Abstract
The ability of a reverse line blot (RLB) assay to identify 13 common species of equine small strongyles (cyathostomins) and to discriminate them from three Strongylus spp. (large strongyles) was demonstrated. The assay relied on the specific hybridization of PCR-amplified intergenic spacer DNA fragments of the nuclear ribosomal DNA to membrane-bound species-specific probes. All cyathostomins examined were unequivocally identified and simultaneously discriminated from each other and from three large strongyles (Strongylus edentatus, Strongylus equinus, and Strongylus vulgaris). This assay will enable the accurate and rapid identification of equine cyathostomins irrespective of their life cycle stage, opening important avenues for a better understanding of their biology and epidemiology and of the pathogenesis of cyathostomin-associated disease. In particular, this RLB method promises to be a powerful diagnostic tool to determine the roles of individual species in the pathogenesis of mixed infections and to elucidate some aspects of cyathostominosis. Also, it could represent a basic step toward the development of a rapid and simple molecular test for the early detection of drug-resistant genotypes of horse strongyle species.
Equine strongyles (Nematoda, Strongylida) belonging to the subfamilies Strongylinae (“large strongyles”) and Cyathostominae (“small strongyles”) are intestinal parasitic nematodes affecting equids worldwide (27, 28, 32).
In the past several decades, the prevalence of Strongylus spp. (large strongyles) has decreased significantly, especially where there is a high level of concern about the impact of the parasitosis on equine health and where the regular antiparasitic treatment of horses is practiced, as in the United States (2, 22). Large strongyles are still present, however, worldwide, e.g., in the United States (2), southeastern Europe (10, 11; D. Traversa, unpublished data), Africa (34), and South America (39).
Nonetheless, the small strongyles (also known as cyathostomes or cyathostomins) are now recognized as the most common nematodes of horses and the principal helminth pathogens of these animals (15, 31). In fact, virtually 100% of horses are infected with small strongyles, and they account for nearly 100% of the helminth egg output of grazing animals (16, 32, 41).
Apart from their worldwide distribution, the major veterinary importance of cyathostomins lies in their high pathogenic potential. After their ingestion, the infective third-stage larvae (L3) undergo some larval development within the wall of the cecum and colon, accumulating in huge numbers. Larval cyathostomes are classified as early L3 (arrested L3), late L3 (which are developing and growing), and developing fourth-stage larvae (L4). Later on, the L4 emerge from the wall of the intestine, molt, and become adults in the lumen of the intestine (5, 6). The simultaneous reactivation of early L3 leads to a clinical syndrome called “larval cyathostominosis,” represented by a severe inflammatory enteropathy affecting the cecum and colon. This disease is characterized by symptoms of various degrees of severity, i.e., severe colitis, protein-losing enteropathy, considerable weight loss, diarrhea, and subcutaneous edema, with a 50% fatality rate despite prompt treatment (5, 6, 13, 30). Moreover the presence of adult small strongyles is associated with various types of colics (33, 37) and with decreased rates of performance, rough hair coat, and debilitation (53).
The accurate identification of horse strongyles irrespective of their life cycle stage is of paramount importance in increasing the understanding of their biology and epidemiology and the pathogenesis of associated diseases. Since the morphological identification of adult cyathostomins can be challenging and that of larval stages is almost impossible, many efforts have been made for the molecular identification of horse strongyles (8, 19, 35). In brief, oligonucleotide primers specific for five common strongylid species were designed within the internal transcribed spacers of the nuclear ribosomal DNA (rDNA) (21). Furthermore, genetic markers within the intergenic spacers (IGS) of rDNA proved to be useful in designing oligonucleotide probes for the molecular identification of four cyathostomin species (20), and in addition, a PCR-enzyme-linked immunosorbent assay was used for the identification of six cyathostome species in the diarrheic feces of infected horses (17) and in the feces of fenbendazole-treated horses (18). Though these methods provided an important step forward, they are relatively time-consuming and are able to discriminate among relatively few cyathostomin species.
Recently, the use of oligonucleotide probes in reverse line blot (RLB) hybridization assays proved to be powerful for the identification of a diverse range of clinically relevant human and veterinary pathogens, with high throughput (14, 40, 42, 44, 54). Briefly, the RLB method enables the nonradioactive hybridization of PCR amplicons with different oligonucleotide probes in a single assay. In this method, oligonucleotide probes are covalently attached to a membrane in parallel lines using a miniblotter and then perpendicularly evaluated against biotin-labeled PCR products in the miniblotter. Hybridization is visualized using peroxidase-labeled streptavidin and chemiluminescence detection. Although species-specific oligonucleotide probes to identify some cyathostomes have been reported (17, 20), the need to increase the number of species able to be molecularly identified by a simple approach adaptable to molecular parasitology laboratories remains. A preliminary RLB assay has recently been validated for the simultaneous molecular identification of eight species of cyathostomes (50, 51). Building on this work, the aim of the present study was to develop an RLB hybridization assay capable of simultaneously identifying the most common large and small equine strongyles.
