Abstract
A real-time PCR targeting the gyrase A subunit gene outside the quinolone resistance-determining region has been developed to detect Arcobacter species. The species identification was done by probe hybridization and melting curve analysis, using fluorescence resonance energy transfer technology. Discrimination between Arcobacter species was straightforward, as the corresponding melting points showed significant differences with the characteristic melting temperatures of 63.5°C, 58.4°C, 60.6°C, and 51.8°C for the Arcobacter butzleri, Arcobacter cryaerophilus, Arcobacter cibarius, and Arcobacter nitrofigilis type strains, respectively. The specificity of this assay was confirmed with pure cultures of 106 Arcobacter isolates from human clinical and veterinary specimens identified by phenotypic methods and 16S rRNA gene sequencing. The assay was then used to screen 345 clinical stool samples obtained from patients with diarrhea. The assay detected A. butzleri in four of these clinical samples (1.2%). These results were confirmed by a conventional PCR method targeting the 16S rRNA gene with subsequent sequencing of the PCR product. In conclusion, this real-time assay detects and differentiates Arcobacter species in pure culture as well as in the competing microbiota of the stool matrix. The assay is economical since only one biprobe is used and multiple Arcobacter species are identified in a single test.
Aerotolerant spirillum/vibrio-like organisms from aborted bovine fetuses were first described by Ellis et al. in 1977 (9) and designated Campylobacter cryaerophila on the basis of their Campylobacter-like morphology, aerotolerance, and growth at 25°C (9). After examining human and veterinary isolates of these aerotolerant campylobacters, Kiehlbauch et al. identified two species, Campylobacter butzleri, corresponding to the majority of human clinical isolates, and Campylobacter cryaerophilus, consisting of two distinct groups (20). Vandamme et al. proposed the genus Arcobacter to encompass aerotolerant campylobacters (36). This genus comprises human and veterinary species, including Arcobacter butzleri, Arcobacter cryaerophilus, Arcobacter cibarius, and Arcobacter skirrowii, and also the type strain from the root of a Spartina plant, Arcobacter nitrofigilis. Two new species, Arcobacter sulfidicus, which inhabits coastal marine water (42), and Arcobacter halophilus, isolated from a hypersaline lagoon (8), represent the diversity of Arcobacter and its capacity to survive in the environment. The genus Arcobacter has been included with the genus Campylobacter in the Campylobacteraceae family, which is part of the Epsilonproteobacteria (11).
Despite the prevalence of Arcobacter species in food specimens, few studies have reported human Arcobacter infections (23). The bacterium was first identified in 2.4% of clinical isolates from 631 Thai children with diarrhea (34). An outbreak of recurrent abdominal cramps in 10 young children in Italy was attributed to A. butzleri (37). Arcobacter butzleri has been linked to sporadic cases of diarrhea and abdominal cramps in patients with chronic diseases (24), such as neonatal bacteremia (27), liver cirrhosis (44), and acute appendicitis (22). More recently, a Belgian study revealed that 3.5% of campylobacters and related organisms isolated from human stool samples over an 8-year period were A. butzleri (38). In one of our previous studies, A. butzleri ranked fourth among the 2,855 strains of Campylobacter-like organisms received during a 17-month surveillance of Campylobacter infections in France (28). These infections were associated with intestinal disorders resembling those caused by Campylobacter jejuni. These observations suggest that the prevalence of this emerging pathogen may be underestimated due to inadequate culture conditions and/or false identification (14, 38). Traditional isolation methods for Arcobacter are similar to those for Campylobacter, although the majority do not grow at 42°C. The problems associated with culture failure or with the standard phenotypic methods of identification have led to the investigation of new approaches for assistance in the preliminary diagnosis of Arcobacter infections. Conventional PCR-based techniques targeting ribosomal genes, such as the 16S RNA gene (13, 15, 32, 41) and the 23S rRNA gene (15, 16, 18, 26), have been widely used for the detection and identification of Arcobacter species. The increasing relevance of nucleic acid amplification tests for the detection and identification of Arcobacter species is now obvious, particularly in light of culture failure and misidentification. With respect to nucleic acid amplification tests, different real-time PCR chemistries are available, with the most frequently used formats being SYBR green, hybridization probes, TaqMan probes, molecular beacons, and Scorpion probes. These PCR formats are usually very sensitive, can be performed in less than 3 hours, and minimize the risk of cross-contamination with other PCR products since all of the steps occur in the same tube and no postamplification handling is necessary. Among these methods, two specific TaqMan assays were recently described for detection of A. butzleri and A. cryaerophilus (4).
