibitor of the cognate mature enzyme (8, 12, 17-19, 22, 24). The C-terminal propeptides of some proteases are also proposed to assist in the secretion of the enzyme (9, 14).
Vibrio vulnificus secretes a broad-specificity metalloprotease that has many biological activities (for a review, see reference 16). We have previously reported the purification and characterization of this enzyme, which we named vEP, from V. vulnificus ATCC 29307. The purified enzyme existed mainly in the form without the C-terminal propeptide, which had been lost through autoprocessing (3). Through N-terminal sequencing of the mature enzyme, the cleavage site for enzyme maturation was identified. Although the precise cleavage point of the C-terminal propeptide was not established, a putative cleavage point was deduced from the result of mass spectrometry analysis of the purified enzyme, and based on the predicted amino acid sequence reported by Jeong et al. (7), Asn314 appears to be the terminal amino acid. The proenzyme of vEP thus comprises a 24-amino-acid putative signal peptide, a 172-amino-acid N-terminal propeptide, a 314-amino-acid mature region possessing protease activity, and a 99-amino-acid C-terminal propeptide. The gene encoding the same enzyme from a different strain of V. vulnificus (L-180) has previously been expressed and purified as a mature active enzyme, mainly in the form retaining the full C-terminal propeptide (15). Upon incubation at 37°C for 24 h, it was mostly processed to a smaller form with the loss of the C-terminal peptide.
vEP belongs to the same family of metalloproteases as thermolysin, the best-characterized representative of this family. The N-terminal propeptide of thermolysin has been shown to inhibit thermolysin with a 50% inhibitory concentration of 14 nM (18). It also inhibited a closely related zinc metallopeptidase produced by Bacillus stearothermophilus with a higher 50% inhibitory concentration (220 nM). The present report describes the cloning, expression, and purification of two forms of vEP as well as the N-terminal propeptide. The interaction between vEP and the N-terminal propeptide is also described.
MATERIALS AND METHODS
Materials.
V. vulnificus strain ATCC 29307 was routinely cultivated in LB medium with 0.5% NaCl. All chromatography columns were obtained from Amersham Biosciences (Uppsala, Sweden). Plasmids pFLAG-ATS (Sigma-Aldrich, St. Louis, MO) and pET-28b (Novagen, Madison, WI) were used for cloning and expression. The medium routinely used for Escherichia coli DH5α or BL21(DE3) carrying recombinant plasmids was LB medium containing either 100 μg/ml ampicillin or 50 μg/ml kanamycin.
DNA manipulation.
Most routine DNA manipulations were performed as described by Sambrook et al. (20). Plasmid DNA was prepared using a plasmid purification kit (QIAGEN, Hilden, Germany). An agarose gel extraction kit and a PCR purification kit (Intron Biotechnology, Seongnam, Korea) were used for the purification of DNA from the gel and PCR sample, respectively.
Cloning of vEP and the N-terminal propeptide.
V. vulnificus genomic DNA was used to clone the full-length vEP gene. Forward primer 5′-CTTCTCGAGATGAAACTCAATCAACGT-3′ (the XhoI site is underlined, and the start codon is boldfaced) and reverse primer 5′-CGGGGTACCTCAATATTGCAGCTTTAA-3′ (the KpnI site is underlined, and the stop codon is boldfaced) were used for PCR amplification of the entire coding region of the vEP gene with the following PCR program: a 2-min hold at 95°C; 30 cycles of 94°C for 1 min, 50°C for 40 s, and 72°C for 2 min; and a hold for 5 min at 72°C to ensure full extension. PCR was performed using a model 9700 Perkin-Elmer thermal cycler. After purification, the DNA was digested with XhoI and KpnI, further purified, and then ligated to XhoI-KpnI-cut pFLAG-ATS to yield the pVEP-45 construct. To construct vEP without the C-terminal propeptide, PCR was conducted using pVEP-45 as a template with the same forward primer but with reverse primer 5′-GTGATGGTGATGGTGATTACCACTTGGCGGCGT-3′ (the Asn314 codon is boldfaced). The same PCR program was used except that the extension time was 1.5 min instead of 2 min. After purification, the DNA was used as a template for a second PCR using the same forward primer but with reverse primer 5′-CGGGGTACCTTAATGGTGATGGTGATGGTGATT-3′ (the KpnI site is underlined, the six histidine codons are italicized, and the stop codon is boldfaced). After purification, the PCR product was digested with XhoI and KpnI and then ligated to XhoI-KpnI-cut pFLAG-ATS to yield the pVEP-34 construct.
The N-terminal propeptide of vEP was amplified from pVEP-45 by PCR with forward primer 5′-ATTCCATATGGCAGAAATGGTGTCCGTC-3′ (the NdeI site is underlined, and the codon for the first amino acid of the N-terminal propeptide is boldfaced) and reverse primer 5′-CGGGATCCGTATGATTT AAGCCATCCCA-3′ (the BamHI site is underlined, and the codon for the last amino acid of the N-terminal propeptide is boldfaced) using the following program: a 2-min hold at 95°C; 30 cycles of 94°C for 1 min, 50°C for 40 s, and 72°C for 1 min; and a hold for 5 min at 72°C to ensure full extension. After purification, the DNA was digested with BamHI and NdeI, purified further, and then ligated to BamHI-NdeI-cut pET-28b to produce the pET-28b-nPP construct.
Protein expression and purification.
