Abstract
Bacillus subtilis encodes seven extracytoplasmic function (ECF) σ factors that regulate partially overlapping regulons related to cell envelope homeostasis and antibiotic resistance. Here, we investigated their physiological role by constructing a mutant set of single, double, triple, and quadruple ECF σ factor deletions in the undomesticated B. subtilis strain NCIB3610. This mutant set was subsequently screened for defects in motility, multicellular differentiation, and sensitivity to more than 200 chemicals by using Phenotype MicroArrays. A quadruple mutant strain, harboring deletions of the sigV, sigY, sigZ, and ylaC gene, behaved indistinguishably from the wild-type strain, indicative of either regulatory redundancy or very specific functions of these four ECF σ factors. In contrast, a triple mutant, inactivated for the sigM, sigW, and sigX genes (but none of the corresponding double mutants), showed a biphasic growth behavior and a complete loss of multicellular differentiation, as judged by both colony formation and the inability to form a pellicle. This triple mutant also displayed a greatly increased sensitivity to detergents and several cell wall antibiotics including β-lactams, polymyxin B, and d-cycloserine. In several cases, these antibiotic-sensitive phenotypes are significantly enhanced in the triple mutant strain relative to strains lacking only one or two σ factors.
The genome of Bacillus subtilis harbors seven extracytoplasmic function (ECF) σ factors. So far, the physiological roles of four of these σ factors have been investigated in some detail (σM, σW, σX, and σY), while the roles of the other three (σV, σZ, and σYlaC) remain elusive. σX was the first ECF σ factor to be analyzed in detail (19, 21). It is induced by inhibitors of peptidoglycan biosynthesis and tunicamycin (17). σX regulates several genes related to cell wall metabolism including the dltA and pssA operons that affect the overall net charge of the cell envelope by incorporating positively charged groups into teichoic acids and the cytoplasmic membrane, respectively. A sigX mutant strain displays increased sensitivity to cationic antimicrobial peptides (8) and is affected in the formation of cell chain clusters during biofilm formation (26). σW is induced by various cell wall antibiotics, alkaline shock, and other stresses affecting the cell envelope (13, 29, 37). It controls a large “antibiosis” regulon involved in mediating resistance to various antibiotics including fosfomycin and the antibiotic peptides sublancin and SdpC (6, 7, 10). σM is activated in response to numerous stresses including high salinity, ethanol, heat, acid, phosphate starvation, superoxide stress, and exposure to cell wall antibiotics such as bacitracin, vancomycin, and cationic antimicrobial peptides (13, 18, 27, 29, 33). A sigM mutant strain is more sensitive to bacitracin, paraquat, and high salinity (9, 11, 27, 33). It has also been demonstrated that in B. subtilis strain W23 σM, together with σX, is involved in teichoic acid biosynthesis and septum formation (28). Identification of two target operons of a fourth ECF σ factor, σY, suggests that this protein may regulate expression of a toxic peptide and the corresponding immunity gene (12).
The ECF σ factors of B. subtilis recognize structurally similar promoter sequences characterized by a highly conserved AAC motif in the −35 region and a CGT motif in the −10 region (17). While our understanding of promoter recognition by this family of σ factors is still incomplete, it is clear that there is the potential for regulatory overlap (9, 20). It has been shown, for example, that the autoregulatory promoter sites for the sigW and sigX genes are specifically recognized by their cognate σ factors but that only one or two base changes in the −10 recognition element lead to sites that can be recognized by both σ factors (30). Similarly, some targets of ECF σ factor regulation have promoter sites that are recognized in vitro and, apparently, also in vivo by more than one holoenzyme species (9, 20). A recently described list of putative σV-dependent genes almost completely overlaps with genes that have already been shown to be under the control of σM, σW, or σX (39). In light of this potentially significant regulatory overlap, it seems likely that the lack of dramatic phenotypes associated with null mutations in many ECF σ factor genes in B. subtilis may be due, in part, to redundancy.
Here, we present studies to determine phenotypes associated with single and multiple mutations in ECF σ factors in B. subtilis strain NCIB3610. Strain NCIB3610 displays complex multicellular behaviors such as swarming motility, pellicle formation (a thick biofilm at the liquid-air interface in standing cultures), and fruiting body formation (2-5, 14, 23-25, 32). Many of these complex, multigenic phenotypes have been lost by mutation in B. subtilis 168 and other laboratory strains but are common among environmental isolates. Thus, NCIB3610 is sometimes referred to as an undomesticated strain. Our findings demonstrate that even a quadruple mutant of four ECF σ factor genes (sigV, sigY, ylaC, and sigZ) is phenotypically silent in a broad range of assays. In contrast, single mutations of the sigM, sigX, and sigW genes display many of the same phenotypes noted previously in strain 168. Remarkably, a triple mutant (sigM sigW sigX), but none of the corresponding single or double mutants, displays several new phenotypes, including growth defects, increased antibiotic sensitivity, and a dramatic loss of the ability to form robust pellicles. These results provide further evidence for regulatory and functional overlap between these three σ factor regulons.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
To assess the role of ECF σ factors in strain NCIB3610, we generated a set of mutant strains as summarized in Table 1. For the mutations already constructed in the 168 derivative CU1065, the mutant alleles were crossed into strain NCIB3610 using SPP1-mediated generalized transduction as described previously (3). To circumvent selection incompatibilities, additional mutants were constructed by long-flanking homology (LFH)-PCR (see below). SPP1 lysates were prepared, and the deletions were introduced into NCIB3610 in a stepwise fashion (Table 1). The success of the allelic replacements was verified by direct colony PCR.
TABLE 1.
