Abstract
Alveolar echinococcosis (AE)—caused by the cestode Echinococcus multilocularis—is a severe zoonotic disease found in temperate and arctic regions of the northern hemisphere. Even though the transmission patterns observed in different geographical areas are heterogeneous, the nuclear and mitochondrial targets usually used for the genotyping of E. multilocularis have shown only a marked genetic homogeneity in this species. We used microsatellite sequences, because of their high typing resolution, to explore the genetic diversity of E. multilocularis. Four microsatellite targets (EmsJ, EmsK, and EmsB, which were designed in our laboratory, and NAK1, selected from the literature) were tested on a panel of 76 E. multilocularis samples (larval and adult stages) obtained from Alaska, Canada, Europe, and Asia. Genetic diversity for each target was assessed by size polymorphism analysis. With the EmsJ and EmsK targets, two alleles were found for each locus, yielding two and three genotypes, respectively, discriminating European isolates from the other groups. With NAK1, five alleles were found, yielding seven genotypes, including those specific to Tibetan and Alaskan isolates. The EmsB target, a tandem repeated multilocus microsatellite, found 17 alleles showing a complex pattern. Hierarchical clustering analyses were performed with the EmsB findings, and 29 genotypes were identified. Due to its higher genetic polymorphism, EmsB exhibited a higher discriminatory power than the other targets. The complex EmsB pattern was able to discriminate isolates on a regional and sectoral level, while avoiding overdistinction. EmsB will be used to assess the putative emergence of E. multilocularis in Europe.
Echinococcus multilocularis is the causative agent of alveolar echinococcosis (AE), a parasitic infection of humans that can be lethal if not appropriately treated. In nature this zoonosis involves different mammalian hosts: carnivores (in Europe mainly foxes [Vulpes vulpes], dogs [Canis lupus familiaris], and raccoon dogs [Nyctereutes procyonoides]) act as definitive hosts (32), and a wide spectrum of rodents are intermediate hosts (34). Humans who are accidentally infected serve as intermediate hosts and may develop AE after a long incubation period (8, 33). Nonhuman primates, such as zoo gorillas (Gorilla spp.) or macaque monkeys (Macaca spp.) can also serve as aberrant intermediate hosts (7, 13, 25). The geographical distribution of the parasite includes large parts of the northern hemisphere: China, Central Asia (12), Hokkaido in Japan (20), Central and Eastern Europe (21, 23, 26, 34), and some parts of North America (14, 27). The extent of both infection and spatial distribution depends on different factors, for example, (i) the probability of a parasite-host encounter (encounter iris), depending on the density of susceptible rodents and carnivores and the human activities and behavior, and (ii) the balance between the immune evasion capacity of the parasite and the host immune response (compatibility iris) (11). This interaction implies a host-parasite arms race that may depend not only on the genetic polymorphism of the host (11, 15, 16) among other components but also on the genetic polymorphism of the parasite. For E. multilocularis, few genetic differences among isolates have been observed with classical nuclear and mitochondrial targets (18). E. multilocularis has shown a variability of at least 10 times less than that of Echinococcus granulosus, the causative agent of cystic hydatidosis (5, 6, 18). The use of more sensitive tools such as microsatellites—fragments of nuclear DNA composed of 1 to 6 bp tandemly repeated—might provide more information about parasite DNA polymorphism; they are already used for genotyping and spatial distribution studies for other species of parasites, such as Leishmania infantum (10). They could help to better identify the spatial-temporal characteristics of the E. multilocularis transmission pattern (3). Analyses performed on the spacers of the U1snRNA gene have shown three distinct genetic profiles for European, North American, and Japanese isolates, but no variability between individual samples of the respective foci has been found (9). A Japanese team documented differences in an adult worm panel collected from Hokkaido Island, but no relationship between this sample panel and geographical position was demonstrated (22). These two publications highlighted the importance of microsatellite analyses in the exploration of the genetic diversity of E. multilocularis. Recently, our collaborative laboratory investigation identified 17 microsatellite targets (4). EmsB, a tandem repeated multilocus microsatellite, was identified and characterized. This microsatellite not only clearly demonstrated a high discriminatory power by identifying samples from different geographical origins (Alaska and Europe) but it also found several similar clusters within the European collection of isolates (4).
In the present investigation, in which the genetic polymorphism of E. multilocularis isolates from Europe, Alaska, China, and Japan was studied, we compared the relevance of four different microsatellite targets. Three of them were taken from a previous work by Bart et al., published in 2006 (4), and a microsatellite sequence published by Nakao et al. in 2003 was used as an independent marker (22).
MATERIALS AND METHODS
Selection of microsatellite targets.
We selected our microsatellite targets from a series of 17 microsatellites published by Bart et al. in 2006 (4). In that study, by using amplification and fragment size analyses, microsatellites were isolated from E. granulosus in order to select markers which would show variations between E. granulosus and E. multilocularis. Two E. granulosus strains were initially tested: an Algerian sheep (strain G1) and a Mauritanian camel (strain G6) isolate; the E. multilocularis Swiss isolate CH5-h (shown in Table 2 of the present study) was also included. Seven microsatellites were subsequently selected and tested on 10 E. multilocularis isolates: CH1-h, CH5-h, 32A-h, 33F-h, 36CH-h, 39CH-h, 40CH-h, 41CH-h, SL1-h, and CND-r (included in Table 2 of the present study). For our study, we selected the three most polymorphic targets: EmsJ (GenBank accession no. GbR AY680845), EmsK (GbR AY680857), and EmsB (GbR AY680860). We also selected an additional independent target, NAK1, from a work published by Nakao et al. (target originally named EMms1; GbR AB100031) (22). For each of the four defined genomic regions, specific primers were designed with Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). The respective characteristics are summarized in Table 1.
TABLE 2.