DH5α cells harboring pVEP-45 or pVEP-34 were inoculated into 50 ml of LB broth plus 100 μg/ml ampicillin and were grown overnight at 37°C. Ten milliliters of this overnight culture was used to inoculate 500 ml of fresh LB broth plus ampicillin and was grown at 37°C until the A600 reached 0.8. A total of 2 liters of cultures was prepared. The target protein was induced by the addition of 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) followed by overnight incubation at 20°C. The cells were harvested by centrifugation, and the periplasmic proteins were isolated by an EDTA-lysozyme treatment adapted from the method described by Zhu et al. (27). The harvested cells were resuspended in 100 ml of 30 mM Tris-HCl (pH 8.0)-20% sucrose-1 mM EDTA-0.3 mg/ml lysozyme-1 mM phenylmethylsulfonyl fluoride and then incubated at 4°C for 30 min. The cell suspension was centrifuged at 18,000 × g for 20 min at 4°C. The supernatant, which contained the periplasmic proteins, was subjected to ammonium sulfate precipitation at 70% saturation. The resulting protein pellet was collected by centrifugation at 35,000 × g for 30 min at 4°C, dissolved in 25 mM Tris-HCl (pH 7.5) containing 1 M NaCl, and then applied to a PD-10 column equilibrated in the same buffer to remove residual (NH4)2SO4. The sample was then applied to a phenyl Sepharose HP column equilibrated with 25 mM Tris-HCl (pH 7.5) containing 1 mM CaCl2 and 1 M NaCl. After a wash with the same buffer, the bound proteins were eluted with a linear gradient of NaCl from 1 to 0 M in the same buffer. Fractions with major protease activity were pooled and further chromatographed on a Superdex 75 10/300 GL column equilibrated in 25 mM Tris-HCl (pH 7.5) containing 1 mM CaCl2 and 0.15 M NaCl. The purified vEP was stored in small aliquots at −20°C.
The N-terminal propeptide was also expressed from BL21(DE3) harboring plasmid pET-28b-nPP under the same conditions as those described above for the expression of vEP, except that induction was carried out at 30°C for 4 h. The cells were then harvested by centrifugation, resuspended in binding buffer (25 mM Tris-HCl [pH 7.5] containing 0.5 M NaCl, 0.1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride), and subjected to three passages through a French press (Thermo Spectronic), followed by sonication using a Misonix XL-2000 cell disruptor set at level 15 for 10 times, 30 s each time, with 1 min of incubation on ice in between. The cell lysate was then centrifuged at 35,000 × g for 30 min at 4°C. The supernatant was filtered through a 0.45-μm-pore-size filter and then applied to a nickel-nitrilotriacetic acid (NTA) column packed with 5 ml of resin that was preequilibrated with binding buffer. The column was washed with 100 ml of binding buffer and with 50 ml of binding buffer plus 50 mM imidazole and was then eluted with a linear gradient of imidazole from 50 to 500 mM in binding buffer. Fractions containing major propeptide proteins were pooled and stored at 4°C.
SDS-PAGE.
The method described by Laemmli (10) was employed for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. 1,10-Phenanthroline was added to the sample to a final concentration of 1 mM before heating to stop any further autodegradation of vEP during heating. Samples were routinely run on a 12% gel, and protein bands in the gel were visualized by staining with Coomassie blue.
Determination of protein concentrations.
Protein concentrations were determined spectrophotometrically at 595 nm using Bradford reagent (Sigma) according to the manufacturer's instructions.
Enzyme activity assay.
The proteolytic activity of vEP was assayed using azocasein as previously described (3). For the cleavage of soluble protein, 10 μg of the substrate was cleaved with 0.3 μg of the enzyme in a total reaction volume of 15 μl containing 50 mM Tris-HCl (pH 7.5) for 30 min at 37°C and then subjected to SDS-PAGE. For the cleavage of the insoluble proteins fibrin and elastin, 2 mg of protein powder was mixed with 1 ml of 50 mM Tris-HCl (pH 7.5) containing 20 μg/ml of the enzyme. The sample was incubated at 37°C on a shaker overnight. Protein aggregates were precipitated by centrifugation at 22,000 × g for 10 min at 4°C, the supernatant was diluted twofold with 50 mM Tris-HCl (pH 7.5), and the presence of soluble proteins/peptides was detected by absorbance at 280 nm. For the inhibition study, the enzyme was incubated with the propeptide for 10 min at 37°C before addition of azocasein prewarmed at 37°C.
RESULTS
Purification of vEP.