Strains used in this study
| B. subtilis strain | Genotype and/or description | Source, reference, or constructiona |
|---|---|---|
| CU1065 | W168 trpC2 attSPβ | Laboratory stock |
| NCIB3610 | undomesticated wildtype strain, ancestor of W168 | 3 |
| HB0009 | CU1065, sigY::mls; donor strain for sigY::mls allele | 12 |
| HB0020 | CU1065 sigW::mls; donor strain for sigW::mls allele | 7 |
| HB0028 | CU1065, sigV::kan; donor strain for sigV::kan allele | 11 |
| HB0029 | CU1065, ylaC::kan; donor strain for ylaC::kan allele | 11 |
| HB0030 | CU1065, sigX::spec sigW::mls | M. Cao (unpublished) |
| HB0031 | CU1065, sigM::kan; donor strain for sigM::kan allele | 13 |
| HB0032 | CU1065, sigZ::kan; donor strain for sigZ::kan allele | 11 |
| HB7007 | CU1065 sigX::spec; donor strain for sigX::spec allele | 19 |
| HB0911 | CU1065 sigV::cat; donor strain for sigV::cat allele | LFH-PCR → CU1065 |
| HB0912 | CU1065 sigY::cat; donor strain for sigY::cat allele | LFH-PCR → CU1065 |
| HB0913 | CU1065 sigZ::cat; donor strain for sigZ::cat allele | LFH-PCR → CU1065 |
| HB0914 | CU1065 ylaC::cat; donor strain for ylaC::cat allele | LFH-PCR → CU1065 |
| HB0915 | CU1065 ylaC::spec; donor strain for ylaC::spec allele | LFH-PCR → CU1065 |
| HB0982 | CU1065 sigM::kan sigW::mls sigX::spec | SPP1HB0031 → HB0030 |
| HB0801 | NCIB3610 sigM::kan | SPP1HB0031 → NCIB3610 |
| HB0802 | NCIB3610 sigV::kan | SPP1HB0028 → NCIB3610 |
| HB0803 | NCIB3610 sigW::mls | SPP1HB0020 → NCIB3610 |
| HB0804 | NCIB3610 sigX::spec | SPP1HB7007 → NCIB3610 |
| HB0805 | NCIB3610 sigY::mls | SPP1HB0031 → NCIB3610 |
| HB0806 | NCIB3610 sigZ::kan | SPP1HB0032 → NCIB3610 |
| HB0807 | NCIB3610 ylaC::kan | SPP1HB0029 → NCIB3610 |
| HB0808 | NCIB3610 ylaC::spec | SPP1HB0915 → NCIB3610 |
| HB0820 | NCIB3610 sigW::mls sigX::spec | SPP1HB7007 → HB0803 |
| HB0822 | NCIB3610 sigY::mls sigV::kan | SPP1HB0028 → HB0805 |
| HB0823 | NCIB3610 sigY::mls sigZ::kan | SPP1HB7007 → HB0805 |
| HB0824 | NCIB3610 sigY::mls ylaC::kan | SPP1HB0029 → HB0805 |
| HB0825 | NCIB3610 sigV::kan sigZ::cat | SPP1HB0911 → HB0802 |
| HB0826 | NCIB3610 sigV::kan ylaC::cat | SPP1HB0914 → HB0802 |
| HB0827 | NCIB3610 sigZ::kan ylaC::cat | SPP1HB0914 → HB0806 |
| HB0828 | NCIB3610 sigM::kan sigX::spec | SPP1HB7007 → HB0801 |
| HB0829 | NCIB3610 sigW::mls sigM::kan | SPP1HB0031 → HB0803 |
| HB0834 | NCIB3610 sigW::mls sigX::spec sigM::kan | SPP1HB0031 → HB0820 |
| HB0838 | NCIB3610 sigY::mls sigV::kan ylaC::spec | SPP1HB0911 → HB0822 |
| HB0839 | NCIB3610 sigY::mls sigV::kan sigZ::cat | SPP1HB0913 → HB0822 |
| HB0840 | NCIB3610 sigY::mls sigZ::kan ylaC::spec | SPP1HB0915 → HB0823 |
| HB0841 | NCIB3610 sigV::kan sigZ::cat ylaC::spec | SPP1HB0915 → HB0825 |
| HB0842 | NCIB3610 sigY::mls sigV::kan ylaC::spec sigZ::cat | SPP1HB0913 → HB0838 |
| HB0844 | NCIB3610 sigW::mls sigX::spec sigM::kan sigV::cat | SPP1HB0911 → HB0834 |
| HB0845 | NCIB3610 sigW::mls sigX::spec sigM::kan sigY::cat | SPP1HB0912 → HB0834 |
| HB0846 | NCIB3610 sigW::mls sigX::spec sigM::kan sigZ::cat | SPP1HB0913 → HB0834 |
| HB0847 | NCIB3610 sigW::mls sigX::spec sigM::kan ylaC::cat | SPP1HB0914 → HB0834 |
LFH- PCR (35) was applied as described previously (27) to construct some of the ECF σ factor deletions using the primers listed in Table 2. Construction of deletion mutants in the nontransformable B. subtilis strain NCIB3610 was achieved by generalized transduction using bacteriophage SPP1 lysates from the respective donor strains as described previously (3).
For strain construction and precultures, B. subtilis strains 168 and NCIB3610 were grown at 37°C in Luria-Bertani (LB) medium (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl per liter of broth) or on LB plates supplemented with 1.5% agar with appropriate selection. Antibiotics were supplemented at the following concentrations: 10 μg ml−1 tetracycline, 100 μg ml−1 spectinomycin, 5 μg ml−1 chloramphenicol, 10 μg ml−1 kanamycin, and 1 μg ml−1 erythromycin plus 25 μg ml−1 lincomycin.
For pellicle formation experiments, 50 μl of mid-log-phase culture was inoculated into 10 ml of minimal MSgg medium (5 mM potassium phosphate, pH 7, 100 mM morpholinepropanesulfonic acid [pH 7], 2 mM MgCl2, 700 μM CaCl2, 50 μM MnCl2, 50 μM FeCl3, 1 μM ZnCl2, 2 μM thiamine, 0.5% glycerol, 0.5% glutamate, 50 μg ml−1 tryptophan, 50 μg ml−1 phenylalanine, and 50 μg ml−1 threonine) and incubated at 22°C (3). For colony architecture analysis, 10 μl of LB precultures was spotted onto minimal MSgg agar plates (dried for 30 min in a laminar airflow prior to spotting) and incubated at 22°C. Swarming motility and sporulation were assayed according to published procedures (16, 25).
LFH-PCR.
