Abstract
Laboratory tests are essential for confirming sporadic cases and outbreaks of rubella. Detection of rubella virus is often necessary to confirm rubella cases and to identify specimens to be used to characterize wild-type rubella viruses. The sensitivities of four methods for detecting rubella virus infection using throat swabs, which had been collected in Henan and Anhui provinces in China, were evaluated. The methods used were reverse transcription (RT)-PCR followed by Southern hybridization using RNA extracted directly from clinical specimens, virus growth in tissue culture followed by virus detection by RT-PCR, low-background immunofluorescence in infected tissue culture cells using monoclonal antibodies to the structural proteins of rubella virus, and a replicon-based method of detecting infectious virus. Among these four methods, direct RT-PCR followed by hybridization was the most sensitive method; the replicon-based method was the least difficult to perform.
Rubella is a mild rash disease with few complications. However, rubella virus infection early in pregnancy often causes death of the fetus and, if the fetus survives, causes congenital defects in about 90% of the newborns. The major defects include deafness, cataracts, and heart disorders, which are collectively known as congenital rubella syndrome (CRS) (12).
Although the elimination of rubella and CRS has been achieved in the United States with an effective vaccination program, in countries without an immunization program or without a good program, rubella is still endemic (11, 21), and explosive outbreaks may occur (9, 13). It was estimated in 2003 that more than 100,000 infants were born with CRS each year worldwide (16). In general, a large proportion of unimmunized populations in areas where rubella is endemic are infected and become immune before puberty. Nevertheless, approximately 3 to 23% of adults remain susceptible in various countries and areas (4, 8, 15, 17).
Routine rubella vaccination is not included in the national immunization program in China, although rubella vaccine is available in certain urban areas. A seroprevalence study in 1993 to 1995 of 2,610 women aged 16 to 30 years in five provinces in China found that only 83.6% were immune to rubella. Thus, CRS is still a public health concern in China (21). An estimate of the incidence of CRS in China in 2005 was at least 20,000 cases per year (14). Several large rubella outbreaks have been reported in different regions since 1987, including Shandong province and Hangzhou city (1987) (25, 27), Shanghai city (1993 to 1994; nearly 60,000 cases), and Beijing city (1994; over 18,000 cases) (21). Examples of recently reported outbreaks include those in Guangxi province (2000; more than 1,200 cases) (23), Yunnan province (2000; more than 2,100 cases) (1), and Anhui province (2001; nearly 5,000 cases) (26).
Two different rubella vaccines are currently available in China. The BRDII attenuated vaccine strain of rubella virus (Tian Tan Biological Products Corporation, Beijing, China) was introduced in 1994 in some parts of China including the city of Shanghai and Shandong province. In addition, an imported measles-mumps-rubella vaccine containing the rubella virus vaccine strain RA27/3 (Merck and Co., Inc., Whitehouse Station, NJ) has been available since 1996 in large cities (20, 24). Since the mid-1990s, despite large outbreaks, the incidence of rubella in China has dramatically decreased (6). Unfortunately, a rubella vaccine has not yet been introduced into the Chinese national immunization program, and the disease is still epidemic in rural areas.
In order to achieve the goal of measles elimination, because rubella is often the final diagnosis of suspected measles cases, the World Health Organization (WHO) has decided to include laboratory testing for rubella in the measles surveillance system and has established a global measles and rubella laboratory network. Approximately 30 to 50% of suspected measles cases turn out to be rubella cases (16).
A common laboratory confirmation of rubella cases is the detection of rubella-specific immunoglobulin M (IgM) in sera from patients with suspected rubella virus infection. In postnatal rubella virus infections, IgM is often not detectable until several days after the rash appears. False-negative results may be obtained if only a single serum sample taken around the day of rash onset is collected, the most likely day for serum collection. However, virus is usually present in the throat or nasopharynx from a few days before until about 5 days after the onset of rash, making virus detection a possible accessory test for diagnosis (2). Furthermore, detection of rubella virus in clinical specimens is necessary for important control activities such as molecular epidemiology.
Rubella virus is the sole member of the genus Rubivirus in the family Togaviridae. The genome of rubella virus is a single-stranded RNA of positive polarity. It contains a 5′-proximal open reading frame (ORF) that encodes nonstructural proteins, which are responsible for viral genome replication, and a 3′-proximal ORF, which encodes three structural proteins, C, E2, and E1 (10). In some studies, reporter genes such as green fluorescent protein (GFP) and chloramphenicol acetyltransferase were used to replace all or part of the 3′-proximal ORF (18). Such modified rubella virus RNAs (replicons) can still replicate inside cultured cells but are not infectious because they lack some or all of the structural proteins necessary for assembly of virions. Expression of the capsid protein (C) has been shown to enhance the replication of replicons (5).
