Abstract
Pulmonary aspergillosis in nonimmunocompromised hosts, although rare, is being increasingly recognized. The diagnosis of pulmonary aspergillosis is difficult, since the recovery of Aspergillus from respiratory samples cannot differentiate colonization from invasion. We assessed the role of bronchoalveolar lavage (BAL) in detecting galactomannan (GM) for diagnosing pulmonary aspergillosis in 73 nonimmunocompromised patients with pulmonary infiltrates for whom the test was ordered. Six patients had pulmonary aspergillosis, two each with acute invasive pulmonary aspergillosis, chronic necrotizing pulmonary aspergillosis, and aspergilloma. All six patients had a BAL GM level of ≥1.18. The sensitivity, specificity, and negative predictive value (NPV) for a BAL GM level of ≥1.0 were 100%, 88.1%, and 100%, respectively. Notably, the positive predictive value (PPV) was only 42.9%, likely reflecting the low prevalence of pulmonary aspergillosis among nonimmunosuppressed patients. The combination of BAL microscopy and culture had a sensitivity and NPV similar to those of BAL GM detection but a higher specificity and PPV (92.5% and 54.6%, respectively). Moreover, a BAL GM test did not identify any cases that were not diagnosed by conventional methods like microscopy and culture. In conclusion, there was no conclusive benefit of determining BAL GM levels in the diagnosis of pulmonary aspergillosis among nonimmunocompromised hosts. Given the likelihood of false-positive results, a BAL GM test should not be ordered routinely in this population.
Invasive infections of lung parenchyma by Aspergillus are rare diseases outside of patients with hematologic malignancies and those who have undergone hematopoietic stem cell transplantation (HSCT) and solid-organ transplantation (SOT) (4, 10, 20). At present, lung biopsies are the diagnostic “gold standard” but are limited by sensitivity and complications (13). While the isolation of Aspergillus species from the respiratory tracts of high-risk patients is predictive of pulmonary aspergillosis (6, 21), culture is limited by poor sensitivity.
A commercially available double-sandwich enzyme-linked immunosorbent assay that detects galactomannan (GM), a cell wall polysaccharide of Aspergillus (Platelia ELISA; Bio-Rad), has been introduced to improve the diagnosis of invasive aspergillosis (5). The sensitivity of a serum GM test is 61% to 71% among HSCT recipients and patients with hematologic malignancies (14) and 30 to 56% among SOT recipients (3, 7, 9). A few studies have assessed the diagnostic utility of bronchoalveolar lavage (BAL) GM testing. Among HSCT recipients and patients with hematologic malignancies, BAL GM testing added to the sensitivity of both BAL culture and serum GM testing (12, 15-18). We recently found similar results among SOT recipients, although false-positive tests due to airway colonization with Aspergillus were common among patients with lung transplants (2).
Over the past decade, invasive aspergillosis has been increasingly recognized in nonimmunosuppressed patients. In these patients, the types of pulmonary aspergillosis include acute invasive pulmonary aspergillosis (IPA), a rapidly progressive infection similar to that of profoundly immunosuppressed hosts (2, 11), chronic necrotizing pulmonary aspergillosis (CNPA), a slowly progressive infection often seen among patients with underlying lung disease (8, 19), and mycetoma or fungus ball. The diagnosis of IPA or CNPA is particularly difficult since the presence of Aspergillus in culture or by microscopy cannot differentiate colonization from invasive disease. The objective of this study was to assess the utility of the BAL GM test in the diagnosis of pulmonary aspergillosis among nonimmunosuppressed patients.
MATERIALS AND METHODS
Identification of patients.
We reviewed all cases at the Shands Teaching Hospital at the University of Florida in which BAL fluid was tested for GM between September 2004 and August 2006. We excluded patients who were immunosuppressed, which was defined as neutropenia (absolute neutrophil count of <1,000 neutrophils/mm3), histories of hematologic malignancy, HSCT, SOT, human immunodeficiency virus infection, and the receipt of corticosteroids or other immunosuppressive agents within 6 months of the diagnosis of pulmonary aspergillosis.