The LFH-PCR technique is derived from a published procedure (35) and was performed as described previously (27). In brief, antibiotic resistance cassettes were amplified from plasmids: the cat cassette from pGEM-cat (38) and the kan, mls, and spec cassettes from pDG780, pDG646, and pDG1726, respectively (15). Two primer pairs were designed to amplify ∼1,000 bp of DNA fragments flanking the region to be deleted at its 5′ and 3′ ends. The resulting fragments are here called “Up” and “Do” fragments, respectively. The 3′ end of the Up fragment as well as the 5′ end of the Do fragment extended into the gene(s) to be deleted in a way that all expression signals of genes up- and downstream of the targeted genes remained intact. Extensions of ∼25 nucleotides were added to the 5′ end of the Up-reverse and the Do-forward primers that were complementary (opposite strand and inverted sequence) to the 5′ and 3′ ends of the amplified resistance cassette. All obtained fragments were purified using a QIAquick PCR purification kit (QIAGEN Sciences, Maryland). A total of 100 to 150 ng of the Up and Do fragments and 250 to 300 ng of the resistance cassette were used together with the specific Up-forward and Do-reverse primers at standard concentrations in a second PCR. In this reaction the three fragments were joined by the 25-nucleotide overlapping complementary ends and simultaneously amplified by normal primer annealing. The PCR products were directly used to transform B. subtilis. Transformants were screened by colony PCR, using the Up-forward primer with a reverse check primer annealing inside the resistance cassette (Table 2). The integrity of the regions flanking the integrated resistance cassettes was verified by sequencing PCR products of ∼1,000 bp amplified from chromosomal DNA of the resulting mutants. Sequencing was performed at the Cornell BioResource Center. All PCRs were done in a total volume of 50 μl (10 μl for colony PCR) using HotStar DNA-Polymerase Mastermix (QIAGEN Sciences, Maryland) or TripleMaster Polymerase Mix (Eppendorf North America, Westbury, NY), according to the manufacturer's procedure. The primers used in this study are listed in Table 2.
TABLE 2.
Oligonucleotides used in this study
| Primer no. | Namea | Sequence |
|---|---|---|
| 1293 | cat fwd | CGGCAATAGTTACCCTTATTATCAAG |
| 1294 | cat rev | CCAGCGTGGACCGGCGAGGCTAGTTACCC |
| 1449 | cat check rev | GTCTGCTTTCTTCATTAGAATCAATCC |
| 1295 | kan fwd | CAGCGAACCATTTGAGGTGATAGG |
| 1296 | kan rev | CGATACAAATTCCTCGTAGGCGCTCGG |
| 1450 | kan-check rev | CTGCCTCCTCATCCTCTTCATCC |
| 1297 | mls fwd | GATCCTTTAACTCTGGCAACCCTC |
| 1298 | mls rev | GCCGACTGCGCAAAAGACATAATCG |
| 1451 | mls-check rev | GTTTTGGTCGTAGAGCACACGG |
| 1587 | spec fwd | ATCGATTTTCGTTCGTGAATACATG |
| 1588 | spec rev | GCAAGGGTTTATTGTTTTCTAAAATCTG |
| 1452 | spec-check rev | CGTATGTATTCAAATATATCCTCCTCAC |
| 751 | sigY-Up fwd | GGCAGATCAATCGCTCCG |
| 1334 | sigY-Up rev | CTTGATAATAAGGGTAACTATTGCCGCTTGTGTATCCAATGACCGTGATCCC |
| 1335 | sigY-Do fwd | CCGTTAGTTGAAGAAGGTTTTTATATTACAGCGGCACTGTAAAGTCCAGAGTTCATAAAGG |
| 1340 | sigY-Do rev | CAGAGGTTGGCGAAAGCAGTCCG |
| 1330 | sigV-Up fwd | GCAAGAATCACCTTTAACAGGCTATGCCG |
| 1331 | sigV-Up rev | CTTGATAATAAGGGTAACTATTGCCGGTCAGTTATGCATGTGACAAGCAACGC |
| 1332 | sigV-Do fwd | CCGTTAGTTGAAGAAGGTTTTTATATTACAGCCTATACAGAGCATTGAAGCTGATGCGC |
| 1333 | sigV-Do-rev | GTGTCTTGTGATACATGGGTATTCCTCC |
| 1342 | sigZ-Up fwd | GCTCAACCGTTCGTGGTCCGATGATCGG |
| 1343 | sigZ-Up rev | CTTGATAATAAGGGTAACTATTGCCGCGGCTGATGAAATTGATCCCATAGATCC |
| 1344 | sigZ-Do fwd | CCGTTAGTTGAAGAAGGTTTTTATATTACAGCGCTGTCACATTGAAGCGGATCGATATGG |
| 1345 | sigZ-Do rev | CAATTCTCCACAGAAAAAATGGCTATGGC |
| 1346 | ylaC-Up fwd | AAAGATCATGCTGATGATGGCGCGG |
| 1347 | ylaC-Up rev | CTTGATAATAAGGGTAACTATTGCCGACAAGTCCTCAATGGAATCCCTATGC |
| 1348 | ylaC-Up rev2 | TATAACATGTATTCACGAACGAAAATCGACAAGTCCTCAATGGAATCCCTATGC |
| 1349 | ylaC-Do fwd | CCGTTAGTTGAAGAAGGTTTTTATATTACAGCCCACATTGCACCGGGCTAGATTAGAGC |
| 1350 | ylaC-Do fwd2 | CAGATTTTAGAAAACAATAAACCCTTGCCCACATTGCACCGGGCTAGATTAGAGC |
| 1351 | ylaC-Do rev | GCTATGTTAACGACTGCCGCAATGACC |
fwd, forward; rev, reverse.
Phenotypic characterization.
Phenotype MicroArray assays were performed by Biolog (Hayward, CA), according to the published procedure (1, 34, 40) using turbidity measurements since, at the time of these analyses (2003), the dyes in use by Biolog were toxic to B. subtilis (B. Bochner, personal communication). Incubation and recording of phenotypic data were performed in the OmniLog station by capturing digital images of the microarray and storing turbidity values in a computer file displayed as a kinetic graph. The Biolog plates used for these analyses were PM9, PM10, PM31A, PM32A, PM33A, PM34B, PM35B, and PM36 as described on the Biolog website (http://www.biolog.com/PM_Maps.html and http://www.biolog.com/PMArchived_Maps.html). The OmniLog-PM software generates time course curves for turbidity and calculates differences in the areas for mutant and control cells. The units are arbitrary. Positive values indicate that the mutant showed greater rates of growth than the control. The differences are averages of values reported for two or more mutants of each type compared with the corresponding control strains. All significant hits (as defined by Biolog) are listed in Table 3 for the comparison of strain HB0834 (ΔsigMWX; hereafter, MWX mutant) versus NCIB3610.