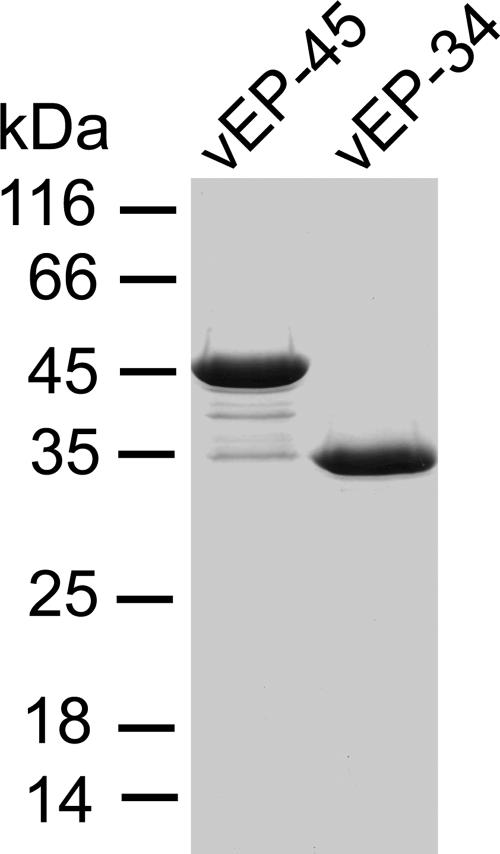
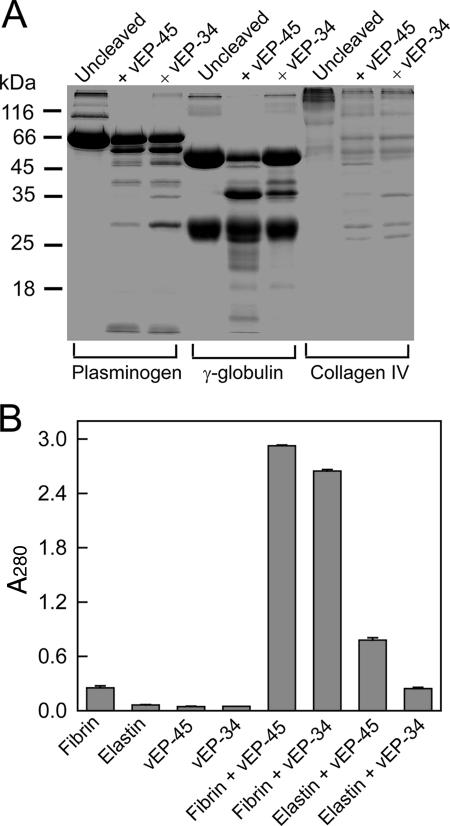
The purification of vEP-45 and vEP-34 is summarized in Table 1. Although vEP-34 was expressed as a His tag fusion protein, it was purified in the same way as vEP-45 for consistency. Figure 1 shows the SDS-PAGE analysis of purified vEP-45 and vEP-34. vEP-45 was obtained mainly as a 45-kDa form, but noticeable minor forms corresponding to the various stages of autoprocessing could be seen. In contrast to the finding reported by Miyoshi et al. (15) for the purification of the enzyme from V. vulnificus L-180, the incorporation of 1 mM CaCl2 in the purification buffer could not completely prevent autoprocessing. vEP-45 and vEP-34 had similar specific activities (16,100 versus 16,200 U/mg) as assayed with azocasein, confirming that the loss of the C-terminal propeptide had no effect on enzyme activity toward a soluble substrate such as azocasein. The expression of vEP-34 was almost double that of vEP-45, and the final yield of the vEP-34 enzyme was more than twice the yield of vEP-45 (Table 1). The difference in proteolytic activity between the two enzymes was compared for three different substrates that could be dissolved in an aqueous buffer. There seemed to be no difference in the patterns and extents of cleavage of plasminogen and collagen (Fig. 2A). However, there was a marked difference in the cleavage of gamma globulin: more of the heavy chains were cleaved by vEP-45 than by vEP-34. Incubation of the insoluble protein fibrin or elastin with either enzyme at 37°C overnight resulted in a noticeable difference in the amount of soluble peptides (detected by absorbance at 280 nm) from that for the substrate-only control (Fig. 2B, fibrin and elastin). Fibrin was a better substrate than elastin for both enzymes. However, vEP-45 was a more efficient enzyme than vEP-34 at cleaving both fibrin and elastin, although the absence of the C-terminal propeptide appeared to decrease the cleavage of elastin more than that of fibrin.
TABLE 1.
Summary of the purification of vEP-45 and vEP-34
| Fraction | Total protein (mg)
|
Total activity (U)a
|
Sp act (U/mg)
|
Yield (%)
|
||||
|---|---|---|---|---|---|---|---|---|
| vEP-45 | vEP-34 | vEP-45 | vEP-34 | vEP-45 | vEP-34 | vEP-45 | vEP-34 | |
| Periplasmic | 75 | 118 | 282,000 | 545,000 | 3,750 | 4,620 | 100 | 100 |
| Phenyl Sepharose HP | 13 | 46 | 194,000 | 509,000 | 14,900 | 11,700 | 69 | 93 |
| Superdex 75 100/300 GL | 4.7 | 23 | 75,900 | 373,000 | 16,100 | 16,200 | 27 | 68 |
One unit is defined as the amount of enzyme that catalyzes the proteolysis of 1 μg of azocasein per min.
FIG. 1.
SDS-PAGE analysis of purified vEP. vEP-45 and vEP-34 resolved from a Superdex 75 100/300 GL column were electrophoresed on a 12% gel under reducing conditions.
FIG. 2.
(A) SDS-PAGE analysis of the cleavage of soluble protein substrates by vEP-45 and vEP-34. A 15-μl sample containing 10 μg of the substrate and 0.3 μg of the enzyme was incubated at 37°C for 30 min and then electrophoresed on a 12% gel. (B) Cleavage of insoluble proteins by vEP. Fibrin or elastin was digested with vEP-45 or vEP-34 as described in Materials and Methods, and the total release of soluble peptides was measured by absorbance at 280 nm. Data are means ± standard deviations from duplicate experiments.
Expression and purification of the N-terminal propeptide.