Main characteristics of E. multilocularis sample panela
| Isolate code | Host species | Geographical origin | Yr of isolation |
|---|---|---|---|
| 1AL-r | Microtus oeconomus | Alaska (St. Lawrence Island) | 1996 |
| 2AL-r | Microtus oeconomus | Alaska (St. Lawrence Island) | 1996 |
| 3AL-r | Microtus oeconomus | Alaska (St. Lawrence Island) | 1996 |
| 4AL-r | Microtus oeconomus | Alaska (St. Lawrence Island) | 1996 |
| 5AL-r | Microtus oeconomus | Alaska (St. Lawrence Island) | 1996 |
| 6AL-r | Microtus oeconomus | Alaska (St. Lawrence Island) | 1996 |
| 7AL-r | Microtus oeconomus | Alaska (St. Lawrence Island) | 1996 |
| 10AL-r | Microtus oeconomus | Alaska (St. Lawrence Island) | 1996 |
| 11AL-r | Microtus oeconomus | Alaska (St. Lawrence Island) | 1996 |
| 13AL-r | Microtus oeconomus | Alaska (St. Lawrence Island) | 1996 |
| 27AL-h | Human | Alaska (St. Lawrence Island) | 1996 |
| 38AL-h | Human | Alaska | 1996 |
| SL1-h | Human | Alaska (St. Lawrence Island) | 1996 |
| CND-r | Rodent | Canada | NDb |
| 2PRC-r | Microtus limnophilus | China (Tibet) | 2001 |
| 5PRC-r | Microtus limnophilus | China (Tibet) | 2002 |
| 6PRC-r | Microtus limnophilus | China (Tibet) | 2002 |
| 7PRC-r | Microtus limnophilus | China (Tibet) | 2002 |
| 9PRC-r | Cricetulus kamensis | China (Tibet) | 2002 |
| 26J-h | Human | Japan | ND |
| E1J-F | Vulpes vulpes | Japan (Hokkaido) | 2005 |
| I3J-F | Vulpes vulpes | Japan (Hokkaido) | 2005 |
| M4J-F | Vulpes vulpes | Japan (Hokkaido) | 2005 |
| N5J-F | Vulpes vulpes | Japan (Hokkaido) | 2005 |
| O4J-F | Vulpes vulpes | Japan (Hokkaido) | 2005 |
| 33F-h | Human | France | ND |
| R04131-1F-F | Vulpes vulpes | France (Ardennes) | 2004 |
| 14CH-r | Arvicola terrestris | Switzerland (Fribourg) | 1995 |
| 15CH-r | Arvicola terrestris | Switzerland (Fribourg) | 1995 |
| 16CH-r | Arvicola terrestris | Switzerland (Fribourg) | 1995 |
| 17CH-r | Arvicola terrestris | Switzerland (Fribourg) | 1995 |
| 18CH-r | Arvicola terrestris | Switzerland (Fribourg) | 1995 |
| 19CH-r | Arvicola terrestris | Switzerland (Fribourg) | 1995 |
| 20CH-r | Arvicola terrestris | Switzerland (Fribourg) | 1995 |
| 21CH-r | Arvicola terrestris | Switzerland (Fribourg) | 1995 |
| 22CH-r | Arvicola terrestris | Switzerland (Fribourg) | 1995 |
| 23CH-r | Arvicola terrestris | Switzerland (Fribourg) | 1995 |
| 24CH-r | Arvicola terrestris | Switzerland (Fribourg) | 1995 |
| 36CH-h | Human | Switzerland | 1982 |
| 37CH-h | Human | Switzerland | 1990 |
| 39CH-h | Human | Switzerland | 1980 |
| 40CH-h | Human | Switzerland | 1994 |
| 41CH-h | Human | Switzerland | 1986 |
| CH1-h | Human | Switzerland | ND |
| CH5-h | Human | Switzerland | ND |
| 29CH-m | Macaque monkey | Switzerland (Basel zoo) | 1997 |
| 31CH-m | Macaque monkey | Switzerland (Basel zoo) | 2003 |
| 34CH-m | Macaque monkey | Switzerland (Zürich zoo) | 1991 |
| 42CH-m | Vervet monkey | Switzerland (Zürich zoo) | 1989 |
| 43CH-m | Vervet monkey | Switzerland (Zürich zoo) | 1990 |
| 45CH-m | Marmoset monkey | Switzerland (Lausanne zoo) | 2003 |
| 3CH-F | Vulpes vulpes | Switzerland | 2001-2003 |
| 52CH-F | Vulpes vulpes | Switzerland | 2001-2003 |
| 64CH-F | Vulpes vulpes | Switzerland | 2001-2003 |
| 35D-h | Human | Germany | ND |
| 101D-F | Vulpes vulpes | Germany | 2001-2003 |
| 116D-F | Vulpes vulpes | Germany | 2001-2003 |
| 121D-F | Vulpes vulpes | Germany | 2001-2003 |
| 126D-F | Vulpes vulpes | Germany | 2001-2003 |
| 141D-F | Vulpes vulpes | Germany | 2001-2003 |
| 32A-h | Human | Austria | 1986 |
| 209A-F | Vulpes vulpes | Austria | 2001-2003 |
| 278A-F | Vulpes vulpes | Austria | 2001-2003 |
| 287A-F | Vulpes vulpes | Austria | 2001-2003 |
| 302PL-F | Vulpes vulpes | Poland | 2001-2003 |
| 310PL-F | Vulpes vulpes | Poland | 2001-2003 |
| 315PL-F | Vulpes vulpes | Poland | 2001-2003 |
| 392PL-F | Vulpes vulpes | Poland | 2001-2003 |
| 525CZ-F | Vulpes vulpes | Czech Rep. | 2001-2003 |
| 535CZ-F | Vulpes vulpes | Czech Rep. | 2001-2003 |
| 559CZ-F | Vulpes vulpes | Czech Rep. | 2001-2003 |
| 425SK-F | Vulpes vulpes | Slovakia | 2001-2003 |
| 435SK-F | Vulpes vulpes | Slovakia | 2001-2003 |
| 480SK-F | Vulpes vulpes | Slovakia | 2001-2003 |
| 402NL-F | Vulpes vulpes | The Netherlands | 2001-2003 |
| 420NL-F | Vulpes vulpes | The Netherlands | 2001-2003 |
| 500 | Meriones unguiculatus | Francec | March 1991 |
| 501 | Meriones unguiculatus | Francec | May 1991 |
| 502 | Meriones unguiculatus | Francec | February 1992 |
Isolate code abbreviations: code number, geographical origin of sample, and animal host (lowercase letter for intermediate hosts [r, rodent; h, human; and m, monkey] and capital letter for definitive hosts [F, fox]). The sample collection contained 13 isolates from Alaska, 1 from Canada, 5 from China, 6 from Japan, 2 from France, 27 from Switzerland, 6 from Germany, 4 from Austria, 4 from Poland, 3 from the Czech Republic, 3 from Slovakia, and 2 from The Netherlands.