MATERIALS AND METHODS
Parasite material.
Adult specimens of three Strongylus species and 13 species of small strongyles were collected from the intestines of horses in southern and eastern Europe and the United States (Table 1). These strongyle species are the ones most commonly seen in most surveys worldwide (2).
TABLE 1.
Horse strongyle species characterized and used in the RLB assay by geographical origin and number of specimens processed
| Strongyle species | Origin | No. processed |
|---|---|---|
| Cyathostominae | ||
| Coronocyclus coronatus | Kentucky | 1 |
| Ukraine | 4 | |
| Poland | 4 | |
| Abruzzo, Italy | 2 | |
| Coronocyclus labiatus | Abruzzo, Italy | 3 |
| Ukraine | 1 | |
| Coronocyclus labratus | Abruzzo, Italy | 4 |
| Poland | 1 | |
| Cyathostomum catinatum | Kentucky | 4 |
| Louisiana | 4 | |
| Abruzzo, Italy | 3 | |
| Ukraine | 5 | |
| Cyathostomum pateratum | Louisiana | 5 |
| Abruzzo, Italy | 4 | |
| Ukraine | 3 | |
| Cylicocyclus ashworthi | Poland | 4 |
| Abruzzo, Italy | 2 | |
| Ukraine | 3 | |
| Cylicocyclus insigne | Louisiana | 2 |
| Abruzzo, Italy | 5 | |
| Ukraine | 4 | |
| Cylicocyclus leptostomum | Poland | 2 |
| Abruzzo, Italy | 1 | |
| Ukraine | 3 | |
| Cylicocyclus nassatus | Kentucky | 4 |
| Louisiana | 2 | |
| Abruzzo, Italy | 3 | |
| Ukraine | 4 | |
| Poland | 5 | |
| Cylicostephanus calicatus | Kentucky | 2 |
| Poland | 1 | |
| Abruzzo, Italy | 4 | |
| Cylicostephanus goldi | Kentucky | 5 |
| Abruzzo, Italy | 1 | |
| Ukraine | 4 | |
| Cylicostephanus longibursatus | Kentucky | 8 |
| Abruzzo, Italy | 2 | |
| Ukraine | 3 | |
| Poland | 4 | |
| Cylicostephanus minutus | Abruzzo, Italy | 3 |
| Poland | 4 | |
| Strongylinae | ||
| Strongylus edentatus | Louisiana | 4 |
| Abruzzo, Italy | 3 | |
| Strongylus equinus | Abruzzo, Italy | 2 |
| Puglia, Italy | 5 | |
| Strongylus vulgaris | Louisiana | 5 |
| Abruzzo, Italy | 3 |
DNA extraction, PCR amplification, and sequencing.
Genomic DNA was isolated from individual parasites using the QIAamp Tissue Kit (QIAGEN GmbH, Germany) (Table 1) and eluted in 25 μl H2O (WATER PCR Product; Sigma, St. Louis, MO).
For sequencing, the IGS were amplified for cyathostomes using primers CY1 (5′-GGTCAAGGTGTTGTATCCAGTAGAG-3′) and CY4 (5′-CGGTACAAAAAGACTTCTACTCG-3′), while for Strongylus spp., primer CY18 (5′-CTTAGACATGCATGGCTTAATC-3′) was used in combination with primer CY26 (5′-GAGCTGGGTTTAGACCGTCGTGAG-3′) (20, 24).
The PCR mixture (50 μl) was prepared with genomic DNA (5 μl), 100 pM of each primer, and 25 μl of Ready Mix REDTaq (Sigma, St. Louis, MO) and rinsed with distilled water.
PCRs were performed in a thermal cycler (model 2700; Applied Biosystems, Foster City, CA) using the following cycling protocol: 10 min at 94°C and 35 cycles at 94°C for 1 min, 50°C for 1 min, and 72°C for 2 min, followed by a final extension at 72°C for 7 min.
The amplicons were separated in 1.5% (wt/vol) agarose gels and stained with ethidium bromide (10 mg/ml).
For each species, selected amplicons were purified using Ultrafree-DA columns (Millipore, Bedford, MA) and then sequenced with a walking strategy using internal primers and the Taq DyeDeoxy Terminator Cycle Sequencing Kit (version 2) (Applied Biosystems, Foster City, CA) in an automated sequencer (ABI-PRISM 377). Sequences were determined in both directions, and the electropherograms were verified by eye.
Sequence analysis and probe design.
Sequences were aligned using the Clustal X program (46). Moreover, the sequences obtained for all strongyles were compared with the IGS of the corresponding species already available in the GenBank database (accession numbers AJ223334, AJ223337 to AJ223340, AJ223342, AJ223344 to AJ223348, and AJ 223727), with the exception of Coronocyclus labratus and Strongylus spp. that were not present in any database.