BAL GM determination.
A BAL GM test was ordered at the discretion of physicians caring for the patients. The BAL fluid was sent on dry ice via overnight mail to MiraVista Diagnostics (Indianapolis, IN) for GM assay using a Platelia Aspergillus enzyme immunoassay (Bio-Rad Laboratories, Redmond, WA). The BAL GM results were made available to the patients’ physicians. Decisions about the institution of antifungal therapy were made by the patients’ physicians and required approval by the infectious disease consultation team.
Case definitions.
The classification of invasive aspergillosis into categories of proven, probable, and possible IPA using modified European Organization for Research and Treatment of Cancer-Mycoses Study Group criteria cannot be applied to our patient population due to the lack of “host factors.” We designed definitions based on the types of pulmonary aspergillosis that are most commonly encountered in the nonimmunosuppressed host.
Mycetoma was defined as a mobile mass lesion within a preexisting pulmonary cavity visualized on a chest X-ray or computed tomography (CT) scan. If Aspergillus spp. were isolated from the sputum or BAL fluid, the diagnosis was proven aspergilloma. In the absence of Aspergillus upon culture, the diagnosis was mycetoma, presumably aspergilloma.
Proven CNPA was defined as a subacute infection associated with the demonstration of septated hyphae invading lung parenchyma on biopsy specimens and accompanied by the growth of Aspergillus in culture. For our purposes, subacute was defined as a duration of pulmonary symptoms of >1 month. The diagnosis was presumed CNPA when invasive hyphae were not demonstrated within the tissue specimen; in this setting, other infectious or noninfectious causes that might account for the pulmonary findings had to be ruled out by the treating physicians using standard diagnostic approaches such as BAL cultures and microscopy with or without transbronchial biopsy, other respiratory cultures, blood cultures or cultures from other sites, and treatment and response to nonfungal therapy. Alternative diagnoses to pulmonary aspergillosis were based on the consensus opinions of the physicians managing the patients as well as the two investigators independently reviewing the cases.
Proven acute IPA was defined as a pulmonary infection (duration of ≤1 month) associated with the demonstration of septated hyphae invading lung parenchyma on biopsy specimens and accompanied by Aspergillus culture growth. The diagnosis was presumed acute IPA when invasive hyphae were not demonstrated within the tissue specimen; in this setting, other infectious or noninfectious causes that might account for the pulmonary findings had to be ruled out.
Data analysis.
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated on a per-patient basis for BAL GM testing, serum GM testing, BAL microscopy, and culture. The optimal cutoff for BAL GM testing was determined by receiver operating characteristic (ROC) analysis. Factors associated with pulmonary aspergillosis were determined using Fisher's exact test and expressed in two-by-two contingency tables; P values of ≤0.05 were considered to be significant.
RESULTS
Description of patient population.
Over a 2-year period at our university medical center, a BAL GM test was ordered for 73 patients who were not known to be immunosuppressed. Twenty-seven patients were previously healthy, and 14 patients had underlying lung diseases (chronic obstructive pulmonary disease, asthma, or bronchiectasis [nine patients]; primary pulmonary hypertension [two patients]; chronic cough [two patients]; and cystic fibrosis [one patient]).
Six patients had pulmonary aspergillosis (Table 1); two of these patients had acute IPA, two patients had CNPA, and two patients had aspergilloma.
TABLE 1.
