Abstract
Although transmissible metallo-β-lactamases (MBLs) are a serious threat to β-lactam antibiotic therapy, the CLSI currently does not recommend testing methods for the detection of MBLs. The aim of this study was to evaluate the capability of double-disk tests (DDTs) by using disks containing a combination of the chelators 2-mercaptopropionic acid (MPA) and Tris-EDTA (TE) to detect MBLs. Sixteen isolates (4 Acinetobacter baumannii isolates, 6 Pseudomonas aeruginosa isolates, 1 Serratia marcescens isolate, 1 Aeromonas hydrophila isolate, 1 Aeromonas veronii isolate, 2 Chryseobacterium meningosepticum isolates, and 1 Stenotrophomonas maltophilia isolate) producing IMP-1, IMP-1-like, IMP-18, GIM-1, SPM-1, VIM-2, VIM-2-like, and chromosomal MBLs and 20 isolates (7 Klebsiella pneumoniae isolates, 3 Escherichia coli isolates, 5 Enterobacter cloacae isolates, 2 S. marcescens isolates, 1 Proteus mirabilis isolate, and 2 A. baumannii isolates) producing non-MBL carbapenemases, AmpC β-lactamases, and extended-spectrum β-lactamases were tested. The DDT method was evaluated by using four types of chelator disks (TE, high-strength TE, MPA, and TE plus 20 μl of MPA [at various concentrations]) and the β-lactams imipenem (IPM), meropenem (MEM), ertapenem (ERT), and ceftazidime (CAZ). DDTs with IPM and a TE disk supplemented with 1:320 MPA detected all MBLs and yielded no false-positive results. Some, but not all, MBL producers were detected in IPM-based tests involving the single chelator TE or MPA alone or by ERT- or CAZ-based tests. IPM-based tests with MPA concentrations other than 1:320 and all MEM-based tests had suboptimal sensitivities or specificities. DDT with IPM and a TE disk supplemented with 20 μl of 1:320 MPA appears to be convenient for the detection of MBLs in the clinical laboratory.
Carbapenems are the drugs of choice for the treatment of infections caused by multiresistant gram-negative bacilli. Carbapenemases involved in acquired resistance are of Ambler molecular classes A, B, and D (4). The class B enzymes, metallo-β-lactamases (MBLs), are the most clinically threatening carbapenemases, because they are capable of hydrolyzing all β-lactams except aztreonam. Gram-negative bacilli producing acquired MBLs have been reported in many countries and are sometimes carbapenem susceptible in vitro under standard conditions, making them difficult to recognize (4, 7). Therefore, accurate detection is important for optimal therapy and infection control precautions. Recently, an E-test MBL strip (AB Biodisk, Solna, Sweden) was recommended for use for screening for MBLs in clinical laboratories, but it has been reported to be unable to detect all MBL-positive members of the family Enterobacteriaceae and also to give false-positive results with some Pseudomonas aeruginosa and Acinetobacter baumannii strains (5, 7). Although various MBL detection techniques have been investigated, there are currently no perfect phenotypic methods for the detection of all transferable MBLs (7).
Here we report on a study performed to evaluate the utility of double-disk tests (DDTs) involving disks containing imipenem (IPM), meropenem (MEM), ertapenem, (ERT), and ceftazidime (CAZ); disks containing low and high concentrations of Tris-EDTA (TE); disks containing 2-mercaptopropionic acid (MPA); and TE disks supplemented with MPA for the detection of MBL producers.
MATERIALS AND METHODS
Bacterial strains.
Sixteen isolates (4 A. baumannii isolates, 6 P. aeruginosa isolates, 1 Serratia marcescens isolate, 1 Aeromonas hydrophila isolate, 1 Aeromonas veronii isolate, 2 Chryseobacterium meningosepticum isolates, and 1 Stenotrophomonas maltophilia isolate) producing IMP-1, IMP-1-like, IMP-18, GIM-1, SPM-1, VIM-2, VIM-2-like, and chromosomal MBLs and 20 isolates (7 Klebsiella pneumoniae isolates, 3 Escherichia coli isolates, 5 Enterobacter cloacae isolates, 2 S. marcescens isolates, 1 Proteus mirabilis isolate, and 2 A. baumannii isolates) producing class A and D carbapenemases (IMI-1, KPC-2, KPC-3, NMC-A, SME-like, and OXA-23), AmpC β-lactamases, and extended-spectrum β-lactamases (ESBLs) were studied (Table 1).