For Cylicocyclus ashworthi, Cylicocyclus goldi, and Cylicostephanus longibursatus, previously designed oligoprobes were selected (20), while for the remaining cyathostomes, species-specific probes were designed to the consensus IGS sequences in regions with the greatest interspecific differences and without intraspecific polymorphisms. Furthermore, an oligonucleotide probe for the three species of Strongylus and a probe common to all cyathostomes were selected (Table 2).
TABLE 2.
Sequences of the diagnostic probes used in the RLB assay, their specificities, and optimal picomol concentrations
| Probe | Sequence (5′-3′) | Specificity | Concn (pmol) | Reference |
|---|---|---|---|---|
| CATD | AATACAATTGTAACATTTTCA | C. catinatum | 3,200 | Present paper |
| NAS2 | GCAAGAACTTCGCTGAAATG | C. nassatus | 1,600 | Present paper |
| INS2 | GTATGTATATGTATCAATGTCTTAA | C. insigne | 400 | Present paper |
| GOL | TCTTAGCATCAGGAGAAAT | C. goldi | 1,600 | 17, 20 |
| LON | GGAGAAATTGGTGGCGACT | C. longibursatus | 400 | 17, 20 |
| LABR2 | GCTGAAATGCCGTGTTAGT | C. labratus | 400 | Present paper |
| LAB2 | GTTCTATTAGGTTGTCTAAGAA | C. labiatus | 1,600 | Present paper |
| PAT2 | AATATTTCAGTAGAAATGCAA | C. pateratum | 1,600 | Present paper |
| LEP1 | CGTGGGGAAAACTTTAGCCA | C. leptostomus | 3,200 | Present paper |
| COR1 | AGAAAATTTTAGCCAAGTGA | C. coronatus | 800 | Present paper |
| ASH | TTGGTCTTACATAGAAAAT | C. ashworthi | 800 | 17, 20 |
| CAL1 | CTTTTATCAGCACTTCTATG | C. calicatus | 800 | Present paper |
| MIN1 | GCAAGAATTTCGCTAAAATG | C. minutus | 800 | Present paper |
| Cya-PAN | GAGACTATCCTATGATCGGGTG | Cyathostominae | 400 | Present paper |
| Str-PAN | CCTGCTGTTTGGTGCGTGA | Strongylus spp. | 400 | Present paper |
The specificities of the sequences of all probes selected were predicted by alignment against all the DNA sequences present in the GenBank database by using the Nucleotide-Nucleotide BLAST software (1).
RLB hybridization.
For each strongyle species, PCRs were carried out as described above using primer CY26 and the biotin-labeled primers CY4B (for cyathostomes) and CY18B (for Strongylus spp.) to obtain at least one IGS amplicon of each species from all geographical areas (Table 1). The diagnostic value of the probes was evaluated in an RLB assay whose conditions (i.e., probe concentration and hybridization time and temperature) were standardized using such IGS amplicons.
All the specific oligonucleotides reported in Table 2 contained an N-terminal N-(trifluoracetamidohexyl-cyanoethyl,N,N-diisopropyl phosphoramidite)-C6 amino linker (Sigma, St. Louis, MO). A Biodyne C blotting membrane (Pall Corporation, Pensacola, FL) was activated by a 10-min incubation in 10 ml of 16% (wt/vol) 1-ethyl-3-(3-dimethylamino-propyl) carbodiimide (Sigma, St. Louis, MO) in demineralized water at room temperature. The membrane was washed for 2 min with distilled water and placed in an MN45 miniblotter (Immunetics Inc., Boston, MA) comprising 45 parallel channels whose bottoms were in contact with the membrane. Optimal probe concentrations were determined by binding various amounts of the probe in such a way that all the probes resulted in similarly intense signals relative to the catch-all control probe. In particular, species-specific oligonucleotide probes were diluted to a 400- to 3,200-pmol/150 μl concentration (Table 2) in 500 mM NaHCO3 (pH 8.4) and were subsequently covalently linked to the membrane with the amino linker by filling the miniblotter slots with the oligonucleotide dilutions. Excess probe not bound to the membrane was removed by aspiration, and then the membrane was incubated for 2 min at room temperature. The membrane was then blocked by incubation in 100 ml of a 100 mM NaOH solution for 6 min at room temperature after the probe solutions were aspirated off, leaving the probes free to react with the cDNA amplicon. The membrane was washed with shaking in 2× SSPE (2.98 M NaCl and 0.02 M EDTA [pH 7.4])-0.1% sodium dodecyl sulfate (SDS) for 5 min at 60°C. Prior to the hybridization tests, the membrane was washed for 5 min at room temperature with 2× SSPE-0.1% SDS and placed in the miniblotter with the slots perpendicular to the previously linked probes. Twenty microliters of each biotinylated IGS amplicon was diluted to an end volume of 150 μl of 2× SSPE-0.1% SDS, denatured at 100°C for 10 min, and immediately cooled on ice. IGS amplicons were placed into the slots and incubated for 60 min at 42°C; the membrane was rotated 90° before the IGS amplicons were incubated in independent channels so each of them was in contact with all the probes. After hybridization, excess amplicons were aspirated, and the blot was washed twice in 2× SSPE-0.5% SDS for 10 min each time at 52°C with shaking. The membrane was then incubated in 10 ml of streptavidin-peroxidase conjugate (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom) diluted in 1:4,000 2× SSPE-0.5% SDS for 30 min at 42°C. The membrane was washed twice with 125 ml of 2× SSPE-0.5% SDS for 10 min each time at 42°C and then with 125 ml of 2× SSPE for 5 min at room temperature. Hybridized IGS amplicons were detected by chemiluminescence by incubating the membrane in 10 ml of an ECL detection fluid (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom) for 1 min and visualized by the exposure of the membrane to an X-ray Hyperfilm ECL (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom) for 30 min to 1 h according to the signal intensity. After use, all amplicons were stripped by washing the membrane twice in 1% SDS for 30 min at 90°C. The membrane was then rinsed in 20 mM EDTA (pH 8.0) and stored in fresh EDTA solution at 4°C for subsequent reuse. The membrane was reused 10 times in subsequent RLB assays to verify the absence of signal loss.