Description of patients with pulmonary aspergillosisa
| Age (yr) (sex) | Underlying disease(s) | Reason(s) for BAL | Antibiotic(s) used prior to or at the time of BAL | CXR/chest CT scan result | BAL GM level(s) | Serum GM level(s) | Microscopy result, TBBX result | Culture | Diagnosis | Treatment | Outcome at follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 66 (M) | CAD, DM, and obesity; admitted with acute pneumococcal sepsis | Fever, septic shock | CFP, Levo, Vanc | Bil parenchymal opacity, diffuse GGO, nodule | 1.18 | 1.41 (+1 day)b | Inflammatory cells (yeasts), not done | BAL, A. flavus; sputum (−1 days),bA. flavus | Proven IPAc | No antifungal | Died, 4 daysb |
| 61 (M) | Previously healthy; admitted with severe upper GI bleed | Shock | CFP, Metro, Vanc, Tim | Nodular infiltrates | 8.44 | 2.58 (−4 days),b 2.69 (−2 days)b | Not done, not done | BAL, A. fumigatus; sputum (−1 days, −2 days),bA. fumigatus | Presumed IPA | VORI until death | Died, 10 daysb |
| 67 (F) | COPD; cavitary lung lesion was found on admission CXR | Respiratory symptoms for 4 mo and cavitary lung lesion | Cipro, Metro | Consolidation with cavitation | 2.0; after antifungal treatment, −0.62 (+19 days)b | 0.05 (+5 days)b | Hyphae, inflammation and necrosis; hyphae seen in tissue | BAL, Candida and A. fumigatus | Proven CNPA | VORI and antibiotics until death | Died, 2 mob (from ruptured AAA) |
| 56 (M) | Healthy, admitted after a stroke; cavitary lung lesion was found on admission CXR | Wt loss, cough, hemoptysis, abnormal CXR | None | Cavitary lesion with surrounding consolidation | 7.41, 7.34d | 0.98 (−2 days)b | Hyphae, chronic and granulomatous inflammation, presence of eosinophils (no hyphae) | BAL, A. fumigatus; sputum (−3 days),bA. fumigatus | Probable CNPA | VORI for 1.3 yr | Lived (F/u, 1.3 yr) |
| 51 (M) | Lung cancer, in remission for 5 yr; admitted for hemoptysis | Fever, respiratory complaints, and hemoptysis | Gati | Cavitary lesion with fungus ball; surrounding consolidation | 1.43, 1.15d | 0.08 (+3 days),b 0.11 (+4 days),b 0.08 (+5 days)b | No hyphae, not done | BAL, Candida and A. fumigatus | Proven aspergilloma; MAI pneumonia and cavitary lesion | VORI for 1 year and Rx for MAI | Lived (F/u, 1 yr) |
| 46 (M) | Crohn's colitis, in remission, MAI pneumonia on treatment; admitted with hemoptysis | Fever, respiratory complaints, and hemoptysis | Mero and meds against MAI | Cavitary lung lesion with fungus ball | 8.89 (LLL), 8.64d (RL) | Not done | Inflammatory cells (no hyphae), chronic and acute inflammation (no hyphae) | A. fumigatus | Proven aspergilloma; MAI cavitary lung lesion | VORI for 6 mo and Rx for MAI | Lived (F/u, 6 mo) |
Abbreviations: TBBX, transbronchial biopsy; M, male; F, female; CAD, coronary artery disease; DM, diabetes mellitus; COPD, chronic obstructive lung disease; AAA, abdominal aortic aneurysm; CXR, chest X ray; Bil, bilateral; GGO, ground glass opacification; MAI, Mycobacterium avium-Mycobacterium intracellularae; RLL, right lower lobe; LLL, left lower lobe; RL, right lung; F/u, follow-up time; Rx, treatment; meds, medications; CFP, cefipime; Levo, levofloxacin; Vanc, vancomycin; Cipro, ciprofloxacin; Gati, gatifloxacin; Metro, metronidazole; Tim, timentin; Mero, meropenem; VORI, voriconazole.
Date given relative to the initial BAL (e.g., +1 day indicates 1 day after BAL, and −1 day indicates 1 day before BAL).
Fungal hyphae were found within the lung parenchyma and blood vessels upon autopsy.
BAL was performed on several lung specimens.
Performance of BAL GM testing.