ND, not documented.
Isolate maintained in vivo in laboratory by several passages in Meriones unguiculatus.
TABLE 1.
Primer sequences and characteristics of microsatellite loci in E. multilocularis
| Primer namesa | Primer sequence | Fragment size (bp) | Annealing temp (°C) | Repetition | Source (accession no.) |
|---|---|---|---|---|---|
| EmsJ A*, EmsJ B | 5′-GAACGCGCTAACCGATTG-3′, 5′-TTAGGAATGGGAAGGTGTCG-3′ | 152-155 | 54 | (CT)n | In-house (AY680845) |
| EmsK A*, EmsK B | 5′-CAGCTCAAAAGAACCCGAAG-3′, 5′-CCAAACTTCCGCTCACTCTG-3′ | 248-250 | 54 | (CA)n | In-house (AY680857) |
| EmsB A*, EmsB C | 5′-GTGTGGATGAGTGTGCCATC-3′, 5′-CCACCTTCCCTACTGCAATC-3′ | 209-241 | 60 | (CA)n(GA)n | In-house (AY680860) |
| NAK1 A*, NAK1 B | 5′-GGTAGCCAATGCTGTGGTTT-3′, 5′-GCGAGGTCACGCAAATGTAT-3′ | 189-201 | 60 | (CCA)n | Nakao et al. (AB100031) |
E. multilocularis isolates.
The panel of 76 E. multilocularis isolates was composed of purified single adult-stage worms obtained from definitive hosts and hepatic metacestode tissue material, which was obtained from intermediate hosts. The adult worms (29 isolates) were taken after necropsies of red foxes; their geographic origins are specified in Table 2. In the metacestode collection (47 isolates), 14 samples were from Alaska and Canada, including 10 specimens obtained in 1995 from Microtus oeconomus originating from a field (of about 100 m by 100 m) just outside of Savoonga, Saint Lawrence Island, Alaska. These particular samples were collected within a period of 3 days. Twenty-four samples were from Switzerland, among them 11 specimens obtained from Arvicola terrestris, all caught in a field (of about 500 m by 500 m) in the Canton of Fribourg over a 1-month period in 1994. Five metacestode tissue samples were isolated from Microtus limnophilus and Cricetulus kamensis, caught in an area of 50 km2 in the vicinity of Tuan-Jie, a city located on the eastern Tibetan plateau (Shiqu County in Western Sichuan, China). Sampling was carried out in July 2001 for sample 2PRC-r and in July 2002 for the other Chinese samples. Fourteen samples were obtained from human AE patients residing in Japan, Alaska, Austria, Switzerland, France, and Germany. Six parasitic lesions were collected from monkeys in Swiss zoos, probably occurring after the animals were fed contaminated grass, mowed close to the zoo.
DNA extraction, PCR, and size polymorphism analysis.
Total genomic DNA was isolated and purified from approximately 50 mg of each of the 47 parasite metacestode tissue samples and from the 29 single adult-stage worm samples, using a DNA Easy tissue kit (QIAGEN, Switzerland). The procedure was carried out according to the manufacturer's protocol. Purified DNA was eluted with 200 μl of elution buffer (provided by the manufacturer) for metacestode samples and 50 μl for adult worms, in order to obtain optimal DNA concentrations. The DNA concentrations were checked with a spectrophotometer apparatus (BioPhotometer; Eppendorf AG, Hamburg, Germany). The DNA samples were then stored at −20°C until use for PCR. Reproducibility of results was checked by performing PCR and fragment analysis in two different laboratories: one in Bern (Switzerland) and the other one in Besançon (France). Amplification by PCR was performed in a 30-μl reaction mixture containing 50 to 100 ng of DNA, 200 μM of each deoxynucleoside triphosphate (GeneAmp dNTPs; Applied Biosystems, Foster City, CA), 0.4 μM of fluorescent forward primer, 5′-labeled specific fluorescence dye, 0.7 μM of classical reverse primers, and 0.5 U of AmpliTaq DNA polymerase enzyme associated with GeneAmp 1× PCR buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2, and 0.001% gelatin) (Applied Biosystems, Foster City, CA) in Bern and 0.5 U of REDTaq DNA polymerase enzyme associated with 1× REDTaq PCR buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl, 1.1 mM MgCl2, and 0.01% gelatin) (Sigma-Aldrich, Saint Louis, MO) for the method carried out in Besançon. The PCR amplification was achieved in a Biometra T3 thermocycler (Whatman Biometra, Goettingen, Germany), under the following conditions: 30 cycles with denaturation at 94°C for 30 s, annealing at 54°C (EmsJ 1/2 and EmsK 1/2) or 60°C (EmsB 1/2 and NAK1 A/B) for 30 s, and extension at 72°C for 30 s to 1 min. PCR products were studied in fragment analysis, to assess the polymorphism of size using automatic sequencers. A comparison was made between the two different systems used, in order to evaluate and demonstrate the independent repeatability of the analyses: an ABI Prism 3100 automatic sequencer (Applied Biosystems, Foster City, CA) was used in Bern, and a Beckman CEQ 8000 (Beckman Coulter, Fullerton, CA) was used in Besançon. Fluorescence signals generated by marked primers were read by colorimetric analysis. Correspondences were established to assess the sizes of the amplified fragments, by using Genotyper 3.7 software for the ABI apparatus and Genetic Analysis System 8.0.52 software for the Beckman apparatus. To establish this comparison, the Pearson correlation coefficient was calculated for each sample between data sets obtained on the two systems. Stability of EmsB profiles was checked by repeated testing (five times) of sample 302 PL-F, selected randomly from the E. multilocularis panel.