TABLE 3.
Phenotype MicroArrray analysis of the MWX strain versus NCIB3610
| Phenotype group and PM no.a | Well(s) | Growth conditions | Differenceb | Mode of actionc |
|---|---|---|---|---|
| Gained phenotypes (resistance) | ||||
| PM32 | A04 | t-Butyl hydroquinone | 43 | Oxidizing agent |
| PM36 | E09, E10 | Neomycin | 27 | Protein synthesis, aminoglycoside |
| PM32 | B01 | Lincomycin | 11 | Protein synthesis, lincosamide |
| Lost phenotypes (sensitivity) | ||||
| PM36 | C02-C04 | 2-Hydroxybenzoic acid | −41 | Anticapsule; multiple antibiotic resistance inducer |
| PM34 | E10, E11 | Warfarin | −55 | Antimicrobial, from plants |
| PM31 | B09-B12 | Fusaric acid | −73 | Chelator, lipophilic |
| PM31 | C03 | 1-Hydroxy-pyridine-2-thione | −22 | Chelator, lipophilic |
| PM35 | C09 | Cinoxacin | −41 | DNA gyrase, DNA topoisomerase |
| PM35 | A06 | Miltefosine | −45 | Fungicide, protein kinase C |
| PM36 | A09-A11 | Ibuprofen | −87 | Inhibitor prostaglandin synthetase |
| PM31 | E11 | Chlorpromazine | −67 | Inhibits cyclic nucleotide phosphodiesterase |
| PM36 | A03 | Alexidine | −79 | Membrane, biguanide, electron transport |
| PM31 | F05-F07 | Lauroylsarcosine | −103 | Membrane, detergent |
| PM31 | H03, H04 | Triton X-100 | −137 | Membrane, detergent |
| PM35 | B04 | CHAPSO | −50 | Membrane, detergent |
| PM35 | B08 | Lauryl sulfobetaine | −24 | Membrane, detergent |
| PM36 | D11, D12 | Dodecyl maltoside | −99 | Membrane, detergent, nonionic |
| PM35 | C04 | Iodoacetate | −64 | Oxidation, sulfhydryl |
| PM10 | A03 | pH 4.5 | −38 | pH |
| PM10 | E08 | pH 9.5 glycine | −23 | pH, deaminase |
| PM10 | F10 | pH 9.5, l-ornithine | −26 | pH, deaminase |
| PM10 | F11 | pH 9.5, l-homoarginine | −38 | pH, deaminase |
| PM10 | F12 | pH 9.5, l-homoserine | −52 | pH, deaminase |
| PM10 | G02 | pH 9.5, l-norleucine | −16 | pH, deaminase |
| PM10 | G03 | pH 9.5, l-norvaline | −35 | pH, deaminase |
| PM10 | B03 | pH 4.5, l-arginine | −49 | pH, decarboxylase |
| PM10 | B04 | pH 4.5, l-asparagine | −32 | pH, decarboxylase |
| PM10 | B05 | pH 4.5, l-aspartic acid | −35 | pH, decarboxylase |
| PM10 | B06 | pH 4.5, l-glutamic acid | −86 | pH, decarboxylase |
| PM10 | B07 | pH 4.5, l-glutamine | −74 | pH, decarboxylase |
| PM10 | B08 | pH 4.5, glycine | −72 | pH, decarboxylase |
| PM10 | B09 | pH 4.5, l-histidine | −71 | pH, decarboxylase |
| PM10 | B10 | pH 4.5, l-isoleucine | −54 | pH, decarboxylase |
| PM10 | C02 | pH 4.5, l-phenylalanine | −56 | pH, decarboxylase |
| PM10 | C04 | pH 4.5, l-serine | −47 | pH, decarboxylase |
| PM10 | C05 | pH 4.5, l-threonine | −57 | pH, decarboxylase |
| PM10 | C06 | pH 4.5, l-tryptophan | −21 | pH, decarboxylase |
| PM10 | C07 | pH 4.5, l-tyrosine | −43 | pH, decarboxylase |
| PM10 | C08 | pH 4.5, l-valine | −59 | pH, decarboxylase |
| PM10 | D02 | pH 4.5, l-norleucine | −34 | pH, decarboxylase |
| PM10 | D03 | pH 4.5, l-norvaline | −82 | pH, decarboxylase |
| PM10 | D04 | pH 4.5, a-amino-N-butyric acid | −23 | pH, decarboxylase |
| PM10 | D07 | pH 4.5, g-hydroxy glutamate | −56 | pH, decarboxylase |
| PM10 | D11 | pH 4.5, trimethylamine-N-oxide | −81 | pH, decarboxylase |
| PM10 | B01 | pH 4.5 | −61 | pH, decarboxylase control |
| PM35 | C06 | Compound 48/80 | −56 | Phospholipase C, ADP ribosylation |
| PM32 | D10-D12 | Cadmium chloride | −35 | Transport, toxic cation |
| PM32 | E10-E12 | Phosphomycin | −82 | Wall |
| PM35 | H07, H08 | Glycine | −60 | Wall |
| PM35 | H12 | Bacitracin, zinc salt | −99 | Wall and membrane |
| PM32 | G03, G04 | Cefotaxime | −131 | Wall, cephalosporin |
| PM32 | G05 | Cefazolin | −90 | Wall, cephalosporin |
| PM32 | H01 | Cefoxitin | −58 | Wall, cephalosporin |
| PM32 | H05 | Cefoperazone | −75 | Wall, cephalosporin |
| PM33 | F02-F04 | Cefsulodin | −149 | Wall, cephalosporin |
| PM33 | F05, F06 | Cephaloridine | −134 | Wall, cephalosporin |
| PM33 | F11, F12 | Cefuroxime | −80 | Wall, cephalosporin |
| PM33 | G09, G10 | Azlocillin | −107 | Wall, β-lactam |
| PM32 | H09, H10 | d-Cycloserine | −100 | Wall, sphingolipid synthesis |
Phenotype MicroArrray (PM) plate numbers are as described on the Biolog website (see Materials and Methods).
Growth measurements were done using turbidity as described in Methods and Materials. Negative values indicate significantly poorer growth of the MWX strain relative to NCIB3610 while positive values indicate better growth of the MWX strain relative to NCIB3610.