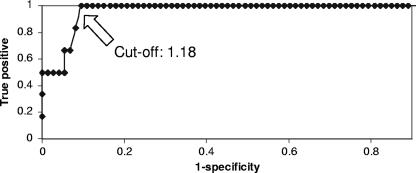
Twenty-two patients had a BAL GM level of ≥0.5, and 15 patients had BAL GM levels of ≥1.0. The sensitivity, specificity, PPV, and NPV of BAL GM testing at various interpretive cutoffs are presented in Table 2. All six patients with pulmonary aspergillosis had BAL GM levels of ≥1.18 (range, 1.18 to 8.89) (Table 1). For three patients, BAL was collected from multiple lung segments, and the GM level was ≥1.15 for each sample. Increasing the cutoff from 0.5 to 1.0 improved the specificity and PPV of the BAL GM test without influencing the sensitivity or NPV. Based on the ROC, the optimal cutoff for positivity was 1.18 (Fig. 1). Excluding the two cases of aspergilloma, which were evident as fungus balls by CT scanning prior to bronchoscopy, the PPV was reduced from 43% to 33% (cutoff, ≥1.0).
TABLE 2.
Performance of tests for diagnosing pulmonary aspergillosis
| Test and cutoff | Sensitivity (%) (no. of positive samples/total no. of samples) (range) | Specificity (%) (no. of positive samples/total no. of samples) (range) | PPV (%) (no. of positive samples/total no. of samples) (range) | NPV (%) (no. of positive samples/total no. of samples) (range) |
|---|---|---|---|---|
| BAL GM | ||||
| ≥0.5 | 100 (6/6) (54.1-100) | 77.6 (52/67) (65.8-86.9) | 28.6 (6/21) (11.3-52.2) | 100 (52/52) (93.2-100) |
| ≥1.0 | 100 (6/6) (54.1-100) | 88.1 (59/67) (77.8-94.7) | 42.9 (6/14) (17.1-71.1) | 100 (59/59) (93.9-100) |
| ≥1.5 | 66.7 (4/6) (22.3-95.7) | 91 (61/67) (81.5-96.6) | 40 (4/10) (12.2-73.8) | 96.8 (61/63) (89.3-99.6) |
| ≥2.0 | 66.7 (4/6) (22.3-95.7) | 94 (63/67) (85.4-98.4) | 50 (4/8) (15.7-84.3) | 96.9 (63/65) (89.3-99.6) |
| ≥2.5 | 50 (3/6) (11.8-88.2) | 95.5 (64/67) (87.5-99.1) | 50 (3/6) (11.8-88.2) | 95.5 (64/67) (87.5-99.1) |
| Serum GMa | ||||
| ≥0.5 | 60 (3/5) (14.7-94.7) | 91.7 (11/12) (61.5-99.8) | 75 (3/4) (19.4-99.4) | 84.6 (11/13) (54.6-98.1) |
| ≥1.0 | 40 (2/5) (5.3-85.3) | 91.7 (11/12) (61.5-99.8) | 66.7 (2/3) (9.4-99.2) | 78.6 (11/14) (49.2-95.4) |
| BAL culture | 66.7 (4/6) (22.3-95.7) | 94 (63/67) (85.4-98.4) | 50 (4/8) (15.7-84.3) | 96.9 (63/65) (89.3-99.6) |
| BAL microscopyb | 80 (4/5) (28.4-99.5) | 96.9 (63/65) (89.3-99.6) | 66.7 (4/6) (22.3-95.7) | 98.4 (63/64) (91.6-100) |
| BAL culture or microscopy | 100 (6/6) (54.1-100) | 92.5 (62/67) (83.4-97.5) | 54.5 (6/11) (23.4-83.2) | 100 (62/62) (94.2-100) |
The serum GM test was performed for only 17 patients.
BAL microscopy was performed for only 70 patients.
FIG. 1.
ROC curve for BAL GM test results.