Genotype determination and statistical analysis.
Alleles from single-locus microsatellite targets (EmsJ, EmsK, and NAK1) were plotted using the results of fragment analyses and specified for homozygote or heterozygote genotypes. To assess the genetic diversity provided by EmsB, the presence and the height of each peak, basically corresponding to alleles, were recorded. Peaks below 10% of the highest peak per run were classified as artifacts and removed from the analysis. The height of each defined peak reflected the number of copies of the microsatellite present in the parasite DNA (4). Because the intensity of signals is dependent on the DNA concentration used for the PCR, normalization for an EmsB profile was achieved by dividing each peak by the sum of all the peaks for a given profile. This method of calculation is an improvement over the method used by Bart et al., which divided each peak by the highest peak of a given profile (4).
Clusters for the EmsB target were identified by hierarchical clustering analysis, using the Euclidean distance and the unweighted-pair group method using average linkages. The stability of clusters was tested by a multiscale bootstrap resampling (B = 1,000), resulting in approximately unbiased P values (28, 29). Dendrograms based on hierarchical clustering were constructed by using pvclust (31), available under the R Project (24). E. granulosus isolates were included in the analysis as outgroup controls (sample 539, a G1 Algerian sheep isolate, and sample 116, a G6 Mauritanian camel isolate [1, 2]). In previous experiments (4), we determined reproducibility and repeatability of EmsB microsatellite analyses by testing one isolate, which had been maintained in vivo in Meriones unguiculatus by serial passages at several-month intervals (samples 500, 501, and 502, detailed in Table 2). Thus, these three samples showed similar EmsB profiles. These results were then used to calculate a genetic threshold, which enabled us to identify the isolate clusters.
The discriminatory power of each of the four microsatellites was assessed using Simpson's index (30), improved by Hunter and Gaston (19), and is described by the following equation:
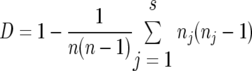 |
where n is the total number of isolates of the sample panel, s is the total number of groups described, and nj is the number of isolates belonging to the jth type.
This index is based on the probability that two unrelated strain samples from a given panel will be placed in different typing groups. A genetic tool has a high discriminating power when the observed value exhibits an index close to 1.
Nucleotide sequence accession numbers.
Nucleotide sequence data reported in this paper are available in the GenBank database under the accession numbers AY680845, AY680857, AY680860, and AB100031.
RESULTS
EmsJ, EmsK, and NAK1 polymorphism.
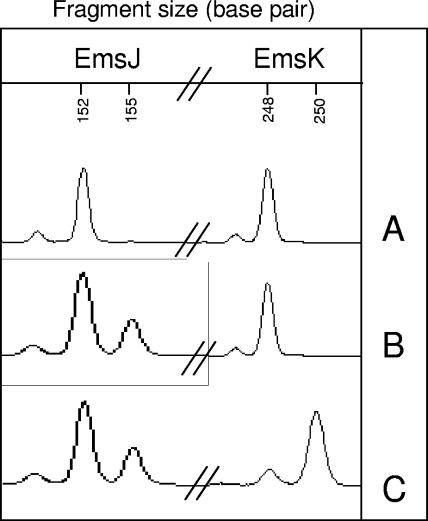
Both EmsJ and EmsK loci exhibited two alleles: 152 and 155 bp and 248 and 250 bp, respectively. These loci, forming three genotypes (A, B, and C) are presented in Fig. 1. Amplification for the EmsJ target was feasible for the entire sample panel (Table 3). For EmsK, one isolate yielded no amplification, even though different Taq polymerases and different running conditions were used (data not shown). The target pair EmsJ and EmsK split the panel into two clusters. The whole European panel presented homozygote genotypes at 152 bp for EmsJ and 248 bp for EmsK (Fig. 1A). The Asian, Alaskan, and Canadian isolates were characterized by heterozygote genotypes at 152 and 155 bp for EmsJ and a homozygote genotype at 248 bp for EmsK (Fig. 1B). Only one Alaskan sample (collected in the field close to Savoonga) differed for EmsK, with an allele at 250 bp (Fig. 1C). The rate of heterozygosity observed was 33.3% for EmsJ and 0% for EmsK. The index of discrimination (D) was 0.37 for EmsJ with two groups and 0.03 for EmsK with two groups.
FIG. 1.
Electrophoregrams of EmsJ (152 and 155 bp) and EmsK (248 and 250 bp) loci, performed with the automatic sequencer ABI Prism 3100 (Applied Biosystems, Foster City, CA). A, European genotype; B, Asian, Alaskan, and Canadian genotypes; C, Alaskan genotype (found for only one rodent).
TABLE 3.
Results of fragment amplification for EmsJ, EmsK, and NAK1a
| Country-host | nb | EmsJ alleles
|
EmsK alleles
|
NAK1 alleles
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Homoz. 152 | Heteroz. 152 + 155 | Homoz. 248 | Homoz. 255 | NA | Homoz. 189 | Homoz. 192 | Homoz. 195 | Homoz. 198 | Homoz. 201 | Heteroz 195 + 198 | Heteroz. 198 + 201 | NA | ||
| AL-r | 10 | 0 | 10 | 9 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 6 |
| AL-h | 3 | 0 | 3 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| CND-r | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| PRC-r | 5 | 0 | 5 | 5 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| J-h | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| J-F | 5 | 0 | 5 | 5 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 |
| F-h | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| F-F | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| CH-r | 11 | 11 | 0 | 11 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 |
| CH-h | 7 | 7 | 0 | 6 | 0 | 1 | 0 | 1 | 1 | 1 | 4 | 0 | 0 | 0 |
| CH-m | 6 | 6 | 0 | 6 | 0 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | 0 |
| CH-F | 3 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 |
| D-h | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| D-F | 5 | 5 | 0 | 5 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 |
| A-h | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| A-F | 3 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 |
| PL-F | 4 | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 |
| CZ-F | 3 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 |
| SK-F | 3 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| NL-F | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
Geographical origins of samples: AL, Alaska; CND, Canada; PRC, China; J, Japan; F, France; CH, Switzerland; D, Germany; A, Austria; PL, Poland; CZ, Czech Republic; SK, Slovakia; NL, The Netherlands. Animal hosts: r, rodent; h, human; m, monkey; F, fox). Homoz., homozygous; Heteroz., heterozygous; NA, no amplification achieved.
n, number of E. multilocularis isolates tested.