Real-time PCR using the fluorescence resonance energy transfer (FRET) hybridization probe method is very promising since it can detect single nucleotide polymorphisms (SNP) with only one biprobe. We have recently described a FRET real-time PCR assay targeting the gyrA gene for rapid and sensitive identification and differentiation of C. jejuni and Campylobacter coli (25). The present study reports the development of a real-time PCR assay using FRET chemistry and targeting the gyrase A gene (gyrA) outside the quinolone resistance-determining region (QRDR) in Arcobacter species. Subsequent identification of the species was based on melting curve analysis (MCA) of the amplified products by detection of the nucleotide changes between the species. The advantages of this new test are the relatively low cost (since only one biprobe is used) and the possible detection and identification of multiple Arcobacter species in a single test.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The following five type strains were used as positive controls: the A. butzleri D2686 type strain (CIP 103493 T, a human isolate), the A. cryaerophilus A169/B type strain (CIP 104014 T, a bovine fetus isolate), the A. cibarius CCUG 48482 type strain (LMG 21996, a chicken skin isolate), the A. skirrowii 449/80 type strain (ATCC 51132, an ovine fetus isolate), and the A. nitrofigilis C1 type strain (CIP 103745, a plant isolate). An additional 106 Arcobacter isolates obtained from human (n = 22) or veterinary (n = 84) sources were employed in this study. Human strains included A. butzleri strain WRI 996/79 (CCUG 10373) and 21 strains (19 A. butzleri and 2 A. cryaerophilus strains) isolated from human clinical specimens, essentially feces from patients with gastroenteritis, from all over France in 2003 and 2004, and sent to the French National Reference Center for Campylobacter and Helicobacter (28). Among the 84 Arcobacter strains isolated from animal sources, 3 strains (A. butzleri Cipolla 4 and A. cryaerophilus strains PC367 and PC249) were obtained from the culture collection of the University of Göteborg, Sweden (CCUG 34397 B, CCUG 12020, and CCUG 12018, respectively), and 81 Arcobacter strains (78 A. butzleri and 3 A. cryaerophilus strains) were obtained from the National Animal Disease Centre (USDA, Agricultural Research Service, Ames, IA).
All arcobacters were grown as previously reported (28). Some of these strains were initially identified as members of the Campylobacteraceae family by phenotypic methods, i.e., morphology, motility, growth in a microaerobic atmosphere, and positive oxidase activity. Identification at the species level was performed by 16S rRNA gene sequencing as previously reported (28).
A panel of bacterial strains was used to evaluate the specificities of the different primers designed during this study. These strains included a large panel of Campylobacter strains, such as C. jejuni CCUG 11284, Campylobacter coli CCUG 145401, Campylobacter fetus UA60, Campylobacter hyointestinalis CCUG 14169, Campylobacter mucosalis CIP 103750, Campylobacter sputorum CCUG 9728, Campylobacter upsaliensis CCUG 14913, and Campylobacter lari CCUG 23947, and Helicobacter strains, such as Helicobacter pullorum CCUG 33839, Helicobacter canadensis NCTC 13221, Helicobacter pylori J99, Helicobacter hepaticus ATCC 51448, Helicobacter bilis ATCC 51630, Helicobacter muridarum ATCC 49282, Helicobacter felis CCUG 28539 T, “Flexispira rappini” (proposed name) CCUG 29176, and Wolinella succinogenes DSMZ 1740. Other enteric bacteria (clinical isolates) commonly isolated from patients (Escherichia coli, Salmonella enterica serovar Enteritidis, and Salmonella enterica serovar Typhimurium) were also tested.
Genomic DNA was isolated by using a QIAamp DNA mini kit (QIAGEN SA, Courtaboeuf, France) and stored at −20°C until required for analysis.
Stool samples.
In total, 345 stool clinical samples were screened with this assay. They were obtained from patients (male/female ratio, 0.56; mean age, 41.4 ± 28.9 years) suffering from diarrhea and presenting themselves at Pellegrin Hospital (Bordeaux, France) between July and September 2005. Genomic DNA was extracted from the stool samples by using the QIAamp DNA stool mini kit (QIAGEN SA) and stored at −20°C.
All stool specimens were also cultured for Arcobacter species by using two methods. The first was inoculation of an enrichment broth (Arco broth) incubated for 24 h at 37°C and subcultured on an Arco agar according to a method previously published (6). The plates were incubated at 37°C in a microaerobic atmosphere for 7 days. The second method was a filtration method using a 0.65-μm filter (Millipore, Billerica, MA) on a blood agar plate without antibiotic incubated for 7 days. Colonies were identified according to standard methods.
PCR and DNA sequencing of the gyrA gene.
The available gyrA sequences (1) from the five A. butzleri strains, D2686, 520H-2004, 1137-2003, 1340-2003, and CCUG 34397 B (GenBank accession numbers DQ464331 to DQ464335, respectively), were aligned with those of the A. cryaerophilus A169/B type strain, the A. cibarius CCUG 48482 type strain, the A. skirrowii 449/80 type strain (GenBank accession numbers DQ464336 to DQ464338, respectively), and the A. nitrofigilis A169/B type strain (GenBank accession number DQ464339) by using multiple sequence alignment with hierarchical clustering (5) (http://prodes.toulouse.inra.fr/multalin/multalin.html). Primers were designed to target a conserved region outside the QRDR. The resulting primer set (F-Arco-FRET5 and R-Arco-FRET5) was designed using web Primer3 software (30) (http://www.broad.mit.edu/cgi-bin/primer/primer3_www.cgi). Use of these primers resulted in the amplification of a 905-bp PCR product. PCR was performed with PWO super yield Taq polymerase (Roche Diagnostics, Meylan, France). The amplification parameters consisted of 1 cycle at 95°C for 5 min, followed by 35 cycles at 95°C for 30 s, 56°C for 30 s, and 72°C for 2 min, and finally 1 cycle at 72°C for 5 min. The 905-bp sequences of the gyrA genes of other clinical Arcobacter isolates (A. butzleri strains 235-2004, 1285-2003, 1188-2003, 1477-2003, 1172-2003, and 1426-2003 and A. cryaerophilus strains 322H-2004, 622H-2004, 492-2004, PC367, and PC249) were amplified using this PCR assay and sequenced on both strands with PCR primers using an Applied Biosystems 3130xl genetic analyzer (Applied Biosystems, Foster City, CA) with a fluorescence BigDye Terminator V1.1 cycle sequencing kit (Applied Biosystems) according to the manufacturer's instructions.