RESULTS
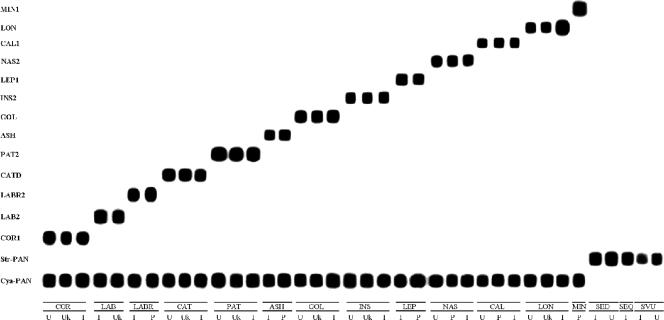
The primer set CY26-CY4 amplified an IGS amplicon of the expected size (ranging from 1.5 kb to 2.5 kb) for all cyathostomes previously tested (24) and one of 1.5 kb for C. labratus. The amplicons produced by the primer set CY26-CY18 were approximately 1.5 kb for Strongylus edentatus, Strongylus equinus, and Strongylus vulgaris. No intraspecific differences in size between IGS amplicons from different geographical areas were visualized on agarose gels. The alignment of the IGS sequences of the different strongyle species examined (Table 1) showed conspicuous interspecific differences with no intraspecific differences between isolates from different geographic regions or between those and the sequences previously deposited in GenBank. These interspecific differences allowed the choice of diagnostic oligonucleotide probes that had been utilized in the RLB assay. In the RLB, the single IGS amplicons hybridized without any nonspecific reaction, i.e., the 13 probes designed within the IGS of the small strongyles were species specific, and the pan-probe for cyathostomes and the probe designed for the Strongylus spp. hybridized with all of the tested small and large strongyles, respectively (Fig. 1).
FIG. 1.
Representative PCR-RLB results for the simultaneous identification of the 13 most diffused species of horse Cyathostominae and their discrimination from Strongylus spp. The DNA samples shown on this membrane are (from left to right) COR, Coronocyclus coronatus; LAB, C. labiatus; LABR, C. labratus; CAT, Cyathostomum catinatum; PAT, C. pateratum; ASH, Cylicocyclus ashworthi; GOL, C. goldi; INS, C. insigne; LEP, C. leptostomum; NAS, C. nassatus; CAL, Cylicostephanus calicatus; LON, C. longibursatus; MIN, C. minutus; SED, Strongylus edentatus; SE, S. equinus; and SVU, S. vulgaris. One specimen from each representative geographical area, i.e., United States (U), eastern Europe (Ukraine [Uk] or Poland [P]), and Italy (I), was included in the representative RLB. See Table 1 for descriptions and specificities of the probes listed on the left.
No loss of signal was detected in the membrane when it was reused in the RLB assay up to 10 times.
DISCUSSION
The RLB assay presented here enables the simultaneous identification to species level of the most common cyathostomes (2, 12, 25, 38, 43), together with their discrimination from Strongylus spp. Since rDNA does not show any change in the course of the parasite life cycle (7, 9), this assay can be applied irrespective of the developmental stage. In fact, despite their major importance, there are still significant gaps in the systematics, biology, epidemiology, pathogenic aspects, and diagnosis of horse strongyles. The lack of a reliable diagnostic test for the most common Cyathostominae and Strongylinae affecting horses has been a major hindrance to basic and applied investigations at species level.