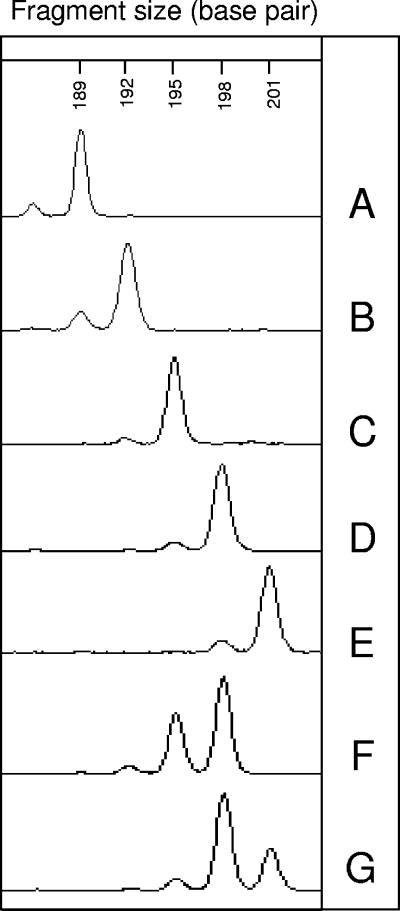
Amplification of the NAK1 target was basically in agreement with previously published data (22). However, a slight size difference of 30 bp was due to the use of primers NAK1 A and NAK1 B (Table 1), which were designed from the sequence available in GenBank (GbR AB100031) before the paper by Nakao et al. was published (22). In addition, two new alleles were found with these primers. Overall, this target presented a total of five alleles, with five homozygote and two heterozygote genotypes (Fig. 2 and Table 3). Some of the Alaskan isolates could not be amplified, despite the use of different sets of primers and different conditions as described above. The investigation of metacestodes from the Chinese rodents yielded one specific allele at 189 bp. An allele at 192 bp was found predominantly in Alaskan and Canadian samples. Among the European and Japanese samples, the 195- and 198-bp alleles were predominant. The 201-bp allele was present only among Swiss isolates. The rate of heterozygosity was 5.88%. The index of discrimination was 0.73 for NAK1, with seven different groups.
FIG. 2.
Electrophoregrams of the NAK1 (192 to 201 bp) locus, performed with the automatic sequencer ABI Prism 3100 (Applied Biosystems, Foster City, CA). Genotypes A to G are shown, and a representative sample of each genotype is indicated in parentheses: A, Chinese samples (2PRC-r); B, Alaskan, Canadian, and Swiss samples (2AL-r); C, Alaskan, Japanese, Swiss, German, Austrian, Czech, and Dutch samples (14CH-r); D, Japanese and European samples (116D-F); E, Swiss samples (CH5-h); F, Alaskan, Swiss, and Austrian samples (32A-h); and G, one Polish sample (392PL-F).
Reproducibility of results was demonstrated by performing the experiments independently at the two research laboratories mentioned above.
EmsB polymorphism.
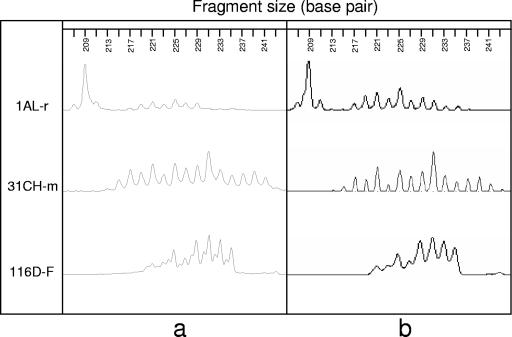
EmsB amplification was successful for the entire sample collection. The EmsB investigations resulted in a complex profile pattern, yielding 17 different alleles (209 bp to 241 bp) as described by Bart et al. in 2006 (4). The Pearson correlation coefficient was determined for each sample between data sets obtained on the Beckman CEQ 8000 and on the ABI Prism 3100. It ranged between 0.92 and 0.99 (P < 0.001). Furthermore, patterns found with the Beckman CEQ 8000 were in agreement with those found with the ABI Prism 3100 as shown in the Fig. 3.
FIG. 3.
Comparison between electrophoregrams performed with the automatic sequencers Beckman CEQ 8000 (a) and ABI Prism 3100 (b) for three isolates arbitrarily selected from the present sample collection: 1AL-r, Alaskan rodent isolate; 31CH-m, Swiss zoo monkey isolate; and 116D-F, German red fox isolate. The Pearson correlation coefficient was determined for results obtained on the two systems and was 0.99 for AL-r, 0.96 for 31CH-m, and 0.99 for 116D-F (P < 0.001).
Repeatability was assessed by performing PCR and fragment analyses of the Polish fox sample 302 PL-F five times with both systems. After normalization of the profiles, standard deviation (σ) of the genetic distance between each repetition was 3.1 × 10−3 with the ABI system versus 6.3 × 10−3 with the Beckman system. The main EmsB profiles are shown in Fig. 4a.
