Abstract
Galactomannan (GM) is a heteropolysaccharide in the cell walls of most Aspergillus and Penicillium species. Cross-reactivity of Cryptococcus neoformans galactoxylomannan in an Aspergillus GM test has also been reported. In this study, we used a Platelia Aspergillus enzyme immunoassay kit (Bio-Rad) to test serum samples obtained from 48 human immunodeficiency virus (HIV)-infected patients (15 with penicilliosis [7 with fungemia alone, 4 with cavitary lung lesions alone, 3 with both fungemia and cavitary lung lesions, and 1 with disseminated disease], 22 with cryptococcosis [11 with fungemia alone, 5 with cavitary lung lesions, 3 with both, and 3 with meningitis alone], and 11 without any invasive fungal infection [control]) for GM levels. None of the patients had aspergillosis or concurrent use of piperacillin-tazobactam or amoxicillin-clavulanate. The median time between diagnosis of fungal infection and collection of serum samples was 0 days for penicilliosis and 1.5 days for cryptococcosis. Of patients with penicilliosis, cryptococcosis, and controls, 73.3%, 13.6%, and 9%, respectively, had GM optical density (OD) indices of >0.5 (P = 0.0001). GM OD indices were higher for penicilliosis (median OD index, 4.419; range, 0.158 to >20) than for cryptococcosis (median, 0.247; range, 0.112 to 3.849) cases (P < 0.001). Patients with fungemic penicilliosis had higher OD indices (median, 10.628; range, 0.401 to >20) than patients with nonfungemic penicilliosis (median, 0.378; range, 0.158 to 4.419) and patients with cryptococcemia (median, 0.231; range, 0.112 to 1.168) (P < 0.001). Of the 15 patients with cavitary lung lesions, those with penicilliosis had higher antigen levels (median OD index, 1.641; range, 0.247 to >20) than those with cryptococcosis (median, 0.227; range, 0.112 to 3.849) (P = 0.011). This study showed that the GM OD index was significantly elevated for HIV patients with penicilliosis. The use of the GM antigen assay may facilitate earlier diagnosis of Penicillium marneffei infection for HIV-infected patients in areas of endemicity.
Invasive fungal infections are common opportunistic infections associated with significant morbidity and mortality for patients with human immunodeficiency virus (HIV) infection, and the risk of invasive fungal infection varies with host immunity as well as environmental exposure (7, 14, 17, 18, 19). Penicillium marneffei and Cryptococcus neoformans are important endemic fungi in Southeast Asia that cause systemic infections in HIV-infected patients, especially those who have low CD4 counts (17, 21). The clinical presentations of P. marneffei infection and cryptococcosis for HIV-infected patients may mimic those of tuberculosis, histoplasmosis, and other infections. Early diagnosis and timely initiation of appropriate therapy are complicated by nonspecific signs and symptoms as well as by difficulties in obtaining tissues for histological recognition. Microbiologic isolation and species identification of the pathogens may be time-consuming (7, 19, 21). Rapid, noninvasive microbiological diagnostic modalities for invasive fungal infections other than determinations of cryptococcal and histoplasma antigens are not available in most parts of the world (2, 3, 9). Although antigen detection to identify P. marneffei infection by using monoclonal antibodies specific for P. marneffei has been reported (2, 3), these tests are not commercially available, which hampers their usefulness.
Galactomannan (GM) is a heteropolysaccharide composed of a nonimmunogenic mannan core and immunoreactive galactofuransyl side chains in the cell walls of most Aspergillus and Penicillium species (10, 11, 15). A double-sandwich enzyme-linked immunosorbent assay for determination of Aspergillus GM was recently approved by the U.S. Food and Drug Administration (FDA) to facilitate early diagnosis of invasive aspergillosis. Studies have shown that a monoclonal antibody against Aspergillus GM reacted with serum and tissue samples from a P. marneffei-infected guinea pig as well as with samples from an HIV-infected patient with penicilliosis (14, 20). In addition, extracts and purified galactoxylomannan of C. neoformans gave positive reactions by the Aspergillus GM test (4). While clinical experience with the Aspergillus GM test in invasive aspergillosis is accumulating (11, 13), data on penicilliosis and cryptococcosis remain limited. In this study, we aimed to compare the GM antigen levels detected by the Aspergillus test for P. marneffei infection versus cryptococcosis in HIV-infected patients.
MATERIALS AND METHODS
Patients and serum samples.