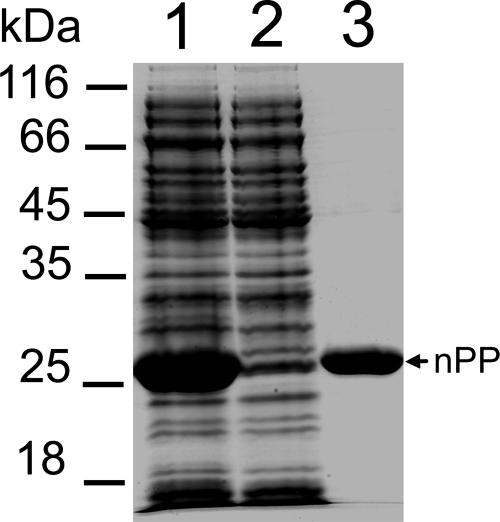
Much of the N-terminal propeptide of vEP expressed as a His tag fusion protein existed as a soluble protein, which enabled the easy purification of the protein by one-step chromatography using a Ni-NTA column. The purified propeptide appeared mainly as a single band on an SDS-PAGE gel (Fig. 3). Although it was expressed as a soluble protein, attempts to concentrate the purified propeptide led to the irreversible aggregation and precipitation of the protein at concentrations higher than 2 mg/ml in a buffer such as 25 mM Tris-HCl (pH 7.5). However, the solubility of the propeptide could be increased about threefold in the presence of a buffer with a pH of 5 or less. Since the assay of vEP was routinely carried out at pH 7.5, the purified propeptide was used without further buffer exchange. The purified N-terminal propeptide is referred to below as nPP.
FIG. 3.
SDS-PAGE analysis of the purification of the N-terminal propeptide. Lane 1, soluble fraction of the cell extract from E. coli BL21(DE3) expressing the propeptide; lane 2, unbound fraction of the cell extract after passage through a Ni-NTA column; lane 3, purified nPP.
Inhibition of vEP.
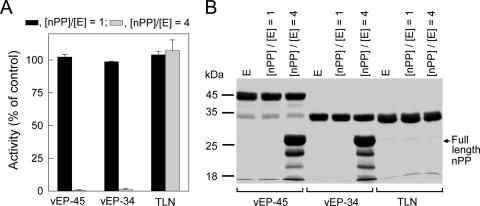
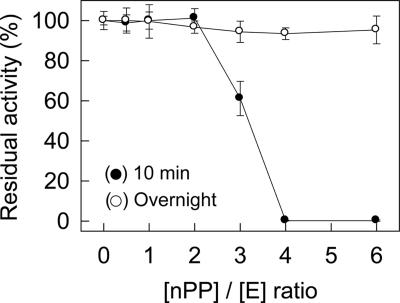
The inhibitory effects of nPP on vEP activity and on the activity of thermolysin (from “Bacillus thermoproteolyticus” [proposed name]) were investigated by incubating nPP with the enzyme for 10 min at 37°C using two different molar ratios of nPP to enzyme ([nPP]/[E], where E stands for enzyme). At an [nPP]/[E] ratio of 1, no inhibition was observed for either vEP or thermolysin (Fig. 4A). At an [nPP]/[E] ratio of 4, total inhibition of vEP activity was observed, whereas no inhibition of thermolysin activity was observed. Thus, nPP appeared to specifically inhibit vEP activity. To investigate whether the lack of inhibition of vEP by nPP at a low [nPP]/[E] ratio and the lack of inhibition of thermolysin could be due to cleavage of nPP, samples containing the enzyme plus nPP were incubated for 10 min at 37°C and then subjected to SDS-PAGE. As shown in Fig. 4B, nPP completely disappeared after incubation with either vEP or thermolysin at an [nPP]/[E] ratio of 1. At an [nPP]/[E] ratio of 4, much of the nPP still remained in the samples containing vEP, but all of the nPP in the sample containing thermolysin had disappeared. To further characterize the inhibition of vEP activity by nPP, vEP-34 was used instead of vEP-45, since the vEP-45 preparation contained other minor forms resulting from autoprocessing. The inhibition of vEP activity versus the concentration of nPP was investigated at 37°C for two different preincubation times. With a short preincubation time (10 min), about 40% inhibition of enzyme activity was obtained with an [nPP]/[E] ratio of 3 (Fig. 5). At higher [nPP]/[E] ratios, total inhibition was observed. However, after overnight preincubation, no inhibition was observed with any [nPP]/[E] ratio, suggesting that the inhibition was reversible.
FIG. 4.
Effects of nPP on the activities of vEP and thermolysin. (A) Protease activities of vEP and thermolysin (TLN) in the absence or presence of different [nPP]/[E] ratios. The enzyme (100 nM) was preincubated with different concentrations of nPP at 37°C for 10 min, and residual activity was measured following the addition of prewarmed azocasein as a substrate. Protease activity was measured with a final enzyme concentration of 80 nM. Residual protease activities of samples containing nPP were expressed as a percentage of the activity of the sample without nPP. Data are means ± standard deviations from two experiments carried out in triplicate. (B) SDS-PAGE of vEP and thermolysin preincubated with different concentrations of nPP. The enzyme (5 μg) was incubated without or with different amounts of nPP ([nPP]/[E] ratios of 1 and 4) in a 15-μl sample volume at 37°C for 10 min and then subjected to SDS-PAGE using a 12% gel. The final enzyme concentrations were 7.4 μM (vEP-45), 9.8 μM (vEP-34), and 9.7 μM (TLN). E, enzyme only.
FIG. 5.