The performances of the serum GM test, BAL microscopy, and culture are presented in Table 2. Of note, both patients with acute IPA and one of two patients with CNPA had serum GM levels of ≥0.5. Overall, the combination of microscopy and culture had a sensitivity and NPV similar to those of BAL GM testing but had a higher specificity and PPV.
Impact of a BAL GM test on the management of patients with BAL GM levels of ≥1.0 but no evidence of pulmonary aspergillosis.
Nine patients had at least one sample with a BAL GM level of ≥1.0 but did not fulfill diagnostic criteria for pulmonary aspergillosis (Table 3). BAL fluid was collected from multiple lung segments of five patients, but only one patient had more than one sample that revealed a GM level of >1.0. Seven patients did not receive antifungal agents; none subsequently developed pulmonary aspergillosis. The other two patients were started on antifungal therapy. The first patient was admitted with signs, symptoms, and radiographic findings that were suggestive of pulmonary tuberculosis. Due to a positive BAL GM test, the treatment for tuberculosis was delayed for 4 days, until the transbronchial biopsy yielded caseating granulomas. This patient subsequently died from disseminated tuberculosis. The second patient was diagnosed with advanced metastatic lung cancer that was deemed untreatable. The patient's physician elected to institute “palliative voriconazole” in response to BAL GM detection since this was the only diagnostic test that was positive for a “treatable disease.”
TABLE 3.
Description of patients with BAL GM levels of ≥1 but with no evidence of pulmonary aspergillosisa
| Age (yr) (sex) | Underlying disease(s) | Reason(s) for BAL | Antibiotic(s) prior to or at the time of BAL | Chest X-ray/chest CAT scan result | BAL GM level(s)b | Serum GM level(s)b | Microscopy result, TBBX result | Culture result | Diagnosis | Treatment (duration) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 52 (M) | Healthy | Fever, respiratory symptoms | CTX, AZI | Micronod IF, air space disease, med LN | 1.04 | Not done | Hyphae, chronic inflammation | Aspergillus fumigatus, Aspergillus niger | CAP | No antifungal, antibiotics | Lived (F/u, 6 mo) |
| 48 (F) | COPD, pulmonary HTN | SOB | CTX, AZI | Multiple nodules, diffuse GGO, hilar LN | 1.33, 0.11c | 0.11 (+3 days), 0.05 (+2 m) | No hyphae, bronchiolitis (interstitial lung disease) | No fungus | Pulmonary HTN | No antifungal, antibiotics and steroid | Died (2 mo) |
| 22 (F) | Healthy with miliary TB | Respiratory symptoms | CTX, AZI | New cavitary lung lesion and tib | 0.32 (−12 days), 1.57 | 0.07 (−12 days), 0.11 | No hyphae, necrotizing granuloma | No fungus | Miliary TB | VORI, 5 days, TB medications | Died (19 days) (from disseminated tuberculosis) |
| 77 (M) | HTN | Fever, respiratory symptoms | CTX, AZI | Airspace disease, hilar and med LN | 1.58 | 0.06 (+3 days) | No hyphae, BOOP | Candida | BOOP | No antifungal, steroid | Lived (F/u, 1 yr) |
| 52 (M) | ICM | Respiratory symptoms | None | New cavitary lesion with surrounding infiltrate, hilar LN | 2.38, 0.15c | 0.15 (+10 days), 0.09 (+12 days), 0.07 (+13 days) | No hyphae, nondiagnostic | No fungus | Lung cancer (adenocarcinoma) | VORI, 3 mo (based on BAL GM), antibiotics | Died (4 mo) (lung cancer) |
| 57 (M) | CAD, HTN | Respiratory symptoms | CTX, AZI | New cavitary lesion, med LN | 2.67 | Not done | No hyphae, acute and organizing pneumonia | Aspergillus terreus | CAP, TB | No antifungal, antibiotics | Lived (F/u, 3 mo) |
| 2 (M) | Healthy | ARDS, fever | CTX, Vanc | Consolidation | 2.89, 0.98c | 0.09 (+3 days), 0.14 (+5 days) | No hyphae, not done | No fungus | MRSA pneumonia on viral pneumonia | No antifungal, antibiotics | Lived (alive at d/c; no F/u available) |