Cases and controls were selected from HIV-infected patients who were hospitalized between January 2000 and December 2006 at the National Taiwan University Hospital, the largest referral hospital for HIV care in Taiwan. Medical records of HIV-infected patients diagnosed with penicilliosis or cryptococcosis in an ongoing prospective cohort study were reviewed using a standardized case record form (17, 18). Infection due to P. marneffei or C. neoformans was diagnosed by identifying the fungus by microscopy and cultures of clinical specimens, including blood, bone marrow aspirate, cerebrospinal fluid, lung aspirate, lymph node biopsy, skin biopsy, and sputum specimens. India ink smears and determinations of cryptococcal antigen levels in clinical specimens by the Latex-Crypto antigen detection system (Immuno Mycologics, Inc., Norman, OK) were also included for the diagnosis of cryptococcosis (17, 21).
Stored serum samples from patients with penicilliosis and cryptococcosis were retrieved for analysis. The serum samples, obtained once the diagnoses of HIV infection and opportunistic infection were made, were stored at −80°C before use. Serum samples from 11 randomly selected HIV-infected patients without active opportunistic infections were obtained as controls. Patients who were receiving piperacillin-tazobactam or amoxicillin-clavulanate within 3 days of serum collection were not included for serum GM detection because both antibiotics have been demonstrated to cause elevation of Aspergillus GM antigen levels (12, 16).
GM antigen detection.
GM levels were determined using the Platelia Aspergillus enzyme immunoassay (Bio-Rad, Marnes-la-Coquette, France) by following the manufacturer's instructions. The test uses a rat anti-GM monoclonal antibody, EB-A2, to recognize the galactofuranoside side chain of the GM molecule (15). Because there was no cutoff value for GM antigen levels in the diagnosis of P. marneffei and C. neoformans infections in the literature, we analyzed the results using three different optical density (OD) cutoff indices: 0.5, 1.0, and 1.5. For the diagnosis of aspergillosis, a cutoff index of 0.5 is accepted by the FDA, while a cutoff index of 0.7 is commonly used in Europe. Besides, 1.5 was the cutoff value initially proposed.
Statistical analysis.
All statistical analyses were performed using SPSS software (version 12.0, 2003; SPSS Inc., Chicago, IL). Categorical variables were compared using a χ2 test or Fisher's exact test, whereas noncategorical variables were compared using Wilcoxon's rank-sum test. Because both invasive fungal infections are often associated with fungemia and cavitary lung lesions in HIV-infected patients (18), subgroup analyses of patients with fungemia and patients with cavitary lung lesions were performed, and comparisons of the GM OD indices of serum samples from patients with penicilliosis and cryptococcosis were stratified by the presence or absence of fungemia and the presence or absence of cavitary lung lesions.
RESULTS
We selected 48 serum samples obtained from 48 HIV-infected patients, including 15 patients with penicilliosis, 22 patients with cryptococcosis, and 11 controls without invasive fungal infection. The clinical characteristics of these 48 patients are shown in Table 1. Among 15 patients with penicilliosis, 7 had fungemia alone, 4 had cavitary lung lesions alone, 3 had both, and 1 had P. marneffei isolated from lymph node aspirate, sputum, and skin biopsy specimens. All of the four cases of cavitary lung lesions alone due to penicilliosis were diagnosed by positive cultures of computed tomography- or sonography-guided lung aspirates. Among 22 patients with cryptococcosis, 11 had fungemia alone, 5 had cavitary lung lesions alone, 3 had both, and 3 had meningitis alone. Serum cryptococcal antigen titers ranged from 1:4 to 1:4,096 (median, 1:512). None of the 48 patients had Aspergillus spp. isolated from their clinical specimens during the 2-month follow-up.
TABLE 1.