Possible effect or mode of action (original Biolog annotation). Not all modes of action are applicable to B. subtilis.
Some of the sensitivities identified by Phenotype MicroArray analysis were subsequently verified by disk diffusion assays. Cultures of NCIB3610, the MWX mutant, and the corresponding single and double mutants were inoculated from fresh overnight cultures (with selection) in LB medium and incubated (without selection) at 37°C with aeration until an optical density at 600 nm of ∼1.0 (late log phase). One hundred milliliters of these cultures was mixed with 3 ml of 0.7% LB soft agar (kept liquid at 50°C) and directly poured onto LB plates (without selection). After 30 min at room temperature (to allow the soft agar to solidify), the plates were dried for 20 min in a laminar airflow hood. After cooling, filter paper disks (5.5-mm diameter) carrying the antibiotics to be tested (5 μl from stock solutions were used per filter disk; antibiotics were at a concentration of 100 mg ml−1 with the exception of polymyxin B at 10 mg ml−1 and moenomycin at 0.25 mg ml−1) were placed on the top of the agar, and the plates were incubated at 37°C overnight. The next day, the diameters of the inhibition zones were measured (after subtraction of the diameter of the filter paper disks).
Position weight matrices.
The promoter consensus sequence alignment was performed with the Weblogo software (http://weblogo.berkeley.edu/). The σX regulon (11 target promoters) (8) and σW regulon (30 promoters) (6, 10) are based on published tabulations. The σM regulon is based on the tabulation of eight promoters listed by Jervis et al. (22) together with 10 recently identified promoters sites (W. Eiamphungporn and J. D. Helmann, unpublished results).
RESULTS AND DISCUSSION
Construction of ECF mutant strains in NCIB3610.
To assess the role of ECF σ factors in strain NCIB3610, we constructed a set of strains including all seven single mutants and selected multiple mutants. Since little is known regarding the roles of four of the ECF σ factors (σV, σY, σZ, and σYlaC), we generated a quadruple mutant (HB0842) to determine if this might reveal previously undisclosed phenotypes. In the case of the other three σ factors (σM, σW, and σX), where considerably more background information is available, we generated all possible single, double, and triple mutant strains. This collection was then screened for defects in multicellular differentiation and motility, as well as sensitivity against a multitude of chemical compounds utilizing Phenotypic MicroArrays (1).
ECF σ factors σM, σW, and σX are important for multicellular differentiation but not motility or sporulation.
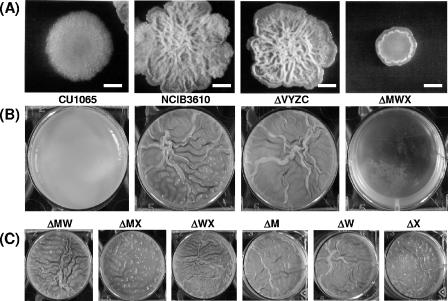
To address the role of ECF σ factors in multicellular differentiation, strains were inoculated in MSgg minimal medium or spotted on MSgg plates to allow pellicle formation or colony differentiation, respectively (3). As expected, the strain 168 derivative CU1065 formed undifferentiated round colonies and only formed a flat, skin-like pellicle. In contrast, NCIB3610 forms complex colony patterns on MSgg plates and thick, wrinkled pellicles in MSgg medium (Fig. 1A and B). The quadruple mutant HB0842 (lacking σV, σY, σZ, and σYlaC) behaved indistinguishably from the corresponding wild type. In contrast, the triple mutant MWX strain completely lost its ability to differentiate on MSgg plates, forming small and relatively uniform-looking colonies and grew very poorly at the liquid-air interface in MSgg medium. This behavior was unique to the MWX triple mutant, since all related double and single mutants behaved just like the wild type (Fig. 1C). These results indicate that these three σ factors have overlapping functions with respect to complex processes of multicellular differentiation.
FIG. 1.
Effects of ECF σ factor deletions on multicellular differentiation and growth. (A) Colony morphology of domesticated (CU1065) and undomesticated (NCIB3610) wild-type strains compared to the quadruple and triple mutants HB0842 (ΔVYZylaC) and MWX, respectively. Strains were grown in LB medium to mid-log phase with selection; 10 μl was spotted on MSgg plates (dried for 30 min in a laminar airflow) and incubated at room temperature for 6 days without selection. Colonies are shown at the same scale. Bar, 2 mm. (B) Pellicle formation of the same strains as in panel A. Ten milliliters of liquid MSgg medium (in six-well plates) was inoculated with 50 μl of mid-log-phase culture and incubated for 6 days at room temperature. The diameter of each well is 35 mm. (C) Pellicle formation of double and single mutants in sigM, sigW, and sigX. All parameters are as above.
Despite the obvious defects in pellicle formation and complex colony architecture, there were no measurable defects for these strains in either sporulation efficiency (in liquid culture) or swarming motility (data not shown). We reasoned that strains lacking additional ECF σ factors might uncover defects in these processes. However, even the four quadruple mutant strains constructed from the MWX triple mutant by individual insertional inactivation of one of the remaining ECF σ factors (Table 1, HB0842 to HB0847) did not have any phenotypic defects in these assays (data not shown). Therefore, these ECF σ factors appear not to be involved in the regulation of motility or sporulation in B. subtilis. However, it remains formally possible that additional defects might be uncovered if all seven ECF σ factors were deleted.
Growth of the MWX triple mutant is affected in LB but not in MSgg medium.
The phenotype of the MWX mutant strain on MSgg plates and in MSgg medium seemed to indicate poor growth (Fig. 1B), at least under the tested conditions. We therefore investigated the growth behavior of various ECF mutant strains. Growth of the MWX triple mutant and the quadruple mutant HB0842 (lacking σV, σY, σZ, and σYlaC) in MSgg medium was unaffected at 37°C with aeration (Fig. 2A). To our surprise, the MWX triple mutant, but none of the component double mutants, showed a reproducible biphasic growth behavior when incubated in LB medium at 37°C with aeration (Fig. 2B and data not shown). The culture grew at a slightly slower rate, and there was a drop in optical density at the end of the logarithmic growth phase. Reinoculation experiments indicate that this is a stable trait of the MWX mutant and does not reflect outgrowth of a mutant subpopulation. Phase-contrast microscopy (data not shown) failed to reveal any dramatic changes in cell morphology, suggesting that the drop in turbidity was likely due to partial lysis of the culture.