| 64 (M) | COPD | Chest pain, s/p falls | None | Mass with necrotic center | 4.98, 6.13c | 0.12 (+4 days) | Yeast, not done | Candida albicans | Disseminated community- acquired MRSA infection | No antifungal, antibiotics | Died (9 days) |
| 54 (F) | Hepatitis C virus with ESLD | Weakness, encephalopathy, respiratory failure | Tim, Vanc | Consolidation (focal), diffuse GGO | 1.26, 0.37c | Not done | Hyphal elements and yeasts, not done | No fungus | Diffuse alveolar hemorrhage | No antifungal | Died (8 days) (hepatorenal syndrome and sepsis) |
TBBX, transbronchial biopsy; M, male; F, female; Micronod IF, micronodular infiltrates; COPD, chronic obstructive pulmonary disease; SOB, shortness of breath; s/p, status post; HTN, hypertension; TB, tuberculosis; ICM, ischemic cardiomyopathy; CAD, coronary artery disease; ESLD, end-stage liver disease; BOOP, bronchiolitis obliterans with organizing pneumonia; LN, lymphadenopathy; GGO, ground glass opacity; med, mediastinal; CAP, community-acquired pneumonia; d/c, discharge; F/u, follow-up; CTX, ceftriaxone; AZI, azithromycin; Tim, timentin; Vanc, vancomycin; VORI, voriconazole.
Date given relative to the initial BAL (e.g., +1 day indicates 1 day after BAL, and −1 day indicates 1 day before BAL).
BAL was performed from several lung segments.
Of note, none of the seven patients with BAL GM levels of between 0.5 and 1.0 were treated with antifungal agents, and none had any further evidence of aspergillosis (data not shown). In addition, none received piperacillin-tazobactam or amoxicillin-clavulanate.
DISCUSSION
A review of the experience at our institution failed to demonstrate a benefit of BAL GM testing in the diagnosis of pulmonary aspergillosis in nonimmunocompromised hosts. A BAL GM test was highly sensitive in this population (100% at a cutoff of ≥1.0), with a good specificity (88.1%) and excellent NPV (100%). The test was limited, however, by a PPV of 43%, which reflects the low prevalence of pulmonary aspergillosis in this population. BAL GM testing was no more sensitive than the combination of BAL microscopy and culture and exhibited a lower PPV than these tests. Moreover, BAL GM testing did not identify any cases of IPA or CNPA that were not diagnosed by conventional methods like microscopy and culture and only increased the likelihood of obtaining false-positive results. Our findings differ from those of previous reports of BAL GM testing among hematologic malignancy, HSCT, and SOT patients, for whom the test generally added to the sensitivity of microscopy and culture and identified cases of pulmonary aspergillosis that were not diagnosed by these methods (2, 12, 15, 16, 18).
This review reiterates that pulmonary aspergillosis is a rare but often unrecognized cause of serious lung disease outside of high-risk groups (1). It is notable that both patients with IPA in our series had serum GM levels of >0.5 concomitantly with a BAL GM level of ≥1.18. These findings suggest that the combination of BAL and serum GM might be useful in subsets of nonimmunocompromised patients for whom IPA is a serious diagnostic consideration. We reported two nonimmunocompromised patients, for example, who developed biopsy-proven IPA following acute pneumonia due to influenza virus (2) and Streptococcus pneumoniae (this report). It is difficult to make definitive recommendations about specific patients who might benefit from the use of the BAL GM test. Nevertheless, clinicians might consider judicious GM testing among certain patients who have ongoing pneumonias of unclear etiology that have not responded to standard therapies.