Baseline characteristics and clinical data of 48 patients enrolled for serum GM testing by the Aspergillus enzyme immunoassay
| Characteristica | Group
|
|||||
|---|---|---|---|---|---|---|
| Penicilliosis (n = 15) | Cryptococcosis (n = 22) | Control (n = 11)b | ||||
| Age (median [range]) | 37.3 (20-81) | 38.1 (23-65) | 35.6 (23-58) | |||
| Gender (female/male) | 2/13 | 0/22 | 0/11 | |||
| Neutrophil count (cells/μl) | ||||||
| Range | 1,530-12,665 | 959-10,943 | 840-7,323 | |||
| Median | 3,680 | 2,482 | 2,522 | |||
| CD4 cell count (cells/μl) | ||||||
| Range | 1-53 | 1-94 | 22-431 | |||
| Median | 6 | 33 | 238 | |||
| Interval between diagnosis of fungal infection and blood sampling (days) | ||||||
| Range | −7 to 6 | 0 to 6 | NA | |||
| Median | 0 | 1.5 | NA | |||
| No. (%) of patients with cavitary lung lesions | 7 (46.7) | 8 (36.4) | NA | |||
| No. of positive cultures/total no. of cultures (%) by specimen type | ||||||
| Blood | 10/15 (66.7) | 14/22 (63.6) | NA | |||
| Bone marrow | 3/6 (50.0) | 1/3 (33.3) | NA | |||
| CSF | 0/4 (0.0) | 12/21 (57.1) | NA | |||
| Lung aspirate | 5/7 (71.4) | 1/4 (25.0) | NA | |||
| Lymph node biopsy | 2/3 (66.7) | 0/2 (0.0) | NA | |||
| Skin biopsy | 4/4 (100) | 0/4 (0.0) | NA | |||
| Sputum | 7/15 (46.7) | 2/22 (9.1) | NA | |||
| No. (%) of patients with concomitant opportunistic infections | ||||||
| Oral candidiasis | 11 (73.3) | 20 (90.9) | 0 | |||
| Disseminated NTM | 4 (26.7) | 2 (9.1) | 0 | |||
| Tuberculosis | 2 (13.3) | 1 (4.6) | 0 | |||
| Salmonellosis | 0 (0.0) | 4 (18.2) | 0 | |||
| CMV retinitis | 2 (13.3) | 1 (4.6) | 0 | |||
| CMV pneumonitis | 1 (6.7) | 1 (4.6) | 0 | |||
| Toxoplasmosis | 0 (0.0) | 1 (4.6) | 0 | |||
| HSV esophagitis | 1 (6.7) | 0 (0) | 0 | |||
| PCP | 0 (0.0) | 3 (13.6) | 0 | |||
CMV, cytomegalovirus; CSF, cerebrospinal fluid; HSV, herpes simplex virus; NTM, nontuberculous mycobacteria; PCP, Pneumocystis carinii (jiroveci) pneumonia.
NA, not applicable.
The median time between diagnosis of invasive fungal infection and collection of serum samples was 0 days (range, −7 to 6 days) for penicilliosis and 1.5 days (range, 0 to 6 days) for cryptococcosis. Fourteen patients with penicilliosis had their serum samples collected before the initiation of antifungal therapy. Of 22 patients with cryptococcosis, 14 had sera collected prior to the initiation of antifungal therapy, 5 within 24 h of the initiation of antifungal therapy, and 3 within 2 days of therapy.
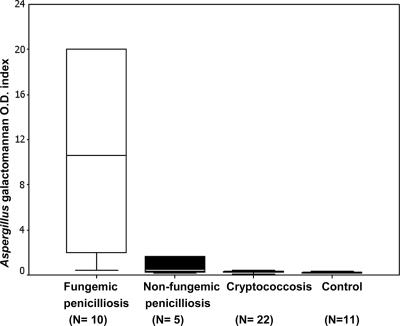
The median OD index was significantly higher for the 15 patients with penicilliosis (4.419) than for the 22 patients with cryptococcosis (0.247) (P < 0.001) and the 11 controls (0.234) (P = 0.001) (Table 2; Fig. 1 and 2). Among patients with fungemia, the 10 patients with penicilliosis also had a higher median OD index than the 14 patients with cryptococcosis (10.628 versus 0.231; P < 0.001) (Table 2; Fig. 2). Among nonfungemic patients, the median OD indices of the penicilliosis and cryptococcosis groups were not significantly different (0.378 versus 0.263; P = 0.464). Among the 15 patients with cavitary lung lesions, the median OD index was higher for the 7 penicilliosis patients (median, 1.641; range, 0.247 to >20) than for the 8 cryptococcosis patients (median, 0.227; range, 0.112 to 3.849) (P = 0.011).
TABLE 2.