Inhibition of vEP by nPP. vEP-34 (100 nM) was incubated without or with different concentrations of nPP at 37°C for 10 min (•) or overnight (○), and the residual proteolytic activity was measured with prewarmed azocasein and with a final enzyme concentration of 80 nM. Data are means ± standard deviations from two experiments carried out in triplicate.
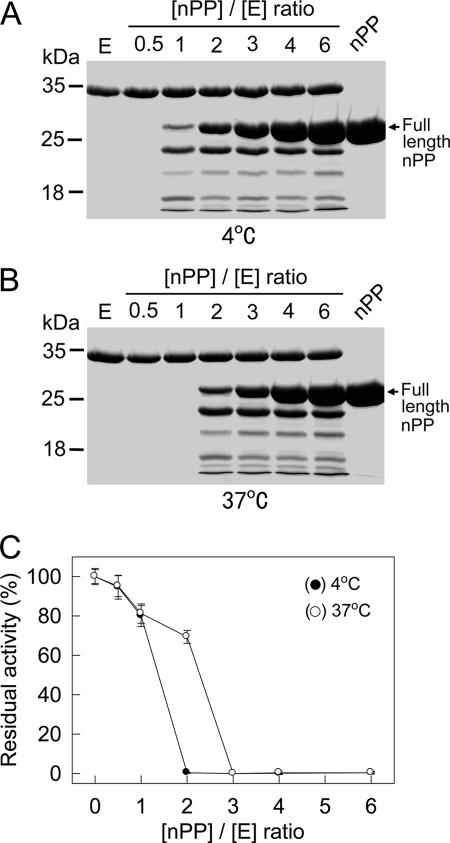
To determine if the cleaved products of nPP could still inhibit vEP activity, nPP was incubated with vEP overnight at an enzyme concentration about 100-fold that used in the activity assay study. SDS-PAGE of samples containing nPP plus vEP showed that nPP was cleaved by the enzyme at both 4°C and 37°C. Complete degradation was observed at [nPP]/[E] ratios of 0.5 and 1 at 4°C and 37°C, respectively (Fig. 6A and B). The degradation of nPP corresponded to the lack of inhibition of vEP activity as shown by an activity assay after the sample was diluted 100-fold into the prewarmed assay mixture (Fig. 6C). Some inhibition (20 to 30%) was observed only with samples that still contained full-length nPP. Complete inhibition was observed only for samples that still had an excess concentration of full-length nPP remaining (an [nPP]/[E] ratio of 2 or higher at 4°C; an [nPP]/[E] ratio of 3 or higher at 37°C).
FIG. 6.
Cleavage of nPP by vEP. vEP-34 (10 μg) was incubated without or with different amounts of nPP in a 30-μl reaction volume at 4°C (A) or 37°C (B) overnight. The final enzyme concentration was 9.8 μM. A 15-μl aliquot of each sample was then subjected to SDS-PAGE using a 12% gel (A and B), while 2-μl aliquots were diluted 100-fold into prewarmed (37°C) assay buffer containing azocasein and incubated for 10 min at 37°C to measure the residual protease activity (C). Data are means ± standard deviations from two experiments carried out in triplicate.
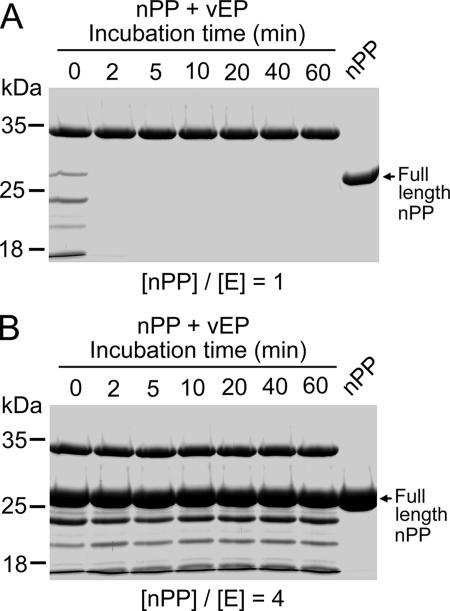
Time-dependent cleavage of nPP by vEP at 37°C showed that cleavage was a rapid process, with significant cleavage occurring even with no incubation (Fig. 7, time zero). The mere addition of vEP (kept on ice) to nPP followed by rapid mixing, which resulted in an elapsed time of about 6 s before addition of the SDS-PAGE sample buffer, caused significant cleavage of nPP for both [nPP]/[E] ratios of 1 and 4. After 2 min at an [nPP]/[E] ratio of 1 (Fig. 7A), nPP had disappeared, whereas at an [nPP]/[E] ratio of 4, the extent of cleavage of nPP remained unchanged throughout the entire 60 min of incubation (Fig. 7B) due to inhibition of vEP activity.
FIG. 7.
Time-dependent cleavage of nPP by vEP. Samples containing 5 μg of vEP-34 (9.8 μM) were incubated with nPP at an [nPP]/[E] ratio of 1 (A) or 4 (B) at 37°C for different times and were then subjected to SDS-PAGE using a 12% gel.
Effect of nPP on the thermal stability of vEP.