FIG. 2.
Growth of NCIB3610 (WT) and the derived triple (HB0834 [WXM]) and quadruple (HB0842 [VYZC]) mutants in MSgg medium (A) and LB medium (B). Ten milliliters of prewarmed medium (without antibiotics) was inoculated with 50 μl of a fresh overnight LB culture (grown with selection) and incubated at 37°C with aeration. Samples were taken at the indicated time points, and the growth was monitored by measuring the optical density at 600 nm (OD600).
A global sensitivity screen revealed new phenotypes linked to the ECF σ factors σM, σW, and σX in HB0834.
ECF σ factors are involved in the maintenance of cell envelope integrity and regulate functions that contribute to the innate resistance of B. subtilis against antibacterial compounds such as bacitracin, fosfomycin, and nisin. However, some compounds (e.g., cephalosporin C) can induce ECF σ-dependent stress responses, and yet single mutants are not more sensitive to these agents (7-10). In light of the known regulatory overlap among ECF σ factors and the results presented above, we reasoned that the roles of ECF σ factors in resistance to some antibiotics may only be apparent in multiple-mutant strains. As one approach to testing a broad range of stress conditions, we examined the sensitivity of NCIB3610, the MWX triple mutant and the quadruple mutant (HB0842) against a variety of chemical stresses using Phenotype MicroArrays.
Phenotype microarrays allow the analysis of hundreds of physiological tests in parallel, thereby enabling the fast and accurate identification of novel traits linked to genetic alterations (1, 34, 40). Remarkably, a comparison of the quadruple mutant HB0842 with the isogenic wild type revealed only three significant differences in growth: the mutant grew better than wild type in the presence of chloramphenicol, neomycin, and piperacillin. The first two phenotypes are explained by the use of chloramphenicol and neomycin resistance cassettes in the strain construction. Thus, despite the deletion of four presumed regulatory proteins (σV, σY, σZ, and σYlaC), the only detectable difference in this assay is a slightly increased resistance to piperacillin.
In contrast, the MWX triple mutant was clearly sensitive to a broad range of chemicals (Table 3). Many of these sensitivities were anticipated from the known phenotypes of the single mutants. This includes, for example, the known sensitivity of sigW mutants to fosfomycin (7) and sigM mutants to bacitracin (9). As expected, resistance to chloramphenicol and kanamycin was detected as gained phenotypes, consistent with the use of these resistance cassettes to delete the genes encoding ECF σ factors. Note that the concentrations of erythromycin and kanamycin in these assays were too low to suppress the growth of B. subtilis and that spectinomycin was not represented on the sensitivity plates available at the time of the analysis.
The most obvious and noteworthy phenotypes detected in the MWX triple mutant were the increased sensitivities to acidic (pH 4.5) or basic (pH 9.5) growth conditions, several detergents {including lauroylsarcosine, Triton X-100, CHAPSO (3-[(3-cholamidopropyl)-dimethylammonio]-2-hydroxy-1-propane- sulfonate), lauryl sulfobetaine, and dodecyl maltoside}, β-lactam antibiotics (including seven different cephalosporins), and d-cycloserine. While the sensitivity to low-pH conditions was anticipated from the phenotype of the sigM single mutant (18), the remaining phenotypes have not previously been associated with mutation of ECF σ factors in B. subtilis. Several of these and related antibacterial compounds were therefore chosen for further in-depth analyses. In addition, several antibiotics previously shown to have ECF σ factor-related resistance determinants (bacitracin, fosfomycin, moenomycin, and nisin; the latter was not present on the phenotype microarray) were also included in our analysis.
σM, σW, and σX play a crucial role in the innate resistance of B. subtilis against inhibitors of cell envelope integrity.
We performed disk diffusion assays for NCIB3610 and the corresponding mutants as described previously (11). The quantified data (average of at least two assays with two independent mutants) for representative compounds are shown in Fig. 3, and the sites of action for several of the cell wall biosynthesis inhibitors are summarized in Fig. 4A. Previous studies indicated that the ECF σ factor-mediated resistance of B. subtilis 168 against bacitracin and fosfomycin is linked to the regulation of the bcrC and fosB genes, respectively. Expression of bcrC is mediated by σM and σX, while fosB is expressed in a σW-dependent manner (7, 9). The present results support the hypothesis that σM is a primary determinant for bacitracin resistance, while σW is key for fosfomycin resistance. However, we additionally observed a further increase in both bacitracin and fosfomycin sensitivity in the MWX triple mutant (Fig. 3).
FIG. 3.
Disk diffusion assays of antibiotic sensitivity. Each bar represents the average zone of inhibition of at least two assays performed with two independent clones of each deletion mutant (deleted sigma factors are shown on the x axis). The y axis shows the zone of inhibition (in millimeters), expressed as total diameter minus diameter of the filter paper disk (5.5 mm). Note that the scale of the individual antibiotics varies for reasons of clarity. NCIB, NCIB3610; SDS, sodium dodecyl sulfate.
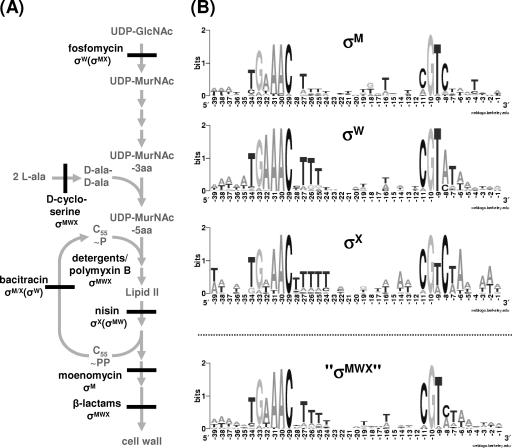
FIG. 4.
(A) Schematic diagram of the steps in peptidoglycan synthesis (in gray) inhibited by various tested antibiotics. The ECF σ factors (or combinations thereof) responsible for mediating resistance against each drug are given. GlcNAc, N-acetylglucosamine; MurNAC, N-acetyl muramic acid; 3aa and 5aa, tri- and pentapeptide side chains, respectively; C55∼P/∼PP, undecaprenyl (pyro)phosphate. (B) Weblogo representation (generated at http://weblogo.berkeley.edu/logo.cgi) of the position weight matrices (promoter consensus sequences) for recognition by σM, σW, and σX. At bottom, a virtual overall consensus for the three ECF σ factors is given which was generated by combining all target promoters of the three corresponding regulons. The promoters used for determining the consensus sequences are derived from either published tabulations (6, 8, 10, 22) or from our unpublished results (for σM).