Serum GM OD indices of patients with penicilliosis, cryptococcosis, and controls
| Patient group | GM OD index
|
No. (%) with a GM OD index of:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Median | >1.5 | >1.0 | >0.5 | <0.5 | |||||||
| Penicilliosis | ||||||||||||
| With fungemia (n = 10) | 0.401->20 | 10.628a | 8 (80.0) | 8 (80.0) | 9 (90.0) | 1 (10.0) | ||||||
| Without fungemia (n = 5) | 0.158-4.419 | 0.378b | 2 (40.0) | 2 (40.0) | 2 (40.0) | 3 (60.0) | ||||||
| All (n = 15) | 0.158->20 | 4.419c | 10 (66.7) | 10 (66.7) | 11 (73.3) | 4 (26.7) | ||||||
| Cryptococcosis | ||||||||||||
| With fungemia (n = 14) | 0.112-1.168 | 0.231a | 0 (0.0) | 1 (7.1) | 1 (7.1) | 13 (92.9) | ||||||
| Without fungemia (n = 8) | 0.115-3.849 | 0.263b | 1 (12.5) | 1 (12.5) | 2 (25.0) | 6 (75.0) | ||||||
| All (n = 22) | 0.112-3.849 | 0.247c | 1 (4.5) | 2 (9.1) | 3 (13.6) | 19 (86.4) | ||||||
| Control (n = 11) | 0.15-1.024 | 0.234 | 0 (0) | 1 (9.1) | 1 (9.1) | 10 (90.9) | ||||||
P < 0.001 for comparison of GM OD indices for Penicillium fungemia and cryptococcal fungemia.
P = 0.464 for comparison of GM OD indices for nonfungemic penicilliosis and nonfungemic cryptococcosis.
P < 0.001 for comparison of GM OD indices for all cases of Penicillium marneffei infection versus all cases of cryptococcosis.
FIG. 1.
GM antigen levels for HIV-infected patients with fungemic penicilliosis, nonfungemic penicilliosis, or cryptococcosis and for controls. The bar in each box represents the median value of the GM OD index for that patient group, while the error bars indicate the range. The upper and lower limits of each box represent the 75th and 25th percentiles, respectively, of the OD index for the group. For values greater than 20, the OD index of 20 is used as the upper limit in the box.
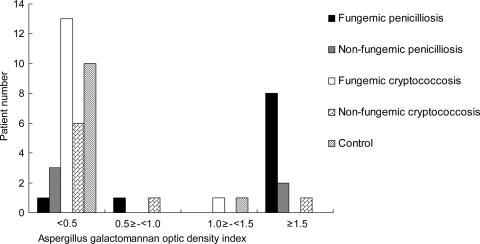
FIG. 2.
Distribution of Aspergillus GM OD indices among HIV-infected patients with penicilliosis, HIV-infected patients with cryptococcosis, and controls.
Of patients with penicilliosis, cryptococcosis, and controls, 73.3%, 13.6%, and 9%, respectively, had GM OD indices higher than 0.5 (P = 0.0001) (Table 2). Of the four patients with penicilliosis who had OD indices below 0.5 (26.7%), one had his serum sample obtained 7 days before the collection of the first positive blood culture, two had nonfungemic penicilliosis with cavitary lung lesions, and one had P. marneffei isolated from lymph node aspirate, sputum, and skin biopsy samples. Of the three patients with cryptococcosis who had OD indices greater than 0.5 (13.6%), one with an OD index of 3.849 presented with a cavitary lung lesion without fungemia; however, tissue biopsy was not performed. The OD indices of the other two patients, who had no cavitary lung lesions, were 0.655 and 1.168, respectively. No microbiological evidence of aspergillosis or penicilliosis was documented during the follow-up period. One of the 11 control patients (9.0%) had an elevated OD index of 1.024. After follow-up for 6 months, he did not develop invasive fungal infections or lung lesions.
DISCUSSION
In this study, we found that patients with fungemia due to P. marneffei had the highest GM OD indices by the Aspergillus enzyme immunoassay and that these levels were significantly higher than those for patients with penicilliosis but without fungemia, patients with cryptococcosis, and control patients.
Since invasive aspergillosis was very rare in our HIV cohort (18), we did not compare GM levels between HIV-infected patients with aspergillosis and those with penicilliosis. Compared with GM OD indices for patients with invasive aspergillosis (1, 11), this study showed relatively high GM antigen levels for patients with fungemic penicilliosis (median, 10.628). This finding is in accordance with the fact that P. marneffei is more likely to be isolated from blood samples of HIV-infected patients than Aspergillus spp. (8, 17).