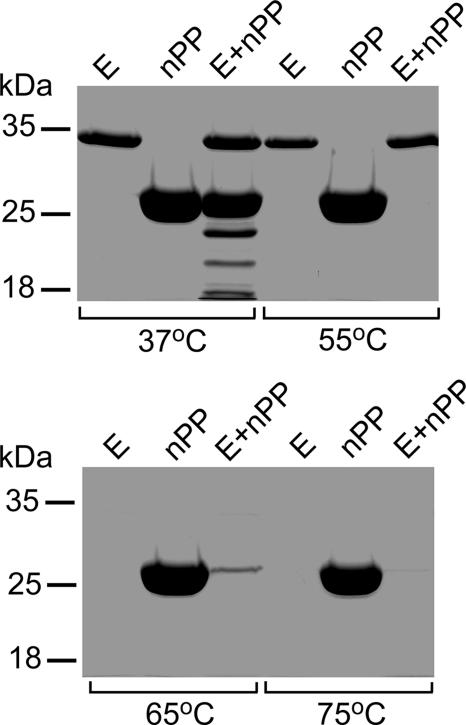
vEP is an enzyme that undergoes rapid autodegradation at high temperatures (65°C and above [unpublished data]). This autodegradation of vEP could be inhibited only by 1,10-phenanthroline at a concentration of 5 mM or higher. The noticeable autodegradation of vEP during the heating step for SDS-PAGE analysis could be prevented by addition of 1 mM 1,10-phenanthroline. To investigate whether the inhibitory effect of nPP on vEP activity could suppress the autodegradation of vEP at high temperatures, vEP was incubated with nPP at an [nPP]/[E] ratio of 4 at four different temperatures. At 37°C, there was some cleavage of nPP, as expected (Fig. 8). This cleavage of nPP was significantly enhanced at 55°C, resulting in complete loss of nPP, although there was little or no autodegradation of vEP. At 65°C, vEP was totally degraded in the absence or presence of nPP. However, little nPP still remained at this temperature when it was incubated with vEP. At 75°C, both vEP and nPP were totally degraded when incubated together. In the absence of vEP, nPP remained intact at all temperatures, confirming that both the cleavage of nPP and the autodegradation of vEP at high temperatures were due to the activity of vEP. At temperatures of 65°C and above, even a high molar ratio of nPP to the enzyme could not prevent the autodegradation of vEP, suggesting that the nPP-vEP complex is not very stable.
FIG. 8.
Effect of nPP on the thermal stability of vEP. vEP-34 (5 μg) and nPP (13.7 μg) were incubated separately or together ([nPP]/[E] ratio, 4) in a 15-μl reaction volume at different temperatures for 20 min and were then subjected to SDS-PAGE using a 12% gel. E, enzyme only.
DISCUSSION
In this paper, we show the expression and purification of two forms of vEP: a full-length enzyme (vEP-45) and a smaller version (vEP-34) lacking the C-terminal propeptide. We also describe the expression and purification of the N-terminal propeptide of vEP. vEP-45 and vEP-34 had similar activities, and both were inhibited by nPP. The absence of the C-terminal propeptide resulted in a loss of efficiency at cleaving insoluble proteins such as fibrin and elastin (Fig. 2B), thus confirming the observation reported previously for the enzyme from V. vulnificus L-180 (15). Due to the autoprocessing activity of vEP-45, which can result in various smaller forms, vEP-34 was used to further characterize the effect of nPP on vEP. The inhibitory effect of nPP on protease activity appeared to be specific for vEP, with no inhibition observed for the closely related enzyme thermolysin. The interaction between the mature protease and its own N-terminal propeptide resulting in inhibition of enzyme activity appears to be a specific interaction. For example, the propeptide of Pseudomonas aeruginosa elastase inhibits only the elastase without affecting thermolysin (8), and the propeptide of α-lytic protease inhibits the closely related Streptomyces griseus proteinase B but not the more distantly related yet structurally similar mammalian pancreatic elastase (1). Similarly, the N-terminal propeptide of a serine protease from Aspergillus fumigatus exhibited strong inhibition only toward its cognate enzyme while displaying no inhibition toward thermolysin or a metalloprotease from Aspergillus flavus (12). Furthermore, the N-terminal propeptide of A. fumigatus serine protease exhibited much less inhibition toward a serine protease from A. flavus despite a high degree of homology (83%) between the two enzymes. The propeptide of porcine carboxypeptidase A inhibits both porcine and bovine carboxypeptidase A enzymes but not porcine carboxypeptidase B (21). In contrast, the propeptides of aspartic proteinases display a very broad inhibitory spectrum (5). The N-terminal propeptide of vibriolysin (a homologue of proaminopeptidase-processing [PA] protease) can assist the refolding of PA protease as well as inhibiting its enzyme activity (23). Furthermore, the inhibition of mature PA protease by the N-terminal propeptide of vibriolysin is stronger than that by its own N-terminal propeptide despite the fact that the two propeptides have only 36% identity.
The kinetics of inhibition of the mature proteases by their respective N-terminal propeptides have been reported for some of the enzymes. For most enzymes, the N-terminal propeptide acts as a competitive inhibitor toward the mature enzyme; examples include subtilisin E (6), carboxypeptidase A (21), a serine protease from A. fumigatus (12), and a metalloprotease from Brevibacillus brevis (22). For some enzymes, such as thermolysin (5) and a PA protease from Aeromonas caviae T-64 (24), the inhibition is of a mixed noncompetitive mode. However, no explanation was given to account for the lack of cross inhibition for these N-terminal propeptides, and no reversible inhibition was mentioned. Since these enzymes are proteases that could cleave other proteins, either with or without substrate specificity, the lack of inhibition exerted by one N-terminal propeptide on a different protease could well result from the destruction of the propeptide through proteolysis. This speculation was supported by the complete degradation of nPP by thermolysin at both low and high [nPP]/[E] ratios (Fig. 4). nPP was also degraded by vEP at a low [nPP]/[E] ratio. At high [nPP]/[E] ratios, an excess of full-length nPP was able to completely inhibit vEP activity (Fig. 4). However, with a low total vEP concentration (e.g., 100 nM) and prolonged incubation (overnight), even the inhibition of vEP activity by nPP at high [nPP]/[E] ratios was totally reversed (Fig. 5), due to degradation of nPP. A study conducted with thermolysin showed that the processing of the proenzyme to the mature enzyme is an autocatalytic and intramolecular event, since a mutation at the mature sequence that inactivated the enzyme resulted in no processing of the proenzyme either in vivo or in vitro (11). The degradation of nPP by vEP suggests that removal of the N-terminal propeptide following processing of the proenzyme can also be an autocatalytic and intramolecular event.