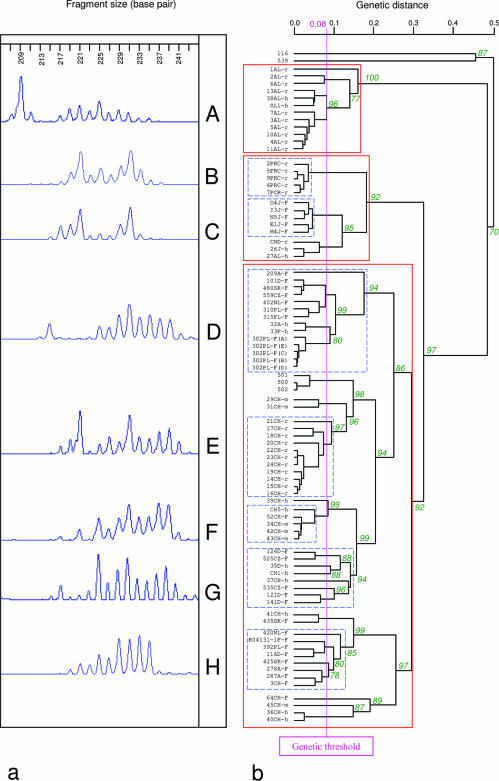
FIG. 4.
E. multilocularis genetic classification according to EmsB results. On the left side (a), an example of electrophoregrams of EmsB loci (209 bp to 241 bp), obtained using the automatic sequencer ABI Prism 3100. On the right side (b), a dendrogram based on EmsB genotypic data, constructed by hierarchical clustering analysis (Euclidian distance, average link clustering method), with pvclust, under the R Project. The approximately unbiased P values (numbers on nodes, in percent) were calculated with a multiscale bootstrap (B = 1,000). The three solid-line boxes show St. Lawrence Island's samples (upper box), the Asian-North American samples (middle box), and the European samples (lower box). The electrophoregrams correspond to (A) the St. Lawrence Island profile in the genetic tree and the dotted-line boxes refer to (B) the Chinese rodent profile, (C) the Japanese fox profile, and (D through H) the main European profiles. Samples 500, 501, and 502 represent a single isolate maintained in vivo by several passages in Meriones unguiculatus. Sample 116 originating from a Mauritanian camel and sample 539 originating from an Algerian sheep were E. granulosus samples and were included as outgroup controls.
ABI data were used to construct a dendrogram (Fig. 4b). Robustness of nodes was tested by multiscale bootstrap resampling (B = 1,000), given an approximately unbiased P value. The outgroup controls, composed of two E. granulosus samples, were distinguished from the other groups by a maximum genetic distance of 0.5.
A genotypic threshold, based on the results given by the cultivated isolates, was created to define the total number of genotypes. This threshold was calculated according to the following formula: x + 3σ (where x represents the average of the genetic distance found between the three samples and σ represents the standard deviation). The calculated average was 0.0266, and standard deviation was 0.0177. The calculated genetic distance value was 0.08. With this method of classification, the index of discrimination was 0.94 for this target, with 29 different groups.
The E. multilocularis panel was divided into three distinct clusters. The Alaskan block was composed of isolates obtained from rodents all caught in the same field and from two Alaskan patients (profile A in Fig. 4). This cluster was genetically distinguished from the other isolates by a value of 0.48. The Asia-Canada-Alaska group (profiles B and C) was clearly distinguishable from the European cluster by a value of 0.32. In Fig. 4, five main European EmsB profiles (D, E, F, G, and H) are shown. Their variations were due to the heights, the numbers, and the sizes of the peaks. For a given profile, variations were caused only by the heights of the peaks. We were able to differentiate several genotypes using our fixed genetic threshold. Thus, 23 genotypes were identified out of the 51 European samples. For example, the cluster formed by profile D was split into four closed genotypes. Profile E was characteristic for the parasites collected from rodents caught in the Fribourg field. Profile F was found among E. multilocularis collected from Swiss foxes, humans, and monkeys.
DISCUSSION
Previous studies (4, 9, 22) have tackled E. multilocularis genetic variability by using microsatellite DNA targets. Microsatellite sequences, due to their high power of discrimination, seemed to be a suitable tool to search for genetic differences not only between geographically distinct endemic areas but also within the areas themselves (4, 22). In the present study, we assessed and compared the discriminatory powers of different microsatellite targets by investigating a large panel of E. multilocularis isolates originating from different endemic foci, such as Saint Lawrence Island (Alaska), Central Europe, the Tibetan Plateau, and Japan. The single-locus microsatellites EmsJ and EmsK provided data about the parasites' genetic diversity that was relevant for discriminating samples over a large geographical range. The European cluster could thus be distinguished from the Alaskan, Canadian, and Asian cluster by two distinctly different genotypes. This combination enabled us to determine the global origins of the samples. Nevertheless, these two microsatellites exhibited a weak discriminatory power on a small scale, and we were unable to compare our results with the heterozygosity previously described by Nakao et al. (22).
With regard to NAK1, its higher level of discriminatory power enabled us to determine genetic polymorphism between the Tibetan, Alaskan, and European-Japanese clusters. On the other hand, a strong similarity was depicted for samples collected from the same field in Switzerland, indicating a possible common origin of contamination by E. multilocularis in that area, as there was also a spatial and temporal homogeneity with regard to the sampling procedure. The same phenomenon was observed for some Alaskan rodents, caught under similar geographically restricted conditions. While these findings were demonstrated with EmsB, they could not be confirmed with the NAK1 target, because these samples did not provide specific amplification products. The lack of amplification has not been clarified, despite the redesign of several primer sets. No reliable geographical or genetic structures were observed among the other European samples with the NAK1 target. More isolates have to be investigated to document the polymorphism level of this target in different areas. The fairly high rate of polymorphism of NAK1 may be linked to the mutation rate which occurs faster in the NAK1 region than in those of the other two single-locus microsatellites. The heterozygosity found by NAK1 in the present study was qualitatively in agreement with results previously published by Haag et al. and Nakao et al. (17, 22). However, the relatively low rate of heterozygosity confirmed that cross-fertilization occurs in the tapeworm, but to a much lesser extent than self-fertilization (17).