The median GM OD index was significantly lower for patients with cryptococcosis than for patients with penicilliosis. However, 3 of 22 patients (13.6%) with cryptococcosis had GM OD indices higher than 0.5. Concomitant penicilliosis cannot be excluded. Alternatively, this finding may be caused by C. neoformans galactoxylomannan, which cross-reacts with the Aspergillus GM antigen assay (4). However, the cross-reactivity was not confirmed in a recent comprehensive study (5). Since determination of serum cryptococcal antigen levels using a latex agglutination test is sensitive and specific for immunocompromised hosts, it will not hamper the usefulness of the GM antigen assay in the diagnosis of invasive fungal infections in HIV-infected patients.
Pulmonary aspergillosis has been reported to be a main cause of cavitary lung lesions for HIV-infected patients (6), and the incidence of aspergillosis among HIV-infected patients was 3.5 cases per 1,000 person-years in a surveillance study between 1990 and 1998 (8). However, of 1,182 HIV-infected patients monitored at this hospital, only 3 patients (0.3%) had invasive aspergillosis diagnosed before the introduction of highly active antiretroviral therapy (18). This and our previous studies showed that a higher proportion of HIV-infected patients with penicilliosis or cryptococcosis had cavitary lung lesions (17, 18). As a result, penicilliosis should be included in the differential diagnosis of cavitary lung lesions for HIV-infected patients, and possibly for other immunocompromised patients, who have GM antigenemia in areas of endemicity (22).
This study has several limitations. First, the sample size is small. More studies are needed to elucidate the role of this GM antigen assay in the diagnosis of penicilliosis in areas of endemicity. Second, diagnosis of nonfungemic penicilliosis may be limited by the relatively low levels of GM in serum. Therefore, bronchoscopy or aspiration/biopsy guided by computed tomography or sonography should be considered for such patients. Third, the GM index was determined by using stored serum samples, and the influence of freezing and thawing on the GM antigen assay is not clear. Fourth, only one serum sample from each patient was used for analysis. A previous report showed that the GM OD index for an HIV-infected patient with penicilliosis declined after treatment for 2 months (14). The temporal trend of GM antigen levels following initiation of antifungal therapies and the question of whether the antigen levels can be used to monitor the response to antifungal therapies remain to be investigated.
In conclusion, our data suggest that the OD index of the GM antigen detected by the Aspergillus enzyme immunoassay is significantly elevated for HIV patients with penicilliosis compared with HIV-infected patients with cryptococcosis or controls. Determination of the GM OD index by the Aspergillus assay may facilitate earlier diagnosis of P. marneffei infection for HIV-infected patients with compatible clinical presentations before the results of microbiologic cultures are available.
Acknowledgments
We thank Christina Tai for technical assistance.
This work was supported by a grant from the National Science Council, Republic of China (NSC 94-2314-B-002-004).
Footnotes
Published ahead of print on 27 June 2007.
REFERENCES
- 1.Boutboul, F., C. Alberti, T. Leblanc, A. Sulahian, E. Gluckman, F. Derouin, and P. Ribaud. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: increasing antigenemia is associated with progressive disease. Clin. Infect. Dis. 34:939-943. [DOI] [PubMed] [Google Scholar]
- 2.Chaiyaroj, S. C., R. Chawengkirttikul, S. Sirisinha, P. Watkins, and Y. Srinoulprasert. 2003. Antigen detection assay for identification of Penicillium marneffei infection. J. Clin. Microbiol. 41:432-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chongtrakool, P., S. C. Chaiyaroj, V. Vithayasai, S. Trawatcharegon, R. Teanpaisan, S. Kalnawakul, and S. Sirisinha. 1997. Immunoreactivity of a 38-kilodalton Penicillium marneffei antigen with human immunodeficiency virus-positive sera. J. Clin. Microbiol. 35:2220-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalle, F., P. E. Charles, K. Blanc, D. Caillot, P. Chavanet, F. Dromer, and A. Bonnin. 2005. Cryptococcus neoformans galactoxylomannan contains an epitope(s) that is cross-reactive with Aspergillus galactomannan. J. Clin. Microbiol. 43:2929-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Jesus, M., E. Hackett, M. Durkin, P. Connolly, A. Casadevall, R. Petraitiene, T. J. Walsh, and L. J. Wheat. 2007. Galactoxylomannan does not exhibit cross-reactivity in the Platelia Aspergillus enzyme immunoassay. Clin. Vaccine Immunol. 14:624-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallant, J. E., and A. H. Ko. 1996. Cavitary pulmonary lesions in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 22:671-682. [DOI] [PubMed] [Google Scholar]