Design of the primers and probes for FRET-PCR identification of Arcobacter species.
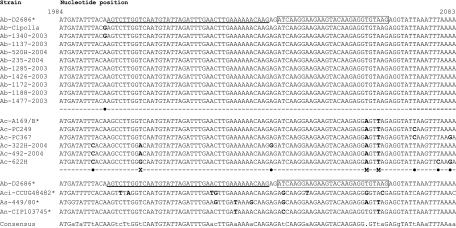
The gyrA sequences (Table 1 and Fig. 1) from A. butzleri (n = 11), A. cryaerophilus (n = 6), A. cibarius (n = 1), A. nitrofigilis (n = 1), and A. skirrowii (n = 1) were aligned and analyzed to identify (i) conserved regions for the primer design and (ii) a specific region for individual probes designed to differentiate the above-mentioned species. LightCycler (LC) probe design software version 1.0 (Roche Diagnostics, Neuilly sur Seine, France) and the LC probe design software module with the mutation search module were used (29). The sensor probe, 5′ labeled with LC-Red 640 and 3′ phosphorylated, hybridized perfectly with the A. butzleri gyrA sequence and presented two, four, three, and two nucleotide mismatches with the A. cryaerophilus, A. cibarius, A. skirrowii, and A. nitrofigilis gyrA sequences, respectively. The anchor probe (melting temperature [Tm] = 65°C), 3′ labeled with fluorescein, hybridized 2 bases upstream from the sensor probe (Tm = 54°C) and frequently presented one or two mismatches (depending on the strain) with the A. cryaerophilus gyrA sequence. As this mismatch(es) is located at the 5′ extremity of the probe, it should not have any influence on the FRET. The anchor probe presented four, three, and one nucleotide mismatch with the A. cibarius, A. skirrowii, and A. nitrofigilis gyrA sequences, respectively. This mismatch(es), located near the 3′ extremity of the sensor probe, could hinder the stability of hybridization and thus the FRET. Two oligonucleotide primers were selected (F1-/R2-FRET5) for the amplification of Arcobacter species.
TABLE 1.
Sequences of the primers and probes designed for FRET-PCR identification of Arcobacter species
| Primer function and designation | Sequence (5′ to 3′) | Nucleotide positionsa |
|---|---|---|
| gyrA gene amplification and sequencing | ||
| F-Arco-FRET5 | TTGAAGATTCTTATGATGAAATTGA (sense) | 1460-1484 |
| R-Arco-FRET5 | TGTATTTCTTCCTGCTTTTCTAATTG (antisense) | 2364-2339 |
| LC-FRET assay | ||
| F1-FRET5 | ATCTTTAGTATTCTTTACAAGAAATGG (sense) | 1830-1856 |
| R2-FRET5 | AACTGTTGTTCGTTTTCCA (antisense) | 2181-2163 |
| S-Ab-FRET5 | Red 640-ATCAAGGAAGAAGTACAAGAGGTGTAAG-p (antisense) | 2039-2066 |
| A-Ab-FRET5 | AGTCTTGGTCAATGTATTAGATTTGAACTTGAAAAAACAAG-F (antisense) | 2036-1996 |
The nucleotide positions of primers and probes were compared to the gyrA gene numbering of Arcobacter butzleri D2682, the type strain.
FIG. 1.
Representation of the internal nucleotide sequences from the gyrA genes of five Arcobacter species and locations of the FRET probes used for real-time PCR amplification. The sequences from nucleotide 1984 to nucleotide 2083 are shown and compared to the numbering for the Arcobacter butzleri D2682 type strain (GenBank accession number DQ464331). The sensor probe (boxed) was labeled at the 5′ end with LC-Red 640-N-hydroxysuccinimide ester, and a 3′ terminal phosphate block was added. The anchor probe (underscored) was labeled at the 3′ end with fluorescein. Asterisks indicate type strains. M indicates nucleotide mismatches with the sensor probe inducing different melting points (with FRET). X indicates silent anchor probe nucleotide mismatches. •, spontaneous point mutation outside the interest region. Nucleotides that have replaced the expected nucleotides are indicated in bold. Ab, Arcobacter butzleri; Ac, Arcobacter cryaerophilus; Aci, Arcobacter cibarius; As, Arcobacter skirrowii; and An, Arcobacter nitrofigilis. The GenBank accession numbers corresponding to the gyrA sequences of A. butzleri strains D2686, Cipolla 4, 1340-2003, 1137-2003, 520H-2004, 235-2004, 1285-2003, 1426-2003, 1172-2003, 1188-2003, and 1477-2003 are DQ464331, DQ464335, DQ464334, DQ464333, DQ464332, EF176585, EF176586, EF176587, EF176588, EF176589, and EF176590, respectively. The GenBank accession numbers corresponding to the gyrA sequences of A. cryaerophilus strains A169/B, PC249, PC367, 322H-2004, 492-2004, and 622H-2004 are DQ464336, EF176591, EF176592, EF176593, EF176594, and EF176595, respectively, and those for A. cibarius strain CCUG 48482, Arcobacter skirrowii strain 449/80, and Arcobacter nitrofigilis strain C1 are DQ464337, DQ464338, and DQ464339, respectively.
Real-time FRET-PCR for identification of Arcobacter species by use of the biprobe.