The EmsB target, referred to as a “tandem repeated multilocus microsatellite,” had a higher discriminatory power than the conventionally used single-locus microsatellites. Indeed, with the 17 alleles described, EmsB enabled us to discriminate single isolates from the same geographic origin, even at the “field” level. The extremely high discriminatory power of this target did not prejudice our study, as only a small genetic distance between samples from the same geographical origin was found, i.e., in the field in Alaska and in the one in Switzerland. When comparing single-locus microsatellites and EmsB results, the hypothesis of a “clonal” contamination of rodents living in the same field by, for example, one or several foxes that were infected by the same parasite isolate is considerably strengthened. In addition, an identical profile was found for different Swiss hosts, as shown in Fig. 4. This result illustrates that the genetic variability exhibited by EmsB is linked to the geographical specificity and not to the host specificity of samples.
Genetic studies using microsatellites are commonly based upon multiplex analyses in which 10 to 15 targets are simultaneously amplified in the same PCR. These analyses are usually carried out in forensic investigations or in filiation studies for livestock animals. E. multilocularis is an organism with a particular reproduction pattern. The very low encountered heterozygosity rate indicates a predominantly self-fertilizing breeding process. This organism does not follow the Hardy-Weinberg principle, and multiplex studies are thus not appropriate. Thus, the EmsB microsatellite proved to be very useful because it provided more information with a single PCR than, for example, 10 single-locus microsatellites together. Furthermore, it yielded a high rate of positive analyses, with nearly 100% of output.
For the first time, a relevant tool is now available to study the temporal and spatial development of the parasite within different host populations, since the similarity between the profiles of definitive and intermediate hosts can be demonstrated.
Using this tool, the question of emergence or reemergence of the infection in several regions of Europe can be addressed (14). Tracking the spread of single genotypes spatially and temporally may help to identify the source of the parasites in recently described new areas of endemicity. Due to the long incubation period of AE in humans, tracing the contamination to its source has been nearly impossible so far. The next challenge will be to superimpose a genetic distribution map on eco-epidemiological data and to construct a risk map for better public health management.
Acknowledgments
We are very grateful to the following persons for providing parasite specimens: Peter Deplazes (Switzerland), Joke van der Giessen (The Netherlands), Thomas Romig (Germany), Andrzej Malczewski (Poland), Pavol Dubinsky (Slovak Republic), Karel Martinek (Czech Republic), Georg Duscher (Austria), Minorou Nakao and Nariaki Nonaka (Japan), and Patrick Giraudoux, Francis Raoul, and Marie-Hélène Guislain (France). We also thank Karen Haag (Brazil) for constructive comments on the manuscript.
This work was supported by the EU EchinoRisk Project QLK2-CT-2001-01995 (BBW no. 00.0586-1), the Swiss National Science Foundation (grant no. 31-111780/1), and the U.S. National Institutes of Health and National Science Foundation (program R01 TW001565-05 “Ecology of Infectious Diseases”).
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Bardonnet, K., R. Piarroux, L. Dia, F. Schneegans, A. Beurdeley, V. Godot, and D. A. Vuitton. 2002. Combined eco-epidemiological and molecular biology approaches to assess Echinococcus granulosus transmission to humans in Mauritania: occurrence of the ‘camel’ strain and human cystic echinococcosis. Trans. R. Soc. Trop. Med. Hyg. 96:383-386. [DOI] [PubMed] [Google Scholar]
- 2.Bart, J. M., K. Bardonnet, M. C. Elfegoun, H. Dumon, L. Dia, D. A. Vuitton, and R. Piarroux. 2004. Echinococcus granulosus strain typing in North Africa: comparison of eight nuclear and mitochondrial DNA fragments Parasitology 128:229-234. [DOI] [PubMed] [Google Scholar]
- 3.Bart, J. M., I. Breyer, B. Gottstein, T. Romig, and R. Piarroux. 2003. Development of molecular tools to explore genetic diversity in Echinococcus multilocularis. Helminthologia 40:117-121. [Google Scholar]
- 4.Bart, J. M., J. Knapp, B. Gottstein, F. El-Garch, P. Giraudoux, M. L. Glowatzki, H. Berthoud, S. Maillard, and R. Piarroux. 2006. EmsB, a tandem repeated multi-loci microsatellite, new tool to investigate the genetic diversity of Echinococcus multilocularis. Infect. Genet. Evol. 6:390-400. [DOI] [PubMed] [Google Scholar]
- 5.Bowles, J., D. Blair, and D. P. McManus. 1992. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 54:165-173. [DOI] [PubMed] [Google Scholar]
- 6.Bowles, J., and D. P. McManus. 1993. Molecular variation in Echinococcus. Acta Trop. 53:291-305. [DOI] [PubMed] [Google Scholar]
- 7.Brack, M., K. Tackmann, F. J. Conraths, and S. Rensing. 1997. Alveolar hydatidosis (Echinococcus multilocularis) in a captive rhesus monkey (Macaca mulatta) in Germany. Trop. Med. Int. Health 2:754-759. [DOI] [PubMed] [Google Scholar]