The PCR-RLB hybridization assay presented here is faster and more convenient than other molecular methods described to date for the identification of equine strongyles, including PCR sequencing, PCR-restriction fragment length polymorphism, PCR-enzyme-linked immunosorbent assay, and species-specific PCRs. In particular, an important advantage of the RLB is its ability to simultaneously identify 13 of the most common cyathostomes, discriminating them from the three Strongylus spp. In fact, the other molecular tools available do not allow this number of taxa to be identified to the species level at the same time (8, 19). Moreover, other methods are relatively costly (e.g., sequencing requires amplicon purification and expensive instruments), time-consuming (e.g., PCR-coupled sequencing requires more than 24 h), and potentially inaccurate in the presence of mixed strongyle species. In comparison, the RLB format provides relative simplicity, low cost, ready availability of the materials and methods, and the ability to simultaneously analyze multiple samples against multiple probes. Furthermore, this method has the capacity to screen up to 43 samples in a single run, and the membrane can be reused up to 10 to 15 times without loss of signal; this is particularly important in further epidemiological and biological studies (see below). Although 13 species-specific probes were used here, up to 43 can be included on the same membrane, providing the flexibility and capacity to simultaneously detect and identify other horse parasites in the future. For example, the recent characterization of the rDNA of equid Habronema stomach worms has allowed the molecular diagnosis in vivo of the infection (48, 49). Given the present emergency status of equid habronemosis in some regions (52), it could be advisable to include Habronema microstoma and Habronema muscae in a future RLB assay for the identification of noncyathostomin equid parasites, as well. For example, it could be possible to molecularly examine fecal samples from Habronema-infected horses collected pre- and posttreatment with anthelmintic drugs, since no detailed drug efficacy data are yet available for both species of stomach worms.
The IGS was chosen as the genetic target for this RLB assay because the level of interspecific differences was previously shown to be greater than the degree of intraspecific variation and much greater than that of the ribosomal internal transcribed spacers (24). Moreover, the diagnostic utility of probes designed within the strongyle IGS has been previously reported (17, 20). However, when the IGS sequence alignment of Cylicocyclus nassatus, Cyathostomum catinatum, and Cylicocyclus insigne with probes previously published (17, 20) was analyzed by using the Nucleotide-Nucleotide BLAST software (1), cross-reactions were predicted, i.e., the sequences of the probes for these three species were present in the IGS of Cylicostephanus minutus (AJ223347), Cylicostephanus calicatus (AJ223338), and Cylicocyclus radiatus (AJ223343), respectively (24). This was not the case with C. ashworthi, C. goldi, and C. longibursatus, and it is the reason why we designed new probes for C. catinatum, C. insigne, and C. nassatus different from those previously reported in the literature (17, 20).
Detailed biology of individual strongyle species at environmental and host stages is lacking, particularly for cyathostomes. As far as free-living stages are concerned, the eggs are indistinguishable at genus or species level and therefore they must be cultured to L3 for their identification. However the L3 can be identified to the species level only for large strongyles, while cyathostomes can be identified only at the subfamily level (36, 45). Moreover, in vitro culture can be challenging because of the variations in larval survival rates under laboratory conditions and the labor and time requirements of the method (3, 8). Also, the identification of L4 to the species level is difficult, if not impossible, and key morphological features are not available for all species (4). The identification of the strongyles involves the morphological examination of the adult stages, but this requires the sacrifice of infected horses or, alternatively, the deworming of animals, which could be limited by individual parasite susceptibilities to the drug and/or by drug resistance (25, 29). Furthermore, the microscopic identification of adult strongyles is problematic and requires considerable experience (26-28, 47).
If one considers the number of species that can be simultaneously detected and identified with its high throughput (up to 43 samples can be tested at the same time), the RLB presented in this paper appears to be the most powerful diagnostic assay currently available for equine strongyles. Therefore, this assay would enable the elucidation of some moot issues regarding the basic biology and epidemiology of horse strongyles. For example, questions about the length of the life cycle, the individual prepatent period, the seasonal fluctuation of fecal egg output in infected horses, and the population dynamics of pasture-living larvae could be addressed.
This RLB method also promises to be a powerful diagnostic tool from clinical and practical standpoints, especially to determine the roles of individual species in the pathogenesis of mixed infections, since the pathogenic potential of each cyathostome is not known. Importantly, this assay would be a powerful tool for elucidating some aspects of the life-threatening cyathostominosis (e.g., the species-specific selective mechanism in gut penetration, association between the individual species, and the occurrence of the clinical disease) and would allow the species-specific diagnosis of this syndrome.
In the past few decades, anthelmintic resistance in cyathostomes worldwide has increased enormously, together with a significant rise in the intensity and prevalence of infections (22, 23, 32). Thus, there is a significant need for the unequivocal and simultaneous discrimination of multiple-drug-resistant species and their distributions. The RLB would be important in future studies carried out to prevent the spread of anthelmintic resistance and to monitor its presence and distribution at the species level. Finally, it could also represent the basis for the development of a rapid and simple molecular test for the early detection of drug-resistant genotypes within each of the individual widespread common strongyle species using appropriate genetic markers.