The PCR and hybridization reactions were carried out in glass capillary tubes (Roche Diagnostics) in an LC thermocycler (Roche Diagnostics). The 7-μl reaction mixture contained 0.7 μl of FastStart DNA master hybridization probe mixture (Roche Diagnostics), 2 mM of MgCl2, 0.7 μM each of the F1-FRET5 and R2-FRET5 primers (Table 1), 0.2 μM of each probe, and 0.7 μl of template DNA. Following an initial denaturation step at 95°C for 10 min with a temperature transition rate of 20°C/s, amplification steps (95°C for 0 s, 60°C for 10 s, and 72°C for 25 s) were repeated for 50 cycles at a temperature transition rate of 20°C/s. Fluorescence was measured at 640 nm after each cycle. This was followed by a melting program of 95°C for 60 s and 38°C for 50 s at a temperature transition rate of 20°C/s and then 80°C for 0 s at a rate of 0.1°C/s with continuous monitoring of the fluorescence. A final step consisted of cooling at 20°C/s to 40°C with a 30-s hold.
PCR amplification of the A. butzleri 16S rRNA gene and sequencing of the 16S rRNA gene.
The primers BUTZ and ARCO, previously reported by Houf et al. (15), were used to produce a 401-bp PCR product, and direct sequencing was achieved on both strands with PCR primers as reported above. Amplified primerless sequences were compared to those in the GenBank database with the BLAST program at the National Center for Biotechnology Information computer server (2).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the gyrA genes of A. butzleri strains 235-2004, 1285-2003, 1426-2003, 1172-2003, 1188-2003, and 1477-2003 are EF176585 to EF176590, respectively, and those for A. cryaerophilus strains PC249, PC367, 322H-2004, 492-2004, and 622H-2004 are EF176591 to EF176595, respectively. The GenBank accession number for the gyrA gene of A. nitrofigilis is DQ464339.
RESULTS
Development of the test.
To identify Arcobacter species, we developed a FRET real-time PCR consisting of amplification of a fragment of the gyrA gene in Arcobacter species with subsequent detection of the species by probe hybridization and MCA.
Alignment of gyrA sequences from 20 Arcobacter strains, including A. butzleri (n = 11), A. cryaerophilus (n = 6), A. cibarius (n = 1), A. nitrofigilis (n = 1), and A. skirrowii (n = 1) strains, was performed in order to design primers and probes for the assay. Numerous nucleotide sequence variations in the gyrA gene were observed among these five Arcobacter species. Because of the constraints imposed by the LC software for the probe design (the highly conserved anchor probe and mismatches located on the sensor probe, a maximum gap of 5 bases between the probes, and restricted Tms for the probes), only one region of the gyrA gene outside the QRDR (nucleotides 1830 to 2364) could be used for the primer and probe design. Because A. butzleri is the most frequently encountered Arcobacter species, the probes used in this study were designed on the A. butzleri gyrA gene.
Identification of control strains and specificity of the assay.
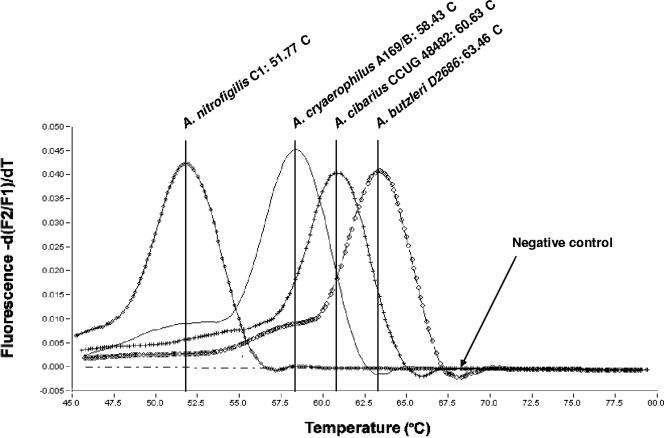
An increase in fluorescence was observed during real-time PCR, with threshold cycle values of approximately 18.5, 20, 25.5, and 28 cycles for the A. butzleri, A. cibarius, A. cryaerophilus, and A. nitrofigilis type strains, respectively (data not shown). MCA of the 352-bp amplicons from the same strains identified a unique melting peak for each species (63.5°C, 60.6°C, 58.4°C, and 51.8°C, respectively) (Fig. 2), allowing unambiguous differentiation of these four species.
FIG. 2.
MCA of the 352-bp amplicon of the gyrA genes of Arcobacter butzleri, Arcobacter cryaerophilus, Arcobacter cibarius, and Arcobacter nitrofigilis obtained with the real-time PCR assay. The melting peaks generated from the dissociation of the fluoroprobes from the Arcobacter species with four different Tms can be observed. Values on the y axis represent the ratio of the first negative derivative of the change in fluorescence [d(F2/F1)] to the variation in temperature (dT).
Then, this assay was applied to pure cultures of 106 Arcobacter isolates (A. butzleri [n = 99] and A. cryaerophilus [n = 7] isolates) obtained from human clinical and veterinary specimens. An excellent agreement between this test, the phenotypic methods, and the 16S rRNA gene sequencing performed on these 106 strains was obtained, suggesting a good specificity and reproducibility for this assay, especially for the identification of A. butzleri and A. cryaerophilus. Maximum differences of 0.5 to 1°C in the melting peak temperatures were observed between different runs (25 runs), resulting from variations in the temperature profile generated by the LC thermocycler. Concerning the species A. cibarius and A. nitrofigilis, only one isolate of each could be tested.