TABLE 1.
Comparison of methods for MBL detection
| Enzyme | Organism (no. of isolates) | No. of isolates with positive result or no. of positive isolates/total no. of isolates evaluated by the following test:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IPM
|
MEM with TE + MPA disksa
|
TE + 1:80 MPA disks
|
|||||||||
| TE disk aloneb | MPA disk alonec | TE + MPA disksa
|
1:80 MPA | 1:320 MPA | 1:640 MPA | ERT | CAZ | ||||
| 1:80 MPAd | 1:320 MPA | 1:640 MPA | |||||||||
| MBLs | |||||||||||
| IMP-1 | A. baumannii (2) | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| IMP-1-like | S. marcescens (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| IMP-18 | P. aeruginosa (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| VIM-2 | A. baumannii (2) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| P. aeruginosa (2) | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | |
| VIM-2-like | P. aeruginosa (1) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| GIM-1 | P. aeruginosa (1) | 1e | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| SPM-1 | P. aeruginosa (1) | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| Chromosomal | A. hydrophila (1) | NDf | ND | ND | 1 | ND | ND | ND | ND | ND | ND |
| Chromosomal | A. veronii (1) | ND | ND | ND | 1 | ND | ND | ND | ND | ND | ND |
| Chromosomal | C. meningosepticum (2) | ND | ND | ND | 2 | ND | ND | ND | ND | ND | ND |
| Chromosomal | S. maltophilia (1) | ND | ND | ND | 1 | ND | ND | ND | ND | ND | ND |
| Total | (16) | 8/11 | 10/11 | 11/11 | 16/16 | 9/11 | 11/11 | 9/11 | 7/11 | 6/11 | 10/11 |
| Non-MBLs | |||||||||||
| ACT-1 | K. pneumoniae (1) | NDg | ND | 1 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| ACT-1 + ESBL | K. pneumoniae (1) | ND | ND | 0 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| CMY | E. coli (1) | ND | ND | 1 | 0 | 0 | 1 | 0 | 0 | ND | ND |
| DHA-1 | K. pneumoniae (1) | ND | ND | 0 | 0 | 0 | 1 | 0 | 0 | ND | ND |
| FOX | K. pneumoniae (1) | ND | ND | 1 | 0 | 0 | 1 | 0 | 0 | ND | ND |
| LAT | E. coli (1) | ND | ND | 1 | 0 | 0 | 1 | 1 | 0 | ND | ND |
| AmpC + porin mutation | E. cloacae (1) | ND | ND | 0 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| SHV-5-like | K. pneumoniae (1) | ND | ND | 1 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| TEM ESBL | P. mirabilis (1) | ND | ND | 1 | 0 | 0 | 1 | 0 | 0 | ND | ND |
| VEB-3 | E. cloacae (1) | ND | ND | 1 | 0 | 0 | 1 | 0 | 0 | ND | ND |
| IMI-1 | E. cloacae (1) | ND | ND | 0 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| KPC-2 | K. pneumoniae (1) | ND | ND | 1 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| KPC-2 + ESBL | K. pneumoniae (1) | ND | ND | 0 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| KPC-3 | E. coli (1) | ND | ND | 0 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| NMC-A | E. cloacae (2) | ND | ND | 1 | 0 | 0 | 2 | 2 | 0 | ND | ND |
| SME-like | S. marcescens (2) | ND | ND | 0 | 0 | 0 | 2 | 2 | 0 | ND | ND |
| OXA-23 | A. baumannii (2) | ND | ND | 0 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| Total | (20) | 9 | 0 | 0 | 10 | 5 | 0 | ||||
| % Sensitivity | 72.7 | 90.9 | 100 | 100 | 81.8 | 100 | 81.8 | 63.6 | 54.5 | 90.9 | |
| % Specificity | NDh | ND | 55 | 100 | 100 | 50 | 75 | 100 | ND | ND | |
The overall sensitivities and specificities of DDTs with 1:160 MPA were 100 and 80%, respectively, for the IPM-based tests and 100 and 60%, respectively, for the MEM-based tests. The sensitivities and specificities for the 1:1,280 MPA-based tests were the same as those with 1:640 MPA.