Unlike IPA and CNPA, the cases of aspergilloma in our series were readily evident upon CT scan and BAL culture. Clearly, BAL GM testing does not have a diagnostic role in routine cases of mycetoma. Moreover, we did not demonstrate an association between elevated BAL GM levels and invasion from mycetomas into local parenchyma, as a tissue biopsy was not performed in one case and failed to demonstrate invasive hyphal elements in the second.
In conclusion, physicians should exercise restraint in ordering and interpreting BAL GM tests for nonimmunocompromised hosts in order to avoid overdiagnosing pulmonary aspergillosis and subjecting patients to unnecessary treatment. The observations from this study should be corroborated in prospective studies.
Acknowledgments
This study was supported by the University of Florida Mycology Research Unit (NIH PO1 AI061537-01 to M. H. Nguyen, C. J. Clancy, and J. R. Wingard).
L. J. Wheat is the President and Director of MiraVista Diagnostics, which performs BAL GM testing as a commercial reference service. The remaining authors do not report any conflict of interest.
Footnotes
Published ahead of print on 27 June 2007.
REFERENCES
- 1.Clancy, C. J., and M. H. Nguyen. 1998. Acute community-acquired pneumonia due to Aspergillus in presumably immunocompetent hosts: clues for recognition of a rare but fatal disease. Chest 114:629-634. [DOI] [PubMed] [Google Scholar]
- 2.Clancy, C. J., R. A. Jaber, H. L. Leather, J. R. Wingard, B. Staley, L. J. Wheat, C. L. Cline, K. H. Rand, D. Schain, M. Baz, and M. H. Nguyen. 2007. Bronchoalveolar lavage galactomannan in diagnosis of invasive pulmonary aspergillosis among solid-organ transplant recipients. J. Clin. Microbiol. 45:1759-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fortun, J., P. Martin-Davila, M. E. Alvarez, A. Sanchez-Sousa, C. Quereda, E. Navas, R. Barcena, E. Vicente, A. Candelas, A. Honrubia, J. Nuno, V. Pintado, S. Moreno, C. Y. Ramon, et al. 2001. Aspergillus antigenemia sandwich-enzyme immunoassay test as a serodiagnostic method for invasive aspergillosis in liver transplant recipients. Transplantation 71:145-149. [DOI] [PubMed] [Google Scholar]
- 4.Holding, K. J., M. S. Dworkin, P. C. Wan, D. L. Hanson, R. M. Klevens, J. L. Jones, P. S. Sullivan, et al. 2000. Aspergillosis among people infected with human immunodeficiency virus: incidence and survival. Clin. Infect. Dis. 31:1253-1257. [DOI] [PubMed] [Google Scholar]
- 5.Hope, W. W., T. J. Walsh, and D. W. Denning. 2005. Laboratory diagnosis of invasive aspergillosis. Lancet Infect. Dis. 5:609-622. [DOI] [PubMed] [Google Scholar]
- 6.Horvath, J. A., and S. Dummer. 1996. The use of respiratory tract cultures in the diagnosis of invasive pulmonary aspergillosis. Am. J. Med. 100:171-178. [DOI] [PubMed] [Google Scholar]
- 7.Husain, S., E. J. Kwak, A. Obman, M. M. Wagener, S. Kusne, J. E. Stout, K. R. McCurry, and N. Singh. 2004. Prospective assessment of Platelia Aspergillus galactomannan antigen for the diagnosis of invasive aspergillosis in lung transplant recipients. Am. J. Transplant. 4:796-802. [DOI] [PubMed] [Google Scholar]
- 8.Kobashi, Y., M. Fukuda, K. Yoshida, N. Miyashita, Y. Niki, and M. Oka. 2006. Chronic necrotizing pulmonary aspergillosis as a complication of pulmonary Mycobacterium avium complex disease. Respirology 11:809-813. [DOI] [PubMed] [Google Scholar]