- 7.Hajjeh, R. A. 1995. Disseminated histoplasmosis in persons infected with human immunodeficiency virus. Clin. Infect. Dis. 21(Suppl. 1):S108-S110. [DOI] [PubMed] [Google Scholar]
- 8.Holding, K. J., M. S. Dworkin, P. C. Wan, D. L. Hanson, R. M. Klevens, J. L. Jones, and P. S. Sullivan for the Adult and Adolescent Spectrum of HIV Disease Project. 2000. Aspergillosis among people infected with human immunodeficiency virus: incidence and survival. Clin. Infect. Dis. 31:1253-1257. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman, L., P. G. Standard, M. Jalbert, P. Kantipong, K. Limpakarnjanarat, and T. D. Mastro. 1996. Diagnostic antigenemia tests for penicilliosis marneffei. J. Clin. Microbiol. 34:2503-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latgé, J. P., H. Kobayashi, J. P. Debeaupuis, M. Diaquin, J. Sarfati, J. M. Wieruszeski, E. Parra, J. P. Bouchara, and B. Fournet. 1994. Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect. Immun. 62:5424-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mennink-Kersten, M. A., J. P. Donnelly, and P. E. Verweij. 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 4:349-357. [DOI] [PubMed] [Google Scholar]
- 12.Mennink-Kersten, M. A., A. Warris, and P. E. Verweij. 2006. 1,3-β-d-Glucan in patients receiving intravenous amoxicillin-clavulanic acid. N. Engl. J. Med. 354:2834-2835. [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer, C. D., J. P. Fine, and N. Safdar. 2006. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin. Infect. Dis. 42:1417-1427. [DOI] [PubMed] [Google Scholar]
- 14.Rimek, D., T. Zimmermann, M. Hartmann, C. Prariyachatigul, and R. Kappe. 1999. Disseminated Penicillium marneffei infection in an HIV-positive female from Thailand in Germany. Mycoses 42(Suppl. 2):25-28. [PubMed] [Google Scholar]
- 15.Stynen, D., J. Sarfati, A. Goris, M. C. Prevost, M. Lesourd, H. Kamphuis, V. Darras, and J. P. Latge. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect. Immun. 60:2237-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sulahian, A., S. Touratier, and P. Ribaud. 2003. False positive test for Aspergillus antigenemia related to concomitant administration of piperacillin and tazobactam. N. Engl. J. Med. 349:2366-2367. [DOI] [PubMed] [Google Scholar]
- 17.Sun, H. Y., M. Y. Chen, C. F. Hsiao, S. M. Hsieh, C. C. Hung, and S. C. Chang. 2006. Endemic fungal infections caused by Cryptococcus neoformans and Penicillium marneffei in patients infected with human immunodeficiency virus and treated with highly active anti-retroviral therapy. Clin. Microbiol. Infect. 12:381-388. [DOI] [PubMed] [Google Scholar]
- 18.Sun, H. Y., M. Y. Chen, S. M. Hsieh, W. H. Sheng, S. Y. Chang, C. F. Hsiao, C. C. Hung, and S. C. Chang. 2006. Changes in the clinical spectrum of opportunistic illnesses in persons with HIV infection in Taiwan in the era of highly active antiretroviral therapy. Jpn. J. Infect. Dis. 59:311-316. [PubMed] [Google Scholar]
- 19.Supparatpinyo, K., C. Khamwan, V. Baosoung, K. E. Nelson, and T. Sirisanthana. 1994. Disseminated Penicillium marneffei infection in southeast Asia. Lancet 344:110-113. [DOI] [PubMed] [Google Scholar]
- 20.Van Cutsem, J., L. Meulemans, F. Van Gerven, and D. Stynen. 1990. Detection of circulating galactomannan by Pastorex Aspergillus in experimental invasive aspergillosis. Mycoses 33:61-69. [DOI] [PubMed] [Google Scholar]
- 21.Vanittanakom, N., C. R. Cooper, Jr., M. C. Fisher, and T. Sirisanthana. 2006. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin. Microbiol. Rev. 19:95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo, P. C., S. K. Lau, C. C. Lau, K. T. Chong, W. T. Hui, S. S. Wong, and K. Y. Yuen. 2005. Penicillium marneffei fungaemia in an allogeneic bone marrow transplant recipient. Bone Marrow Transplant. 35:831-833. [DOI] [PubMed] [Google Scholar]