The specificities of the PCR primers F1-/R2-FRET5 were tested using other bacterial species, such as related non-Arcobacter species or other bacteria which could be present in stool samples. The FRET signal as well as the expected 352-bp amplicon observed by agarose gel electrophoresis analysis was obtained only for A. butzleri, A. cryaerophilus, A. cibarius, and A. nitrofigilis. They were not observed in A. skirrowii or in related bacteria which could be present in stool samples, including Campylobacter, Helicobacter, and Wolinella (see Materials and Methods). These results confirm the excellent specificities of the primers and the discriminatory power of the hybridization probe assay.
The reverse PCR R2-FRET5 primer presents a T/A mismatch at its 3′ end on the A. skirrowii gyrA gene (data not shown), which explains the lack of amplification for this species. Consequently, a new set of primers was designed for A. skirrowii amplification, using the same probes and identical reaction conditions so that the assay could be run concurrently. MCA of the 394-bp amplicon from A. skirrowii revealed the presence of a double curve, which was difficult to interpret (not shown). This bimodal curve may result from an unstable hybridization of both the anchor and the sensor probes on the gyrA gene of A. skirrowii since nucleotide mismatches are located at the 3′ and 5′ extremities of the anchor and sensor probes, respectively (Fig. 1), thus preventing a good FRET. Considering this result, only A. butzleri, A. cryaerophilus, A. cibarius, and A. nitrofigilis can be identified by this assay.
Comparison with standard nucleic acid amplification tests.
The standard PCR described by Houf et al. (15) for species identification of A. butzleri and A. cryaerophilus strains as well as identification at the species level by sequencing of the 16S rRNA gene (28) was performed on all of the strains. A complete agreement was found (data not shown).
Evaluation of the sensitivity of the test.
The sensitivity of the PCR was evaluated using 10-fold serial dilutions of a bacterial suspension of A. butzleri in brucella broth, which was quantified by culture on specific media and subsequent colony counts. PCRs with the DNA extracted from the bacterial suspensions yielded regression curves with slopes between −2.946 and −3.512, spanning the slope value of −3.33, which corresponds to the maximum efficiency. Although this assay was able to detect fewer than 30 CFU, the regression curve was linear only from 3 × 107 to 3 × 102 CFU with reaction volumes of 20 μl or 7 μl.
Moreover, this assay always led to a positive result with the use of the conventional boiling method, in contrast to standard PCR (data not shown).
Detection of Arcobacter species from human stool samples.
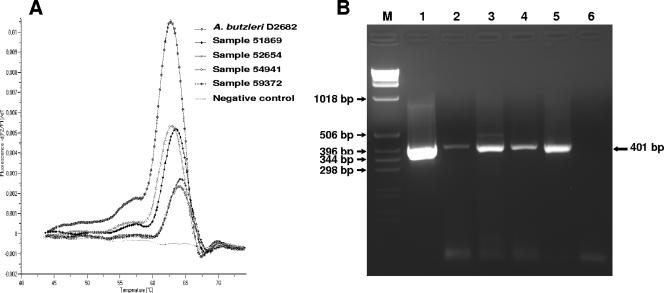
Of the 345 stool samples obtained from patients with diarrhea, this assay was able to detect A. butzleri DNA in four cases (∼1.2%), with the Tms ranging from 62.86°C to 64.22°C, compared to the A. butzleri D2686 type strain, which exhibited a Tm of 62.72°C (Fig. 3A). Furthermore, agarose gel electrophoresis of the amplified products confirmed the presence of the 352-bp amplicon for the four positive fecal samples (data not shown). The presence of A. butzleri DNA in these four samples was also confirmed by the standard PCR assay described by Houf et al. (15). This PCR assay yielded the expected 401-bp PCR product (Fig. 3B) for these four positive samples, whereas no amplification was observed with the 50 negative stool samples tested as negative controls (data not shown). Sequencing of the 401-bp PCR products revealed that the amplified 16S rRNA gene sequences from these four positive stool samples corresponded to A. butzleri, with 100% identity on the entire length of the primerless amplicon sequence. These results concur with those obtained with the real-time FRET-PCR assay for the direct detection of A. butzleri in feces.
FIG. 3.
PCR analysis of DNA extracted from stool samples. (A) MCA of the 352-bp amplicon of the gyrA gene. (B) Agarose gel electrophoresis of PCR products from Arcobacter butzleri strain D2686 and human stool samples obtained with primers ARCO and BUTZ for amplification of 401 bp from the 16S rRNA gene. M, 1-kb ladder; lane 1, A. butzleri strain D2686 as a reference; lanes 2 to 5, human stool samples 51669, 52654, 54941, and 59372, respectively; lane 6, negative control; d(F2/F1)/dT, ratio of the first negative derivative of the change in fluorescence to the variation in temperature.
Conversely, none of the stool samples was positive for Arcobacter species by culture.