The dissociation of the propeptide from the mature enzyme following secretion and maturation is necessary to release the active enzyme. For P. aeruginosa elastase, the dissociation of the propeptide from the mature enzyme is a well-coordinated process, and a host-specific factor is required to induce this event, since expression of P. aeruginosa elastase in Pseudomonas putida resulted in a substantial amount of the secreted form, but the propeptide-enzyme complex failed to dissociate spontaneously (2). In the absence of this factor, the dissociation of the complex is dynamically disfavored but may occasionally occur spontaneously. With vEP, the dissociation of the propeptide-enzyme complex may simply occur as a result of the degradation of the propeptide by the mature enzyme without requiring a host-specific factor.
We have previously reported that vEP is a highly unstable enzyme at high temperatures, resulting in complete autodegradation of the enzyme (3). So far, only 1,10-phenathroline could suppress this autodegradation activity at concentrations of 5 mM or above (unpublished data). Inhibition of vEP by nPP could not prevent the autodegradation activity of vEP, which appeared to be stimulated by high temperatures (Fig. 8). High temperatures such as 65°C or above might have a denaturing effect on nPP and thereby facilitate its rapid degradation by vEP. High temperatures could also cause the aggregation of nPP into an insoluble form, and therefore no preheating treatment of nPP was performed to assess the effect of heat treatment on the cleavage of nPP by vEP. Unfolding of vEP itself could also occur at temperatures of 55°C, as seen by the slight decrease in the intensities of the vEP bands compared with those observed at 37°C (Fig. 8). At higher temperatures, more-extensive unfolding of vEP might have occurred, thereby contributing to its complete autodegradation (Fig. 8).
The inhibition of vEP by nPP appeared to be mediated by full-length nPP and not by fragments of nPP bearing the correct sequence, such as a peptide inhibitor. The correct conformation of nPP appeared to be important for inhibition, and thus, cleavage of nPP by vEP would result in the loss of this conformation, leading to the loss of inhibition. Expression of different truncated forms of nPP and testing for their abilities to inhibit vEP activity would provide definitive confirmation. Attempts to determine the kinetics of inhibition have so far been unsuccessful due to the fact that nPP is both an inhibitor and a substrate. Understanding how nPP interacts with vEP at the molecular level, including the elucidation of the binding site for nPP and vEP, may help with finding a specific inhibitor for this enzyme. Inhibitors for thermolysin such as Z-Phe-OH have no effect on vEP (unpublished data), and therefore, an inhibitor specific for vEP is of vital interest in a broad area of study relating to this enzyme both in vitro and in vivo.
Acknowledgments
This work was supported by the Ministry of Science and Technology of Korea, by KOSEF through the Research Center for Proteineous Materials (RCPM) of Chosun University, and by research funds from Chosun University, 2006.
Footnotes
Published ahead of print on 20 July 2007.
REFERENCES
- 1.Baker, D., J. L. Silen, and D. A. Agard. 1992. Protease pro region required for folding is a potent inhibitor of the mature enzyme. Proteins 12:339-344. [DOI] [PubMed] [Google Scholar]
- 2.Braun, P., W. Bitter, and J. Tommassen. 2000. Activation of Pseudomonas aeruginosa elastase in Pseudomonas putida by triggering dissociation of the propeptide-enzyme complex. Microbiology 146:2565-2572. [DOI] [PubMed] [Google Scholar]
- 3.Chang, A. K., H. Y. Kim, J. E. Park, P. Acharya, I. S. Park, S. M. Yoon, H. J. You, K. S. Hahm, J. K. Park, and J. S. Lee. 2005. Vibrio vulnificus secretes a broad-specificity metalloprotease capable of interfering with blood homeostasis through prothrombin activation and fibrinolysis. J. Bacteriol. 187:6909-6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuda, R., H. Horiuchi, A. Ohta, and M. Takagi. 1994. The prosequence of Rhizopus niveus aspartic proteinase-I supports correct folding and secretion of its mature part in Saccharomyces cerevisiae. J. Biol. Chem. 269:9556-9561. [PubMed] [Google Scholar]
- 5.Fusek, M., M. Mares, J. Vagner, Z. Voburka, and M. Baudys. 1991. Inhibition of aspartic proteinases by propart peptides of human procathepsin D and chicken pepsinogen. FEBS Lett. 287:160-162. [DOI] [PubMed] [Google Scholar]
- 6.Hu, Z., K. Haghjoo, and F. Jordan. 1996. Further evidence for the structure of the subtilisin propeptide and for its interactions with mature subtilisin. J. Biol. Chem. 271:3375-3384. [DOI] [PubMed] [Google Scholar]