Resistance against β-lactams is mostly mediated by the concerted action of σW and σX. Overall, the effect is more pronounced with penicillins (Fig. 3, ampicillin), while only a weak to moderate effect was observed for cephalosporins (Fig. 3, cephalosporin C). Comparable results were obtained for penicillin G and carbenicillin (penicillins), as well as cefotaxim/ceoxitin (cephalosporins) (data not shown). The most pronounced example of a concerted action of all three ECF σ factors was d-cycloserine, where increased sensitivity was observed only in the triple mutant, while all single and double mutants behaved indistinguishably from the wild-type strain NCIB3610 (Fig. 3). Similarly, sensitivity to nisin, sodium dodecyl sulfate, polymyxin B, and Triton X-100 was in each case most dramatic for the triple mutant (Fig. 3; also data not shown).
Concluding remarks.
The results presented here emphasize the complex and overlapping roles of ECF σ factors in B. subtilis physiology. One surprising result of this study is the lack of any phenotype for a quadruple mutant inactivated for four of the seven B. subtilis ECF σ factors (σV, σY, σZ, and σYlaC). The σY regulon has been found to consist of its own (autoregulated) operon, possibly encoding a toxic peptide and accompanying export machinery, and a single unlinked gene (ybgB) encoding a protein similar to immunity proteins that protect against toxic peptides (6, 12). No regulatory overlap was observed with any other ECF σ factors, and its physiological role is unclear. Interestingly, genes activated by the overexpression of σV have been identified, and most appear to overlap with the known targets for σM, σW, and σX (39). Thus, σV may, under some growth conditions, also contribute to expression of some of the same genes. The roles of the σZ and σYlaC factors are presently unclear, although it has been suggested that a ylaC mutant strain may be more sensitive to oxidative stress (31).
A key finding of the present analyses relates to the regulatory overlap, and the corresponding functional consequences, for the σX, σW, and σM factors. The novel phenotypes revealed in the MWX triple mutant strain with respect to pellicle formation, colony morphology, and antibiotic sensitivity reveal a clear functional overlap. In principle, these results could be due to the control of separate, but functionally complementary, target genes by each of these σ factors. For example, a function needed for pellicle formation may be provided by any of three genes that are specifically transcribed as part of the σX, σW, and σM regulons. Alternatively, there may be one gene, or possibly more, that is essential for pellicle formation and is transcribed from a promoter that can be recognized by any of these three ECF σ factors. The actual situation may reflect some combination of these possibilities, although we favor the latter model. There are already several published examples of promoter sites that can be recognized by more than one ECF σ factor (9, 20, 22, 28). This overlap is perhaps not too surprising as the deduced consensus sequences for recognition by σM, σW, and σX (Fig. 4B) are quite similar. Moreover, recent global analyses in Escherichia coli have revealed an unexpected extent of promoter overlap even among more distantly related σ factors (36). Ongoing studies to determine the complete regulons controlled by each of these regulators and the extent of regulon overlap will hopefully shed light on this and related questions.
Acknowledgments
We thank Daniel Kearns (Indiana University) for phage SPP1 and advice on NCIB3610 genetics, Reinhold Brückner and Regine Hakenbeck (Kaiserslautern) for the generous gift of various β-lactam antibiotics, and Dave Popham (Virginia Tech) for the gift of moenomycin.
This work was supported by grant GM-47446 from the National Institutes of Health (to J.D.H.) and by grant MA 3269 from the Deutsche Forschungsgemeinschaft and grants from the Fonds der Chemischen Industrie (to T.M.).
Footnotes
Published ahead of print on 3 August 2007.
REFERENCES
- 1.Bochner, B. R., P. Gadzinski, and E. Panomitros. 2001. Phenotype microarrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 11:1246-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branda, S. S., F. Chu, D. B. Kearns, R. Losick, and R. Kolter. 2006. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 59:1229-1238. [DOI] [PubMed] [Google Scholar]
- 3.Branda, S. S., J. E. Gonzalez-Pastor, S. Ben-Yehuda, R. Losick, and R. Kolter. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 98:11621-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branda, S. S., J. E. Gonzalez-Pastor, E. Dervyn, S. D. Ehrlich, R. Losick, and R. Kolter. 2004. Genes involved in formation of structured multicellular communities by Bacillus subtilis. J. Bacteriol. 186:3970-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branda, S. S., S. Vik, L. Friedman, and R. Kolter. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20-26. [DOI] [PubMed] [Google Scholar]
- 6.Butcher, B. G., and J. D. Helmann. 2006. Identification of Bacillus subtilis σW-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by bacilli. Mol. Microbiol. 60:765-782. [DOI] [PubMed] [Google Scholar]
- 7.Cao, M., B. A. Bernat, Z. Wang, R. N. Armstrong, and J. D. Helmann. 2001. FosB, a cysteine-dependent fosfomycin resistance protein under the control of σW, an extracytoplasmic-function σ factor in Bacillus subtilis. J. Bacteriol. 183:2380-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, M., and J. D. Helmann. 2004. The Bacillus subtilis extracytoplasmic-function σX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides. J. Bacteriol. 186:1136-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao, M., and J. D. Helmann. 2002. Regulation of the Bacillus subtilis bcrC bacitracin resistance gene by two extracytoplasmic function σ factors. J. Bacteriol. 184:6123-6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao, M., P. A. Kobel, M. M. Morshedi, M. F. Wu, C. Paddon, and J. D. Helmann. 2002. Defining the Bacillus subtilis σW regulon: a comparative analysis of promoter consensus search, run-off transcription/macroarray analysis (ROMA), and transcriptional profiling approaches. J. Mol. Biol. 316:443-457. [DOI] [PubMed] [Google Scholar]