TE alone, tests with both standard BD-manufactured TE disks and also disks to which additional TE solution (1:16 or undiluted) was added.
MPA alone, tests with blank disks to which MPA solution (1:80 or 1:320) was added.
The MPA dilutions tested were 1:80, 1:160, 1:320, 1:640, and 1:1,280.
The unsupplemented TE disk failed to detect one GIM-1-producing P. aeruginosa isolate, whereas the high-strength TE disk detected this isolate.
ND, tests with chromosomal MBL producers were not done by these methods because they were shown to be suboptimal with isolates that produced plasmid-mediated MBLs.
ND, tests with non-MBL producers were not done because initial tests with MBL-producing isolates indicated that this test was too insensitive for further investigation.
ND, specificity could not be determined.
Detection of MBLs.
Four types of disks containing chelating agents were investigated. These were (i) TE disks (BD Diagnostic Systems, Sparks, MD) premoistened with 20 μl saline; (ii) TE disks supplemented with 20 μl of 1:80, 1:160, 1:320, 1:640, and 1:1,280 dilutions of MPA (T31003-5G; Sigma-Aldrich Corp., St. Louis, MO); (iii) high-strength TE disks, i.e., TE disks supplemented with 20 μl of either undiluted TE or a 1:16 dilution of 100× TE (T-9285; Sigma-Aldrich Corp.); and (iv) MPA disks, i.e., blank disks supplemented with 20 μl of a 1:80 or 1:320 dilution of MPA. The test strains were adjusted to a McFarland 0.5 standard and inoculated as lawns onto Mueller-Hinton agar plates (Oxoid Ltd., Basingstoke, England). Disks containing 10 μg IPM, MEM, or ERT or 30 μg CAZ (BD Diagnostic Systems) were placed on the surface, and the chelator disk was placed 10 mm (edge to edge) from the β-lactam disk. After incubation overnight at 37°C, any clear extension of the inhibition zone around the carbapenem disk toward the TE disk was interpreted as a positive result.
RESULTS
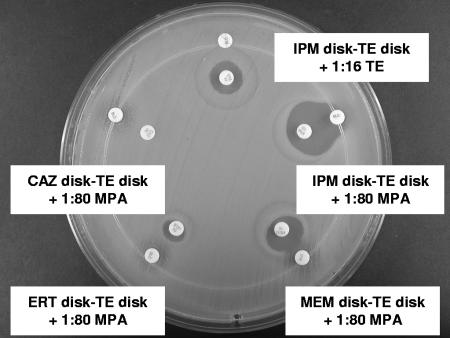
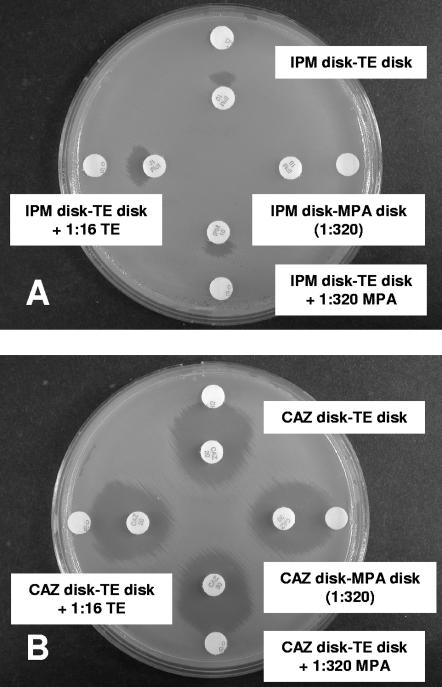
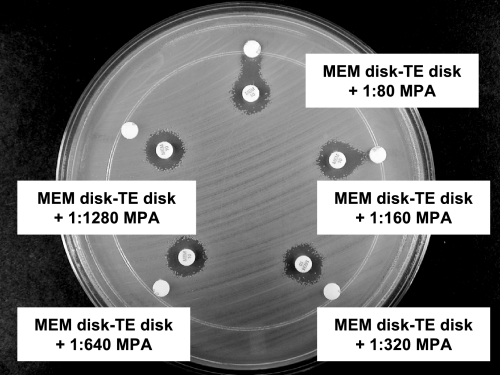
As shown in Table 1, the IPM-based test with a TE disk supplemented with 1:320 MPA provided the most sensitive (100%) and specific (100%) test for MBL detection. In tests with IPM, the unsupplemented or high-strength TE disks did not detect IMP-1 in two isolates of A. baumannii (Fig. 1) and SPM-1 in one isolate of P. aeruginosa. MPA disks alone failed to detect a VIM-2-like MBL in one isolate of P. aeruginosa (Fig. 2A). Three of the five chromosomal MBL-producing isolates (two C. meningosepticum isolates and one S. maltophilia isolate) yielded strongly positive results with IPM and a TE disk supplemented with 1:320 MPA, but two