- 7.Jeong, K. C., H. S. Jeong, J. H. Rhee, S. E. Lee, S. S. Chung, A. M. Starks, G. M. Escudero, P. A. Gulig, and S. H. Choi. 2000. Construction and phenotypic evaluation of a Vibrio vulnificus vvpE mutant for elastolytic protease. Infect. Immun. 68:5096-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kessler, E., and M. Safrin. 1994. The propeptide of Pseudomonas aeruginosa elastase acts an elastase inhibitor. J. Biol. Chem. 269:22726-22731. [PubMed] [Google Scholar]
- 9.Kim, D. W., and H. Matuzawa. 2000. Requirement for the COOH-terminal pro-sequence in the translocation of aqualysin I across the cytoplasmic membrane in Escherichia coli. Biochem. Biophys. Res. Commun. 277:216-220. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Marie-Claire, C., B. P. Roques, and A. Beaumont. 1998. Intramolecular processing of prothermolysin. J. Biol. Chem. 273:5697-5701. [DOI] [PubMed] [Google Scholar]
- 12.Markaryan, A., J. D. Lee, T. D. Sirakova, and P. E. Kolattukudy. 1996. Specific inhibition of mature fungal serine proteinases and metalloproteinases by their propeptides. J. Bacteriol. 178:2211-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIver, K. S., E. Kessler, J. C. Olson, and D. E. Ohman. 1995. The elastase propeptide functions as an intramolecular chaperone required for elastase activity and secretion in Pseudomonas aeruginosa. Mol. Microbiol. 18:877-889. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki, H., N. Yanagida, S. Horinouchi, and T. Beppu. 1989. Characterization of the precursor of Serratia marcescens serine protease and COOH-terminal processing of the precursor during its excretion through the outer membrane of Escherichia coli. J. Bacteriol. 171:6566-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyoshi, S., H. Wakae, K. Tomochika, and S. Shinoda. 1997. Functional domains of a zinc metalloprotease from Vibrio vulnificus. J. Bacteriol. 179:7606-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyoshi, S. 2006. Vibrio vulnificus infection and metalloprotease. J. Dermatol. 33:589-595. [DOI] [PubMed] [Google Scholar]
- 17.Nirasawa, S., Y. Nakajima, Z. Z. Zhang, M. Yoshida, and K. Hayashi. 1999. Intramolecular chaperone and inhibitor activities of a propeptide from a bacterial zinc aminopeptidase. Biochem. J. 341:25-31. [PMC free article] [PubMed] [Google Scholar]
- 18.O'Donohue, M. J., and A. Beaumont. 1996. The roles of the prosequence of thermolysin in enzyme inhibition and folding in vitro. J. Biol. Chem. 271:26477-26481. [DOI] [PubMed] [Google Scholar]
- 19.Ramos, C., J. R. Winther, and M. C. Kielland-Brandt. 1994. Requirement of the propeptide for the in vivo formation of active yeast carboxypeptidase Y. J. Biol. Chem. 269:7006-7012. [PubMed] [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 21.Segundo, B. S., M. C. Martinez, M. Vilanova, C. M. Cuchillo, and F. X. Aviles. 1982. The severed activation segment of porcine pancreatic procarboxypeptidase A is a powerful inhibitor of the active enzyme. Isolation and characterization of the activation peptide. Biochim. Biophys. Acta 707:74-80. [DOI] [PubMed] [Google Scholar]
- 22.Serkina, A. V., T. F. Gorozhankina, A. B. Shevelev, and G. G. Chestukhina. 1999. Propeptide of the metalloprotease of Brevibacillus brevis 7882 is a strong inhibitor of the mature enzyme. FEBS Lett. 456:215-219. [DOI] [PubMed] [Google Scholar]
- 23.Tang, B., S. Nirasawa, M. Kitaoka, C. Marie-Claire, and K. Hayashi. 2003. General function of N-terminal propeptide on assisting protein folding and inhibiting catalytic activity based on observations with a chimeric thermolysin-like protease. Biochem. Biophys. Res. Commun. 301:1093-1098. [DOI] [PubMed] [Google Scholar]
- 24.Tang, B., S. Nirasawa, M. Kitaoka, and K. Hayashi. 2002. The role of the N-terminal propeptide of the pro-aminopeptidase processing protease: refolding, processing, and enzyme inhibition. Biochem. Biophys. Res. Commun. 296:78-84. [DOI] [PubMed] [Google Scholar]
- 25.van den Hazel, H. B., M. C. Kielland-Brandt, and J. R. Winther. 1993. The propeptide is required for in vivo formation of stable active yeast proteinase A and can function even when not covalently linked to the mature region. J. Biol. Chem. 268:18002-18007. [PubMed] [Google Scholar]
- 26.Zhang, Z. Z., S. Nirasawa, Y. Nakajima, M. Yoshida, and K. Hayashi. 2000. Function of the N-terminal propeptide of an aminopeptidase from Vibrio proteolyticus. Biochem. J. 350:671-676. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu, Z., D. Sun, and V. L. Davidson. 1999. Localization of periplasmic redox proteins of Alcaligenes faecalis by a modified general method for fractionating gram-negative bacteria. J. Bacteriol. 181:6540-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu, X. L., Y. Ohta, F. Jordan, and M. Inouye. 1989. Pro-sequence of subtilisin can guide the refolding of denatured subtilisin in an intermolecular process. Nature 339:483-484. [DOI] [PubMed] [Google Scholar]