- 8.Bresson-Hadni, S., R. Piarroux, B. Bartholomot, J. P. Miguet, G. Mantion, and D. Vuitton. 2005. Echinococcose alvéolaire—alveolar echinococcosis. EMC Hépato-Gastroentérologie 2:86-104. [Google Scholar]
- 9.Bretagne, S., B. Assouline, D. Vidaud, R. Houin, and M. Vidaud. 1996. Echinococcus multilocularis: microsatellite polymorphism in U1snRNA genes. Exp. Parasitol. 82:324-328. [DOI] [PubMed] [Google Scholar]
- 10.Bulle, B., L. Millon, J. M. Bart, M. Gallego, F. Gambarelli, M. Portus, L. Schnur, C. L. Jaffe, S. Fernandez-Barredo, J. M. Alunda, and P. Piarroux. 2002. Practical approach for typing strains of Leishmania infantum by microsatellite analysis. J. Clin. Microbiol. 40:3391-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Combes, C. 1997. Fitness of parasites: pathology and selection. Int. J. Parasitol. 27:1-10. [DOI] [PubMed] [Google Scholar]
- 12.Craig, P. S. 2006. Epidemiology of human alveolar echinococcosis in China. Parasitol. Int. 55(Suppl.):S221-S225. [DOI] [PubMed] [Google Scholar]
- 13.Deplazes, P., and J. Eckert. 2001. Veterinary aspects of alveolar echinococcosis—a zoonosis of public health significance. Vet. Parasitol. 98:65-87. [DOI] [PubMed] [Google Scholar]
- 14.Eckert, J., F. J. Conraths, and K. Tackmann. 2000. Echinococcosis: an emerging or re-emerging zoonosis? Int. J. Parasitol. 30:1283-1294. [DOI] [PubMed] [Google Scholar]
- 15.Eiermann, T. H., F. Bettens, P. Tiberghien, K. Schmitz, I. Beurton, S. Bresson-Hadni, R. W. Ammann, S. F. Goldmann, D. A. Vuitton, B. Gottstein, and P. Kern. 1998. HLA and alveolar echinococcosis. Tissue Antigens 52:124-129. [DOI] [PubMed] [Google Scholar]
- 16.Gottstein, B., F. Bettens, A. J. Parkinson, and F. Wilson. 1996. Immunological parameters associated with susceptibility or resistance to alveolar hydatid disease in Yupiks/Inupiats. Arctic Med. Res. 55:14-19. [PubMed] [Google Scholar]
- 17.Haag, K. L., A. M. Araujo, B. Gottstein, and A. Zaha. 1998. Selection, recombination and history in a parasitic flatworm (Echinococcus) inferred from nucleotide sequences. Mem. Inst. Oswaldo Cruz 93:695-702. [DOI] [PubMed] [Google Scholar]
- 18.Haag, K. L., A. Zaha, A. M. Araujo, and B. Gottstein. 1997. Reduced genetic variability within coding and non-coding regions of the Echinococcus multilocularis genome. Parasitology 115:521-529. [DOI] [PubMed] [Google Scholar]
- 19.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito, A., T. Romig, and K. Takahashi. 2003. Perspective on control options for Echinococcus multilocularis with particular reference to Japan. Parasitology 127(Suppl.):S159-S172. [PubMed] [Google Scholar]
- 21.Kern, P., K. Bardonnet, E. Renner, H. Auer, Z. Pawlowski, R. W. Ammann, and D. A. Vuitton. 2003. European echinococcosis registry: human alveolar echinococcosis, Europe, 1982-2000. Emerg. Infect. Dis. 9:343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakao, M., Y. Sako, and A. Ito. 2003. Isolation of polymorphic microsatellite loci from the tapeworm Echinococcus multilocularis. Infect. Genet. Evol. 3:159-163. [DOI] [PubMed] [Google Scholar]
- 23.Piarroux, M., S. Bresson-Hadni, I. Capek, J. Knapp, J. Watelet, J. Dumortier, A. Abergel, A. Minello, A. Gérard, J. Beytout, R. Piarroux, B. Kantelip, E. Delabrousse, V. Vaillant, D. Vuitton, and P. L. R. Francechino. 2006. Surveillance de l'échinococcose alvéolaire en France: bilan de cinq années d'enregistrement 2001-2005. Bull. Epidemiol. Hebd. 27-28/2006:206-208. [Google Scholar]
- 24.R Development Core Team. 2005. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org.
- 25.Rehmann, P., A. Grone, A. Lawrenz, O. Pagan, B. Gottstein, and L. N. Bacciarini. 2003. Echinococcus multilocularis in two lowland gorillas (Gorilla g. gorilla). J. Comp. Pathol 129:85-88. [DOI] [PubMed] [Google Scholar]
- 26.Romig, T., A. Dinkel, and U. Mackenstedt. 2006. The present situation of echinococcosis in Europe. Parasitol. Int. 55(Suppl.):S187-S191. [DOI] [PubMed] [Google Scholar]
- 27.Schantz, P. M., C. F. von Reyn, T. Welty, and M. G. Schultz. 1976. Echinococcosis in Arizona and New Mexico. Survey of hospital records, 1969-1974. Am. J. Trop. Med. Hyg. 25:312-317. [DOI] [PubMed] [Google Scholar]
- 28.Shimodaira, H. 2002. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 51:492-508. [DOI] [PubMed] [Google Scholar]
- 29.Shimodaira, H. 2004. Approximately unbiased test of regions using multistep-multiscale bootstrap resampling. Ann. Stat. 32:2616-2641. [Google Scholar]
- 30.Simpson, E. H. 1949. Measurement of diversity. Nature (London) 163:688. [Google Scholar]
- 31.Suzuki, R., and H. Shimodaira. 2005. pvclust: hierarchical clustering with p-values. R package version 1.0-3. http://www.is.titech.ac.jp/∼shimo/prog/pvclust/.
- 32.Thompson, R. C., C. M. Kapel, R. P. Hobbs, and P. Deplazes. 2006. Comparative development of Echinococcus multilocularis in its definitive hosts. Parasitology 132:709-716. [DOI] [PubMed] [Google Scholar]
- 33.Vuitton, D. A., S. L. Zhang, Y. Yang, V. Godot, I. Beurton, G. Mantion, and S. Bresson-Hadni. 2006. Survival strategy of Echinococcus multilocularis in the human host. Parasitol. Int. 55(Suppl.):S51-S55. [DOI] [PubMed] [Google Scholar]
- 34.Vuitton, D. A., H. Zhou, S. Bresson-Hadni, Q. Wang, M. Piarroux, F. Raoul, and P. Giraudoux. 2003. Epidemiology of alveolar echinococcosis with particular reference to China and Europe. Parasitology 127(Suppl.):S87-S107. [PubMed] [Google Scholar]