isolates (one A. hydrophila isolate and one A. veronii isolate) yielded only weakly positive results. Higher MPA concentrations (1:80 and 1:160) were associated with false-positive results in tests with IPM and some class A carbapenemase-, AmpC-, and ESBL-producing isolates; and lower MPA concentrations (1:640 and 1:1,280) reduced the sensitivity of the test. MEM-based tests with lower MPA concentrations (1:320 to 1:1,280) had suboptimal sensitivities (63.6 to 81.8%), while those with higher MPA concentrations (1:80 to 1:320) had suboptimal specificities (50 to 75%) (Table 1 and Fig. 3). ERT-based tests were too insensitive (54.5%) with the highest MPA concentration (1:80). CAZ-based tests exhibited larger inhibition zones in tests with five P. aeruginosa isolates, including one VIM-2-like MBL-producing P. aeruginosa isolate, than the corresponding IPM-based tests (Fig. 2), but were less sensitive because they failed to detect one IMP-1-producing A. baumannii isolate (Fig. 1).
FIG. 1.
IMP-1-producing A. baumannii. Positive test results (expanded inhibition zones) were obtained with MPA-supplemented TE disks in tests with IMP, MEM, and ERT. The results of the tests with the MPA-supplemented TE disk with CAZ and the high-strength TE disk with IMP were negative.
FIG. 2.
VIM-2-like MBL-producing P. aeruginosa. (A) IPM-based tests showed positive results with different chelating agents, excluding MPA alone. (B) CAZ-based tests showed positive results with all chelating agents used and inhibition zones larger than those obtained by the IPM-based tests.
FIG. 3.
False-positive results in tests with NMC-A-producing E. cloacae by using MEM and various concentrations of MPA added to the TE disks. Tests with high concentrations of MPA ranging from 1:80 to 1:320 showed false-positive results.
DISCUSSION
It is necessary to perform MBL detection tests with suspicious isolates because these enzymes cannot be identified by routine susceptibility tests. Suspicious isolates are those with significantly reduced susceptibility to carbapenems, e.g., E. coli and Klebsiella isolates with imipenem MICs of 1 μg/ml or higher; Enterobacter, Citrobacter freundii, and S. marcescens isolates with imipenem MICs of 4 μg/ml or higher; and P. aeruginosa isolates with imipenem MICs of 8 μg/ml or higher. Tests involving chelating agents such as EDTA and MPA are useful because the chelators are specific inhibitors of MBLs but not of other β-lactamases (1-3, 5-8). A single chelating agent may sometimes not adequately inhibit all MBLs in certain pathogens, making it necessary to use a mixture of chelating agents for the reliable detection of MBLs.