Acknowledgments
We are grateful to Eugene Lyons (Department of Veterinary Science, Gluck Equine Research Center, University of Kentucky) and Tetiana Kuzmina (Department of Parasitology, Schmalhausen Institute of Zoology, Kyiv, Ukraine) for providing some specimens of strongyles. D.T. thanks Annunziata Giangaspero (Faculty of Agronomy, University of Foggia, Italy) for supporting the preliminary work on the RLB assay presented here.
D.T. and V.A.K. are grateful to the Accademia Nazionale dei Lincei (Rome, Italy) and the National Academy of Sciences of Ukraine (Kyiv, Ukraine) for supporting their scientific collaboration on equine cyathostomes.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman, M. R., D. D. French, and T. R. Klei. 2002. Gastrointestinal helminths of ponies in Louisiana: a comparison of species currently prevalent with those present 20 years ago. J. Parasitol. 88:1130-1134. [DOI] [PubMed] [Google Scholar]
- 3.Dobson, R. J., E. H. Barnes, S. D. Birclijin, and J. H. Gill. 1992. The survival of Ostertagia circumcincta and Trichostrongylus colubriformis in faecal culture as a source of basis in apportioning egg counts to worm species. Int. J. Parasitol. 22:1005-1008. [DOI] [PubMed] [Google Scholar]
- 4.Dvojnos, G. M., and V. A. Kharchenko. 1990. Morphology and differential diagnostics of parasitic larvae of some Strongylidae (Nematoda) of horses. Angew. Parasitol. 31:15-28. [PubMed] [Google Scholar]
- 5.Eysker, M., J. H. Boersema, and F. N. Kooyman. 1989. Emergence from inhibited development of cyathostome larvae in ponies following failure to remove them by repeated treatments with benzimidazole compounds. Vet. Parasitol. 34:87-93. [DOI] [PubMed] [Google Scholar]
- 6.Eysker, M., J. H. Boersema, and F. N. Kooyman. 1990. Seasonally inhibited development of cyathostomine nematodes in Shetland ponies in The Netherlands. Vet. Parasitol. 36:259-264. [DOI] [PubMed] [Google Scholar]
- 7.Gasser, R. B., and S. E. Newton. 2000. Genomic and genetic research on bursate nematodes: significance, implications and prospects. Int. J. Parasitol. 30:509-534. [DOI] [PubMed] [Google Scholar]
- 8.Gasser, R. B., G. C. Hung, N. B. Chilton, and I. Beveridge. 2004. Advances in developing molecular-diagnostic tools for strongyloid nematodes of equids: fundamental and applied implications. Mol. Cell Probes 18:3-16. [DOI] [PubMed] [Google Scholar]
- 9.Gasser, R. B., N. B. Chilton, and I. Beveridge. 1993. Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Res. 21:2525-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gawor, J. J. 2000. Occurrence of Strongylidae (Nematoda: Strongyloidea) in Polish horses “tarpans” from Popielne Reserve. Wiad. Parazytol. 46:87-92. [PubMed] [Google Scholar]
- 11.Gawor, J. J., S. Kornas, V. A. Kharchenko, B. Nowosad, and M. Skalska. 2006. Intestinal parasites and health problems in horses in different breeding systems. Med. Weter. 62:331-334. [Google Scholar]
- 12.Gawor, J. J. 1995. The prevalence and abundance of internal parasites in working horses autopsied in Poland. Vet. Parasitol. 58:99-108. [DOI] [PubMed] [Google Scholar]
- 13.Giles, C. J., K. A. Urquhart, and J. A. Longstaffe. 1985. Larval cyathostomiasis (immature Trichonema-induced enteropathy): a report of 15 clinical cases. Equine Vet. J. 17:196-201. [DOI] [PubMed] [Google Scholar]
- 14.Gubbels, J. M., A. P. de Vos, M. van der Weide, J. Viseras, L. M. Schouls, E. de Vries, and F. Jongejan. 1999. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J. Clin. Microbiol. 37:1782-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herd, R. P. 1990. Equine parasite control—problems associated with intensive anthelmintic therapy. Equine Vet. Educ. 2:41-47. [Google Scholar]
- 16.Herd, R. P., T. B. Miller, and A. A. Gabel. 1981. A field evaluation of pro-benzimidazole, benzimidazole, and non-benzimidazole anthelmintics in horses. J. Am. Vet. Med. Assoc. 179:686-691. [PubMed] [Google Scholar]
- 17.Hodgkinson, J. E., J. R. Lichtenfels, T. S. Mair, P. Cripps, K. L. Freeman, Y. H. Ramsey, S. Love, and J. B. Matthews. 2003. A PCR-ELISA for the identification of cyathostomin fourth-stage larvae from clinical cases of larval cyathostominosis. Int. J. Parasitol. 33:1427-1435. [DOI] [PubMed] [Google Scholar]