DISCUSSION
Several studies described the detection of Arcobacter in the stools of animals. In most, culture was carried out to isolate the strain; then, nucleic acid amplification tests, such as PCR and/or sequencing, were performed for species identification (3, 28, 39). Concerning human specimens, Kulkarni et al. investigated the optimal method for detection of Campylobacter-like organisms from stool samples by comparing selective culture with membrane filtration and PCR (21). They concluded that membrane filtration was the best method for detection of Arcobacter species in stool samples, but this conclusion was based on the detection of a single positive sample by culture. In contrast, the results obtained by Engberg et al. showed the difficulty in screening human stool samples for the presence of Arcobacter species, since no single method can successfully identify isolates from all taxa (10). A more recent study showed that among 255 patients hospitalized with gastrointestinal complaints in South Africa, A. butzleri, A. cryaerophilus, and A. skirrowii were detected by PCR in 7.5, 3.5, and 2% of the stool specimens, respectively (31). The authors emphasized the usefulness of molecular techniques in the identification of Arcobacter species compared to that of the culture method. Molecular techniques constitute an important advance in the rapid diagnosis of gastrointestinal infections when the organisms are fastidious. Among the numerous molecular techniques, real-time PCR is an important tool for the rapid and unequivocal detection of bacterial species in complex human clinical samples, such as stool samples.
The PCR format described herein has shown its ability to detect specific Arcobacter species, with the exception of A. skirrowii, in a specific and reliable way. This method is rapid and convenient and, moreover, in comparison to the real-time PCR method previously described (4) is economical since it has the advantage of using only one biprobe in a low final volume. Such a method should facilitate the detection of the emerging pathogenic Arcobacter species. Of the most popular real-time detection formats currently used (SYBR green, hybridization probes, TaqMan probes, molecular beacons, and Scorpion probes), the SYBR green format was excluded since (i) the specificity is not optimal, as no probe is used, (ii) the identification of several species in a single test remains difficult as several primer sets are necessary, and (iii) distinct Tms are also necessary to identify each species without ambiguity. For the identification of the two most frequently encountered Arcobacter species, two specific real-time PCRs using TaqMan chemistry were recently reported (4). These TaqMan assays require a mixture of probes (each labeled twice, as is the case for molecular beacons and Scorpion probes), producing a cost twice as high as that of FRET hybridization probe chemistry with the SNP detection format, for which only one biprobe (each probe labeled once) has to be used. For this reason, a FRET hybridization probe PCR with the SNP detection format was chosen. Consequently, the primer set and biprobe were designed to amplify a gene common to the Arcobacter species but presenting ample sequence divergences for species differentiation by MCA. Concerning the target gene, the 16S rRNA gene speciation of Arcobacter species seems satisfactory since several techniques were developed successfully (13, 15, 32, 41). However, 16S rRNA gene-based taxonomy is not highly discriminant for Epsilonproteobacteria. Indeed, discordant 16S and 23S rRNA gene phylogenies among Helicobacter species (7) as well as a lack of discrimination among Campylobacter species (12) were reported. Therefore, in this study ribosomal genes were not chosen as a possible target for the PCR. The gyrA gene encoding a subunit of DNA gyrase, an essential bacterial enzyme (33, 35, 40), was reported to be an important tool for bacterial phylogeny (17, 19, 43). Furthermore, a FRET real-time PCR targeting the gyrA gene was previously used as a rapid and sensitive method for identification and differentiation of C. jejuni and C. coli (25), which motivated our choice for this approach.
The results obtained with the 345 stool specimens tested with this PCR assay showed a higher sensitivity than those for culture since no positive culture could be obtained from these specimens. However, slightly higher Tms were observed for the three positive stool samples than for the control isolates. Indeed, 10% of the extracted stool sample was directly used for the assay whereas DNA extracted from the strains was highly diluted before use. These Tm differences observed for the stool samples may be due to the stool composition modifying the final salt concentration and/or the pH of the PCR mix.
With regard to the previously reported PCR assays (4, 13, 15, 16, 18, 26, 32, 41), this FRET real-time PCR assay is of interest for detection and identification of A. butzleri, A. cryaerophilus, A. cibarius, and A. nitrofigilis in a single test. This assay detected Arcobacter species in 1.2% of the stool samples from 345 patients with diarrhea, which is higher than previous estimates for France and Belgium obtained by culture (28, 38). This demonstrates the utility of molecular techniques in the diagnosis of human Arcobacter infections and highlights the fact that human Arcobacter infection is underestimated. Interestingly, another study performed by PCR supports our results since Arcobacter species were detected in 12.9% of the stools of diarrheic patients from South Africa (31). The latest study also revealed the presence of Arcobacter species in 3% of the stools of asymptomatic children from South Africa. Moreover, an Arcobacter-selective isolation procedure revealed the presence of A. cryaerophilus in 1.4% of the fecal samples of asymptomatic humans in Switzerland (14). It would be interesting to use the FRET real-time PCR developed in this study to determine the prevalence of Arcobacter species in healthy subjects in different countries of the world.
Acknowledgments
We are grateful to Leila Labadi, Christine Camou, and Brigitte Tauzin for their technical assistance. Khalil Abdelbaqi was the recipient of a doctoral fellowship of the French government program for the Centre Régional des œuvres Universitaires et Scolaires.
Footnotes
Published ahead of print on 25 July 2007.