- 11.Cao, M., C. M. Moore, and J. D. Helmann. 2005. Bacillus subtilis paraquat resistance is directed by σM, an extracytoplasmic function sigma factor, and is conferred by YqjL and BcrC. J. Bacteriol. 187:2948-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao, M., L. Salzberg, C. S. Tsai, T. Mascher, C. Bonilla, T. Wang, R. W. Ye, L. Marquez-Magana, and J. D. Helmann. 2003. Regulation of the Bacillus subtilis extracytoplasmic function protein σY and its target promoters. J. Bacteriol. 185:4883-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao, M., T. Wang, R. Ye, and J. D. Helmann. 2002. Antibiotics that inhibit cell wall biosynthesis induce expression of the Bacillus subtilis σW and σM regulons. Mol. Microbiol. 45:1267-1276. [DOI] [PubMed] [Google Scholar]
- 14.Chu, F., D. B. Kearns, S. S. Branda, R. Kolter, and R. Losick. 2006. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol. Microbiol. 59:1216-1228. [DOI] [PubMed] [Google Scholar]
- 15.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 16.Harwood, C. R., and S. M. Cutting. 1990. Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
- 17.Helmann, J. D. 2002. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46:47-110. [DOI] [PubMed] [Google Scholar]
- 18.Horsburgh, M. J., and A. Moir. 1999. σM, an ECF RNA polymerase sigma factor of Bacillus subtilis 168, is essential for growth and survival in high concentrations of salt. Mol. Microbiol. 32:41-50. [DOI] [PubMed] [Google Scholar]
- 19.Huang, X., A. Decatur, A. Sorokin, and J. D. Helmann. 1997. The Bacillus subtilis σX protein is an extracytoplasmic function sigma factor contributing to survival at high temperature. J. Bacteriol. 179:2915-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, X., K. L. Fredrick, and J. D. Helmann. 1998. Promoter recognition by Bacillus subtilis σW: autoregulation and partial overlap with the σX regulon. J. Bacteriol. 180:3765-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, X., and J. D. Helmann. 1998. Identification of target promoters for the Bacillus subtilis σX factor using a consensus-directed search. J. Mol. Biol. 279:165-173. [DOI] [PubMed] [Google Scholar]
- 22.Jervis, A. J., P. D. Thackray, C. W. Houston, M. J. Horsburgh, and A. Moir. 2007. SigM-responsive genes of Bacillus subtilis and their promoters. J. Bacteriol. 189:4534-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kearns, D. B., F. Chu, S. S. Branda, R. Kolter, and R. Losick. 2005. A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 55:739-749. [DOI] [PubMed] [Google Scholar]
- 24.Kearns, D. B., F. Chu, R. Rudner, and R. Losick. 2004. Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol. Microbiol. 52:357-369. [DOI] [PubMed] [Google Scholar]
- 25.Kearns, D. B., and R. Losick. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49:581-590. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi, K. 2007. Bacillus subtilis pellicle formation proceeds through genetically defined morphological changes. J. Bacteriol. 189:4920-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascher, T., N. G. Margulis, T. Wang, R. W. Ye, and J. D. Helmann. 2003. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol. Microbiol. 50:1591-1604. [DOI] [PubMed] [Google Scholar]
- 28.Minnig, K., J. L. Barblan, S. Kehl, S. B. Moller, and C. Mauel. 2003. In Bacillus subtilis W23, the duet σX σM, two sigma factors of the extracytoplasmic function subfamily, are required for septum and wall synthesis under batch culture conditions. Mol. Microbiol. 49:1435-1447. [DOI] [PubMed] [Google Scholar]
- 29.Pietiäinen, M., M. Gardemeister, M. Mecklin, S. Leskela, M. Sarvas, and V. P. Kontinen. 2005. Cationic antimicrobial peptides elicit a complex stress response in Bacillus subtilis that involves ECF-type sigma factors and two-component signal transduction systems. Microbiology 151:1577-1592. [DOI] [PubMed] [Google Scholar]
- 30.Qiu, J., and J. D. Helmann. 2001. The −10 region is a key promoter specificity determinant for the Bacillus subtilis extracytoplasmic-function σ factors σX and σW. J. Bacteriol. 183:1921-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryu, H. B., I. Shin, H. S. Yim, and S. O. Kang. 2006. YlaC is an extracytoplasmic function (ECF) sigma factor contributing to hydrogen peroxide resistance in Bacillus subtilis. J. Microbiol. 44:206-216. [PubMed] [Google Scholar]
- 32.Stanley, N. R., and B. A. Lazazzera. 2005. Defining the genetic differences between wild and domestic strains of Bacillus subtilis that affect poly-γ-dl-glutamic acid production and biofilm formation. Mol. Microbiol. 57:1143-1158. [DOI] [PubMed] [Google Scholar]
- 33.Thackray, P. D., and A. Moir. 2003. SigM, an extracytoplasmic function sigma factor of Bacillus subtilis, is activated in response to cell wall antibiotics, ethanol, heat, acid, and superoxide stress. J. Bacteriol. 185:3491-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Eiff, C., P. McNamara, K. Becker, D. Bates, X. H. Lei, M. Ziman, B. R. Bochner, G. Peters, and R. A. Proctor. 2006. Phenotype microarray profiling of Staphylococcus aureus menD and hemB mutants with the small-colony-variant phenotype. J. Bacteriol. 188:687-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wach, A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12:259-265. [DOI] [PubMed] [Google Scholar]
- 36.Wade, J. T., D. C. Roa, D. C. Grainger, D. Hurd, S. J. Busby, K. Struhl, and E. Nudler. 2006. Extensive functional overlap between sigma factors in Escherichia coli. Nat. Struct. Mol. Biol. 13:806-814. [DOI] [PubMed] [Google Scholar]
- 37.Wiegert, T., G. Homuth, S. Versteeg, and W. Schumann. 2001. Alkaline shock induces the Bacillus subtilis σW regulon. Mol. Microbiol. 41:59-71. [DOI] [PubMed] [Google Scholar]
- 38.Youngman, P. 1990. Use of transposons and integrational vectors for mutagenesis and construction of gene fusions in Bacillus subtilis, p. 221-266. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
- 39.Zellmeier, S., C. Hofmann, S. Thomas, T. Wiegert, and W. Schumann. 2005. Identification of σV-dependent genes of Bacillus subtilis. FEMS Microbiol. Lett. 253:221-229. [DOI] [PubMed] [Google Scholar]
- 40.Zhou, L., X.-H. Lei, B. R. Bochner, and B. L. Wanner. 2003. Phenotype microarray analysis of Escherichia coli K-12 mutants with deletions of all two-component systems. J. Bacteriol. 185:4956-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]