- 9.Kwak, E. J., S. Husain, A. Obman, L. Meinke, J. Stout, S. Kusne, M. M. Wagener, and N. Singh. 2004. Efficacy of galactomannan antigen in the Platelia Aspergillus enzyme immunoassay for diagnosis of invasive aspergillosis in liver transplant recipients. J. Clin. Microbiol. 42:435-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marr, K. A., T. Patterson, and D. Denning. 2002. Aspergillosis. Pathogenesis, clinical manifestations, and therapy. Infect. Dis. Clin. N. Am. 16:875-894. [DOI] [PubMed] [Google Scholar]
- 11.Meersseman, W., S. J. Vandecasteele, A. Wilmer, E. Verbeken, W. E. Peetermans, and E. Van Wijngaerden. 2004. Invasive aspergillosis in critically ill patients without malignancy. Am. J. Respir. Crit. Care Med. 170:621-625. [DOI] [PubMed] [Google Scholar]
- 12.Musher, B., D. Fredricks, W. Leisenring, S. A. Balajee, C. Smith, and K. A. Marr. 2004. Aspergillus galactomannan enzyme immunoassay and quantitative PCR for diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. J. Clin. Microbiol. 42:5517-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nosari, A., P. Oreste, R. Cairoli, M. Montillo, G. Carrafiello, A. Astolfi, G. Muti, L. Marbello, A. Tedeschi, E. Magliano, and E. Morra. 2001. Invasive aspergillosis in haematological malignancies: clinical findings and management for intensive chemotherapy completion. Am. J. Hematol. 68:231-236. [DOI] [PubMed] [Google Scholar]
- 14.Pfeiffer, C. D., J. P. Fine, and N. Safdar. 2006. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin. Infect. Dis. 42:1417-1427. [DOI] [PubMed] [Google Scholar]
- 15.Salonen, J. O., M. R. Terasjarvi, and J. Nikoskelainen. 2000. Aspergillus antigen in serum, urine and bronchoalveolar lavage specimens of neutropenic patients in relation to clinical outcome. Scand. J. Infect. Dis. 32:485-490. [DOI] [PubMed] [Google Scholar]
- 16.Sanguinetti, M., B. Posteraro, L. Pagano, G. Pagliari, L. Fianchi, L. Mele, M. La Sorda, A. Franco, and G. Fadda. 2003. Comparison of real-time PCR, conventional PCR, and galactomannan antigen detection by enzyme-linked immunosorbent assay using bronchoalveolar lavage fluid samples from hematology patients for diagnosis of invasive pulmonary aspergillosis. J. Clin. Microbiol. 41:3922-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seyfarth, H. J., P. Nenoff, J. Winkler, R. Krahl, U. F. Haustein, and J. Schauer. 2001. Aspergillus detection in bronchoscopically acquired material. Significance and interpretation. Mycoses 44:356-360. [DOI] [PubMed] [Google Scholar]
- 18.Siemann, M., and M. Koch-Dorfler. 2001. The Platelia Aspergillus ELISA in diagnosis of invasive pulmonary aspergillosis (IPA). Mycoses 44:266-272. [PubMed] [Google Scholar]
- 19.Vahid, B., and P. Marik. 2007. Fatal massive hemoptysis in a patient on low-dose oral prednisone: chronic necrotizing pulmonary aspergillosis. Respir. Care 52:56-58. [PubMed] [Google Scholar]
- 20.Warris, A., A. Bjorneklett, and P. Gaustad. 2001. Invasive pulmonary aspergillosis associated with infliximab therapy. N. Engl. J. Med. 344:1099-1100. [PubMed] [Google Scholar]
- 21.Yu, V. L., R. R. Muder, and A. Poorsattar. 1986. Significance of isolation of Aspergillus from the respiratory tract in diagnosis of invasive pulmonary aspergillosis. Results from a three-year prospective study. Am. J. Med. 81:249-254. [DOI] [PubMed] [Google Scholar]