REFERENCES
- 1.Abdelbaqi, K., A. Ménard, V. Prouzet-Mauleon, F. Bringaud, P. Lehours, and F. Mégraud. 2007. Nucleotide sequence of the gyrA gene of Arcobacter species and characterization of human ciprofloxacin resistant clinical isolates. FEMS Immunol. Med. Microbiol. 49:337-345. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atabay, H. I., M. Waino, and M. Madsen. 2006. Detection and diversity of various Arcobacter species in Danish poultry. Int. J. Food Microbiol. 109:139-145. [DOI] [PubMed] [Google Scholar]
- 4.Brightwell, G., E. Mowat, R. Clemens, J. Boerema, D. J. Pulford, and S. L. On. 2006. Development of a multiplex and real time PCR assay for the specific detection of Arcobacter butzleri and Arcobacter cryaerophilus. J. Microbiol. Methods 17:17. [DOI] [PubMed] [Google Scholar]
- 5.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer, E., J. J. Tilburg, D. L. Woodward, H. Lior, and W. M. Johnson. 1996. A selective medium for the isolation of Arcobacter from meats. Lett. Appl. Microbiol. 23:64-66. [DOI] [PubMed] [Google Scholar]
- 7.Dewhirst, F. E., Z. L. Shen, M. S. Scimeca, L. N. Stokes, T. Boumenna, T. T. Chen, B. J. Paster, and J. G. Fox. 2005. Discordant 16S and 23S rRNA gene phylogenies for the genus Helicobacter: implications for phylogenetic inference and systematics. J. Bacteriol. 187:6106-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donachie, S. P., J. P. Bowman, S. L. On, and M. Alam. 2005. Arcobacter halophilus sp. nov., the first obligate halophile in the genus Arcobacter. Int. J. Syst. Evol. Microbiol. 55:1271-1277. [DOI] [PubMed] [Google Scholar]
- 9.Ellis, W. A., S. D. Neill, J. J. O'Brien, H. W. Ferguson, and J. Hanna. 1977. Isolation of Spirillum/Vibrio-like organisms from bovine fetuses. Vet. Rec. 100:451-452. [DOI] [PubMed] [Google Scholar]
- 10.Engberg, J., S. L. On, C. S. Harrington, and P. Gerner-Smidt. 2000. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for campylobacters. J. Clin. Microbiol. 38:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrity, G. M., J. A. Bell, and T. Lilburn. 2005. Family II. Helicobacteraceae fam. nov., 2nd ed., vol. 2. Springer, New York, NY.
- 12.Gorkiewicz, G., G. Feierl, C. Schober, F. Dieber, J. Kofer, R. Zechner, and E. L. Zechner. 2003. Species-specific identification of campylobacters by partial 16S rRNA gene sequencing. J. Clin. Microbiol. 41:2537-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harmon, K. M., and I. V. Wesley. 1996. Identification of Arcobacter isolates by PCR. Lett. Appl. Microbiol. 23:241-244. [DOI] [PubMed] [Google Scholar]
- 14.Houf, K., and R. Stephan. 2007. Isolation and characterization of the emerging foodborn pathogen Arcobacter from human stool. J. Microbiol. Methods 68:408-413. [DOI] [PubMed] [Google Scholar]
- 15.Houf, K., A. Tutenel, L. De Zutter, J. Van Hoof, and P. Vandamme. 2000. Development of a multiplex PCR assay for the simultaneous detection and identification of Arcobacter butzleri, Arcobacter cryaerophilus, and Arcobacter skirrowii. FEMS Microbiol. Lett. 193:89-94. [DOI] [PubMed] [Google Scholar]
- 16.Hurtado, A., and R. J. Owen. 1997. A molecular scheme based on 23S rRNA gene polymorphisms for rapid identification of Campylobacter and Arcobacter species. J. Clin. Microbiol. 35:2401-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurtle, W., E. Bode, D. A. Kulesh, R. S. Kaplan, J. Garrison, D. Bridge, M. House, M. S. Frye, B. Loveless, and D. Norwood. 2004. Detection of the Bacillus anthracis gyrA gene by using a minor groove binder probe. J. Clin. Microbiol. 42:179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabeya, H., Y. Kobayashi, S. Maruyama, and T. Mikami. 2003. One-step polymerase chain reaction-based typing of Arcobacter species. Int. J. Food Microbiol. 81:163-168. [DOI] [PubMed] [Google Scholar]
- 19.Kasai, H., T. Tamura, and S. Harayama. 2000. Intrageneric relationships among Micromonospora species deduced from gyrB-based phylogeny and DNA relatedness. Int. J. Syst. Evol. Microbiol. 50:127-134. [DOI] [PubMed] [Google Scholar]
- 20.Kiehlbauch, J. A., D. J. Brenner, M. A. Nicholson, C. N. Baker, C. M. Patton, A. G. Steigerwalt, and I. K. Wachsmuth. 1991. Campylobacter butzleri sp. nov. isolated from humans and animals with diarrheal illness. J. Clin. Microbiol. 29:376-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni, S. P., S. Lever, J. M. Logan, A. J. Lawson, J. Stanley, and M. S. Shafi. 2002. Detection of Campylobacter species: a comparison of culture and polymerase chain reaction based methods. J. Clin. Pathol. 55:749-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau, S. K., P. C. Woo, J. L. Teng, K. W. Leung, and K. Y. Yuen. 2002. Identification by 16S ribosomal RNA gene sequencing of Arcobacter butzleri bacteraemia in a patient with acute gangrenous appendicitis. Mol. Pathol. 55:182-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehner, A., T. Tasara, and R. Stephan. 2005. Relevant aspects of Arcobacter spp. as potential foodborne pathogen. Int. J. Food Microbiol. 102:127-135. [DOI] [PubMed] [Google Scholar]
- 24.Lerner, J., V. Brumberger, and V. Preac-Mursic. 1994. Severe diarrhea associated with Arcobacter butzleri. Eur. J. Clin. Microbiol. Infect. Dis. 13:660-662. [DOI] [PubMed] [Google Scholar]
- 25.Ménard, A., F. Dachet, V. Prouzet-Mauléon, M. Oleastro, and F. Mégraud. 2005. Development of a real-time fluorescence resonance energy transfer PCR to identify the main pathogenic Campylobacter spp. Clin. Microbiol. Infect. 11:281-287. [DOI] [PubMed] [Google Scholar]
- 26.Moreno, Y., S. Botella, J. L. Alonso, M. A. Ferrus, M. Hernandez, and J. Hernandez. 2003. Specific detection of Arcobacter and Campylobacter strains in water and sewage by PCR and fluorescent in situ hybridization. Appl. Environ. Microbiol. 69:1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.On, S. L., A. Stacey, and J. Smyth. 1995. Isolation of Arcobacter butzleri from a neonate with bacteraemia. J. Infect. 31:225-227. [DOI] [PubMed] [Google Scholar]
- 28.Prouzet-Mauléon, V., L. Labadi, N. Bouges, A. Ménard, and F. Mégraud. 2006. Arcobacter butzleri: underestimated enteropathogen. Emerg. Infect. Dis. 12:307-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche Applied Science. 2001. LightCycler probe design software, version 1.0. Roche Applied Science, Mannheim, Germany.