- 18.Hodgkinson, J. E., K. L. Freeman, J. R. Lichtenfels, S. Palfreman, S. Love, and J. B. Matthews. 2005. Identification of strongyle eggs from anthelmintic-treated horses using a PCR-ELISA based on intergenic DNA sequences. Parasitol. Res. 95:287-292. [DOI] [PubMed] [Google Scholar]
- 19.Hodgkinson, J. E. 2006. Molecular diagnosis and equine parasitology. Vet. Parasitol. 136:109-116. [DOI] [PubMed] [Google Scholar]
- 20.Hodgkinson, J. E., S. Love, J. R. Lichtenfels, S. Palfreman, Y. H. Ramsey, and J. B. Matthews. 2001. Evaluation of the specificity of five oligoprobes for identification of cyathostomin species from horses. Int. J. Parasitol. 31:197-204. [DOI] [PubMed] [Google Scholar]
- 21.Hung, G. C., R. B. Gasser, I. Beveridge, and N. B. Chilton. 1999. Species-specific amplification by PCR of ribosomal DNA from some equine strongyles. Parasitology 119:69-80. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan, R. M. 2002. Anthelmintic resistance in nematodes of horses. Vet. Res. 33:491-507. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan, R. M. 2004. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 20:477-481. [DOI] [PubMed] [Google Scholar]
- 24.Kaye, J. N., S. Love, J. R. Lichtenfels, and J. B. McKeand. 1998. Comparative sequence analysis of the intergenic spacer region of cyathostome species. Int. J. Parasitol. 28:831-836. [DOI] [PubMed] [Google Scholar]
- 25.Kuzmina, T. A., V. A. Kharchenko, A. I. Starovir, and G. M. Dvojnos. 2005. Analysis of the strongylid nematodes (Nematoda: Strongylidae) community after deworming of brood horses in Ukraine. Vet. Parasitol. 131:283-290. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenfels, J. R., E. P. Hoberg, and D. S. Zarlenga. 1997. Systematics of gastrointestinal nematodes of domestic ruminants: advances between 1992 and 1995 and proposals for future research. Vet. Parasitol. 72:225-245. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenfels, J. R., L. M. Gibbons, and R. C. Krecek. 2002. Recommended terminology and advances in the systematics of the Cyathostominea (Nematoda: Strongyloidea) of horses. Vet. Parasitol. 107:337-342. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenfels, J. R., V. A. Kharchenko, R. C. Krecek, and L. M. Gibbons. 1998. An annotated checklist by genus and species of 93 species level names for 51 recognized species of small strongyles (Nematoda: Strongyloidea: Cyathostominea) of horses, asses and zebras of the world. Vet. Parasitol. 79:65-79. [DOI] [PubMed] [Google Scholar]
- 29.Lind, E. O., M. Eysker, O. Nilsson, A. Uggla, and J. Hoglund. 2003. Expulsion of small strongyle nematodes (cyathostomin spp.) following deworming of horses on a stud farm in Sweden. Vet. Parasitol. 115:289-299. [DOI] [PubMed] [Google Scholar]
- 30.Love, S., and J. B. McKeand. 1997. Cyathostominosis: practical issue of treatment and control. Equine Vet. Educ. 9:253-256. [Google Scholar]
- 31.Love, S., D. Murphy, and D. Mellor. 1999. Pathogenicity of cyathostome infection. Vet. Parasitol. 85:113-122. [DOI] [PubMed] [Google Scholar]
- 32.Lyons, E., S. Tolliver, and J. Drudge. 1999. Historical perspective of cyathostomes: prevalence, treatment and control programs. Vet. Parasitol. 85:97-112. [DOI] [PubMed] [Google Scholar]
- 33.Mair, T. S., D. G. Sutton, and S. Love. 2000. Caecocaecal and caecocolic intussusceptions associated with larval cyathostominosis in four young horses. Equine Vet. J. 32:77-80. [DOI] [PubMed] [Google Scholar]
- 34.Matthee, S., R. C. Krecek, and S. A. Milne. 2000. Prevalence and biodiversity of helminth parasites in donkeys from South Africa. J. Parasitol. 86:756-762. [DOI] [PubMed] [Google Scholar]
- 35.Matthews, J. B., J. E. Hodgkinson, S. M. J. Dowdall, and C. J. Proudman. 2004. Recent developments in research into the Cyathostominae and Anoplocephala perfoliata. Vet. Res. 35:371-381. [DOI] [PubMed] [Google Scholar]
- 36.Ministry of Agriculture, Fisheries and Food. 1986. Technical bulletin no. 18. Manual of veterinary parasitological laboratory techniques. Her Majesty's Stationary Office, London, United Kingdom.