The IPM-based test involving TE alone did not detect two IMP-1-producing A. baumannii isolates (Fig. 1) and one SPM-1-producing P. aeruginosa isolate, whereas The IPM-based test involving MPA alone failed to detect one VIM-2-like MBL-producing P. aeruginosa isolate. Positive tests with these isolates were obtained only when an MPA-supplemented TE disk was used. The finding that two chelators were needed was consistent with the findings presented in the report of Lee et al. (3) that EDTA disks were more sensitive than sodium mercaptoacetic acid (SMA) disks for the detection of MBL-producing pseudomonads, while SMA disks provided more sensitive tests for the detection of MBL-producing acinetobacters. The MPA concentration used for supplementation of the TE disks was critical, with a 1:320 dilution providing optimal specificity and sensitivity.
The tests with the MEM, ERT, and CAZ disks yielded some inaccurate results and were less satisfactory than the tests with IPM disks. Arakawa et al. (1) reported that CAZ disks used in conjunction with MPA provided optimal MBL detection because MBL producers were usually highly resistant to CAZ (MIC, >64 μg/ml). However, Lee et al. (3) reported that MBL production by some Acinetobacter isolates was difficult to detect by tests based on CAZ disks and attributed this to the possibility that the isolates may have additional CAZ resistance mechanisms, such as ESBL and AmpC production. In our study, the MBL activity of one Acinetobacter isolate was not detected with the CAZ disk, although this isolate was highly resistant to CAZ (MIC, >64 μg/ml).
Our findings with 16 MBL-producing isolates that produced IMP-1, IMP-1-like, IMP-18, GIM-1, SPM-1, VIM-2, VIM-2-like, and chromosomal MBLs suggest that a convenient and accurate screening method suitable for use in clinical laboratories for the detection of MBL producers may be provided by use of the IPM and TE disk supplemented with 20 μl of 1:320 MPA. Because only a modest number of isolates that produced MBLs were included in this study, it is necessary for further evaluation of this method to be undertaken.
Acknowledgments
We thank the following microbiologists for isolates used in this study: K. Lee, Yonsei University College of Medicine, Seoul, Korea; M. Inoue, Kitasato University School of Medicine, Sagamihara, Japan; and L. M. Deshpande, JMI Laboratories, North Liberty, IA.
Footnotes
Published ahead of print on 27 June 2007.
REFERENCES
- 1.Arakawa, Y., N. Shibata, K. Shibayama, H. Kurokawa, T. Yagi, H. Fujiwara, and M. Goto. 2000. Convenient test for screening metallo-β-lactamase-producing gram-negative bacteria by using thiol compounds. J. Clin. Microbiol. 38:40-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee, K., Y. Chong, H. B. Shin, Y. A. Kim, D. Yong, and J. H. Yum. 2001. Modified Hodge and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin. Microbiol. Infect. 7:88-91. [DOI] [PubMed] [Google Scholar]
- 3.Lee, K., Y. S. Lim, D. Yong, J. H. Yum, and Y. Chong. 2003. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-β-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 41:4623-4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordmann, P., and L. Poirel. 2002. Emerging carbapenemases in gram-negative aerobes. Clin. Microbiol. Infect. 8:321-331. [DOI] [PubMed] [Google Scholar]
- 5.Segal, H., and B. G. Elisha. 2005. Use of Etest MBL strips for the detection of carbapenemases in Acinetobacter baumannii. J. Antimicrob. Chemother. 56:598. [DOI] [PubMed] [Google Scholar]
- 6.Walsh, T. R., A. Bolmstrom, A. Qwarnstrom, and A. Gales. 2002. Evaluation of a new Etest for detecting metallo-β-lactamases in routine clinical testing. J. Clin. Microbiol. 40:2755-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-β-slactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yong, D., K. Lee, J. H. Yum, H. B. Shin, G. M. Rossolini, and Y. Chong. 2002. Imipenem-EDTA disk method for differentiation of metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 40:3798-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]