- 30.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365-386. [DOI] [PubMed] [Google Scholar]
- 31.Samie, A., C. L. Obi, L. J. Barrett, S. M. Powell, and R. L. Guerrant. 2007. Prevalence of Campylobacter species, Helicobacter pylori and Arcobacter species in stool samples from the Venda region, Limpopo, South Africa: studies using molecular diagnostic methods. J. Infect. 54:558-566. [DOI] [PubMed] [Google Scholar]
- 32.Snaidr, J., R. Amann, I. Huber, W. Ludwig, and K. H. Schleifer. 1997. Phylogenetic analysis and in situ identification of bacteria in activated sludge. Appl. Environ. Microbiol. 63:2884-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sternglanz, R., S. DiNardo, K. A. Voelkel, Y. Nishimura, Y. Hirota, K. Becherer, L. Zumstein, and J. C. Wang. 1981. Mutations in the gene coding for Escherichia coli DNA topoisomerase I affect transcription and transposition. Proc. Natl. Acad. Sci. USA 78:2747-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor, D. N., J. A. Kiehlbauch, W. Tee, C. Pitarangsi, and P. Echeverria. 1991. Isolation of group 2 aerotolerant Campylobacter species from Thai children with diarrhea. J. Infect. Dis. 163:1062-1067. [DOI] [PubMed] [Google Scholar]
- 35.Trucksis, M., and R. E. Depew. 1981. Identification and localization of a gene that specifies production of Escherichia coli DNA topoisomerase I. Proc. Natl. Acad. Sci. USA 78:2164-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandamme, P., E. Falsen, R. Rossau, B. Hoste, P. Segers, R. Tytgat, and J. De Ley. 1991. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int. J. Syst. Bacteriol. 41:88-103. [DOI] [PubMed] [Google Scholar]
- 37.Vandamme, P., P. Pugina, G. Benzi, R. Van Etterijck, L. Vlaes, K. Kersters, J. P. Butzler, H. Lior, and S. Lauwers. 1992. Outbreak of recurrent abdominal cramps associated with Arcobacter butzleri in an Italian school. J. Clin. Microbiol. 30:2335-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandenberg, O., A. Dediste, K. Houf, S. Ibekwem, H. Souayah, S. Cadranel, N. Douat, G. Zissis, J. P. Butzler, and P. Vandamme. 2004. Arcobacter species in humans. Emerg. Infect. Dis. 10:1863-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Driessche, E., K. Houf, F. Vangroenweghe, L. De Zutter, and J. Van Hoof. 2005. Prevalence, enumeration and strain variation of Arcobacter species in the faeces of healthy cattle in Belgium. Vet. Microbiol. 105:149-154. [DOI] [PubMed] [Google Scholar]
- 40.Wang, B., and H. K. Kuramitsu. 2003. Assessment of the utilization of the antisense RNA strategy to identify essential genes in heterologous bacteria. FEMS Microbiol. Lett. 220:171-176. [DOI] [PubMed] [Google Scholar]
- 41.Wesley, I. V., L. Schroeder-Tucker, A. L. Baetz, F. E. Dewhirst, and B. J. Paster. 1995. Arcobacter-specific and Arcobacter butzleri-specific 16S rRNA-based DNA probes. J. Clin. Microbiol. 33:1691-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirsen, C. O., S. M. Sievert, C. M. Cavanaugh, S. J. Molyneaux, A. Ahmad, L. T. Taylor, E. F. DeLong, and C. D. Taylor. 2002. Characterization of an autotrophic sulfide-oxidizing marine Arcobacter sp. that produces filamentous sulfur. Appl. Environ. Microbiol. 68:316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto, S., H. Kasai, D. L. Arnold, R. W. Jackson, A. Vivian, and S. Harayama. 2000. Phylogeny of the genus Pseudomonas: intrageneric structure reconstructed from the nucleotide sequences of gyrB and rpoD genes. Microbiology 146:2385-2394. [DOI] [PubMed] [Google Scholar]
- 44.Yan, J. J., W. C. Ko, A. H. Huang, H. M. Chen, Y. T. Jin, and J. J. Wu. 2000. Arcobacter butzleri bacteremia in a patient with liver cirrhosis. J. Formos. Med. Assoc. 99:166-169. [PubMed] [Google Scholar]