- 37.Murphy, D., and S. Love. 1997. The pathogenic effects of experimental Cyathostome infections in ponies. Vet. Parasitol. 70:99-110. [DOI] [PubMed] [Google Scholar]
- 38.Ogbourne, C. P. 1976. The prevalence, relative abundance and site distribution of nematodes of the subfamily Cyathostominae in horses killed in Britain. J. Helminthol. 50:203-214. [DOI] [PubMed] [Google Scholar]
- 39.Pereira, J. R., and S. S. Vianna. 2006. Gastrointestinal parasitic worms in equines in the Paraiba Valley, State of Sao Paulo, Brazil. Vet. Parasitol. 140:289-295. [DOI] [PubMed] [Google Scholar]
- 40.Playford, E. G., F. Kong, Y. Sun, H. Wang, C. Halliday, and T. C. Sorrel. 2006. Simultaneous detection and identification of Candida, Aspergillus, and Cryptococcus species by reverse line blot hybridization. J. Clin. Microbiol. 44:876-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reinemeyer, C. R., S. A. Smith, A. A. Gabel, and R. P. Herd. 1984. The prevalence and intensity of internal parasites of horses in the USA. Vet. Parasitol. 15:75-83. [DOI] [PubMed] [Google Scholar]
- 42.Rombout, Y. B., S. Bosch, and J. W. Van Der Giessen. 2001. Detection and identification of eight Trichinella genotypes by reverse line blot hybridization. J. Clin. Microbiol. 39:642-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva, A. V. M., H. M. A. Costa, H. A. Santos, and R. O. Carvalho. 1999. Cyathostominae (Nematoda) parasites of Equus caballus in some Brazilian states. Vet. Parasitol. 86:15-21. [DOI] [PubMed] [Google Scholar]
- 44.Sparagano, O. A., G. Carelli, L. Ceci, V. Shkap, T. Molad, F. Vitale, G. R. Loria, S. Reale, S. Caracappa, A. Bouattour, S. Almeira, J. Castella, E. Corchero, and M. Habela. 2002. Pan-Mediterranean comparison for the molecular detection of Theileria annulata. Ann. N. Y. Acad. Sci. 969:73-77. [DOI] [PubMed] [Google Scholar]
- 45.Thienpont, D., F. Rochette, and O. F. D. Vanprijs. 1986. Diagnosis helminthiasis by coprological examination, p. 17-69. Beerse Janssen Research Foundation, Beerse, Belgium.
- 46.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tolliver, S. G. 2000. A practical method of identification of the North American cyathostomes (small strongyles) in equids in Kentucky, p. 1-37. University of Kentucky, Lexington, KY.
- 48.Traversa, D., A. Giangaspero, P. Galli, B. Paoletti, D. Otranto, and R. B. Gasser. 2004. Specific identification of Habronema microstoma and Habronema muscae (Spirurida, Habronematidae) by PCR using markers in ribosomal DNA. Mol. Cell Probes 18:215-221. [DOI] [PubMed] [Google Scholar]
- 49.Traversa, D., A. Giangaspero, R. Iorio, D. Otranto, B. Paoletti, and R. B. Gasser. 2004. Semi-nested PCR for the specific detection of Habronema microstoma or Habronema muscae DNA in horse faeces. Parasitology 129:733-739. [DOI] [PubMed] [Google Scholar]
- 50.Traversa, D., O. A. Sparagano, N. Thanantong, R. Iorio, and A. Giangaspero. 2006. Molecular identification of equine strongyles by a reverse line blot hybridization assay, p. 50. In Proceedings of the 158th Meeting of the Society of General Microbiology. Society of General Microbiology, Warwick, United Kingdom.
- 51.Traversa, D., O. A. Sparagano, N. Thanantong, R. Iorio, B. Paoletti, A. Gatti, and A. Giangaspero. 2006. Reverse line blot hybridization assay for the molecular speciation of strongyles affecting horses, p. 73. In Proceedings of the XI International Conference of Parasitology. British Society of Parasitology, Glasgow, United Kingdom.
- 52.Traversa, D., R. Iorio, G. Capelli, B. Paoletti, D. Otranto, R. Bartolini, and A. Giangaspero. 2006. Molecular cross-sectional survey of gastric habronemosis in horses. Vet. Parasitol. 141:285-290. [DOI] [PubMed] [Google Scholar]
- 53.Uhlinger, C. A. 1991. Equine small strongyles: epidemiology, pathology, and control. Compend. Cont. Educ. Pract. Vet. 13:863-869. [Google Scholar]
- 54.Xiong, L., F. Kong, Y. Yang, J. Cheng, and G. L. Gilbert. 2006. Use of PCR and reverse line blot hybridization macroarray based on 16S-23S rRNA gene internal transcribed spacer sequences for rapid identification of 34 mycobacterium species. J. Clin. Microbiol. 44:3544-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]