Several laboratory techniques were used in the present work to detect rubella virus in clinical specimens, including reverse transcription (RT)-PCR using rubella virus RNA extracted from a clinical specimen followed by Southern hybridization (RT-PCR plus hybridization), RT-PCR using RNA recovered from infected tissue culture (culture plus RT-PCR), a low-background immunofluorescent assay (IFA) to detect viral proteins in infected tissue culture cells, and a replicon-based method to detect infectious virus (replicon cells). Culture plus RT-PCR is well established for detecting infectious virus in clinical samples. The IFA for rubella virus-infected cells used here was implemented using monolayer culture and monoclonal antibodies to the rubella proteins, since both were necessary to reduce background; low background is essential for good specificity in IFA detection of rubella virus-infected cells. The sensitive RT-PCR plus hybridization and the replicon-based diagnosis are newly developed methods for detecting rubella virus. Work showing a proof of concept that replicons can be used for the detection of rubella virus-infected cells was reported previously (19). For the current study, a rubella virus replicon cell line was established with BHK cells expressing C lacking the first 8 amino acids and using a rubella virus replicon capable of expressing GFP. All four methods were compared using 22 throat swab samples from patients from Henan and Anhui provinces with clinically diagnosed rubella virus infection.
MATERIALS AND METHODS
Clinical specimens.
Twenty-two throat swabs specimens were collected from patients in China with clinically suspected rubella virus infection (Table 1). Thirteen specimens were collected from an outbreak in 2001 and 2002 in Henan province, and nine were collected from a single outbreak in 2000 and 2001 in Anhui province.
TABLE 1.
Epidemiologic information and laboratory results from clinical rubella cases
| Sample | Province | No. of days after rash when specimen was collected | Rubella IgM result | City, yrc | Direct detection
|
Cell-based detection (virus isolation) resulta
|
|||
|---|---|---|---|---|---|---|---|---|---|
| RT-PCR | RT-PCR+ hybridization | RT-PCR | IFA | Replicon | |||||
| RVTS17 | Henan | 1 | − | Xinyang, 2001 | − | + | P1− P2− P3− | − | − |
| RVTS18 | Henan | 3 | + | Xinyang, 2001 | + | + | P1+ | + | + |
| RVTS19 | Henan | 5 | − | Xinyang, 2001 | + | + | P1+ | + | + |
| RVTS20 | Henan | 1 | + | Xinyang, 2001 | + | + | P1+ | + | + |
| RVTS21 | Henan | 2 | + | Xinyang, 2001 | + | + | P1+ | + | + |
| RVTS22 | Henan | 5 | + | Xinyang, 2001 | − | + | P1− P2− P3− | − | − |
| RVTS23 | Henan | 1 | − | Xinyang, 2001 | + | + | P1− P2− P3− | − | − |
| RVTS24 | Henan | 1 | NAb | Puyang, 2002 | + | + | P1− P2− P3− | − | − |
| RVTS25 | Henan | 0 | NA | Puyang, 2002 | + | + | P1+ | + | + |
| RVTS26 | Henan | 0 | + | Xuchang, 2002 | + | + | P1+ | + | + |
| RVTS27 | Henan | 0 | + | Xuchang, 2002 | + | + | P1+ | + | + |
| RVTS28 | Henan | 1 | + | Xuchang, 2002 | + | + | P1+ | + | + |
| RVTS29 | Henan | 1 | + | Xuchang, 2002 | − | + | P1+ | + | + |
| RVTS30 | Anhui | 2 | + | Heifei, 2001 | + | + | P1+ | + | + |
| RVTS31 | Anhui | 1 | + | Shucheng, 2001 | + | + | P1+ | + | + |
| RVTS32 | Anhui | 2 | + | Shucheng, 2001 | − | + | P1+ | + | + |
| RVTS33 | Anhui | 3 | + | Anqing, 2000 | − | + | P1+ | + | + |
| RVTS34 | Anhui | 3 | + | Anqing, 2000 | − | + | P1+ | + | + |
| RVTS35 | Anhui | 2 | + | Heifei, 2000 | − | − | P1− P2− P3− | − | − |
| RVTS36 | Anhui | 2 | + | Dangshan, 2000 | + | + | P1+ | + | + |
| RVTS37 | Anhui | 2 | + | Dangshan, 2000 | + | + | P1+ | + | + |
| RVTS38 | Anhui | 2 | + | Dangshan, 2000 | + | + | P1+ | + | + |
P2 and P3 refer to the passage numbers in Vero cell cultures.
Not available because patient was not tested for rubella virus-specific IgM.
Specimens from outbreaks in Henan (2001 and 2002) and Anhui (2000 and 2001).
IgM testing.
Indirect rubella IgM kits used in Henan province were purchased from Hangzhou Everlong Biochemical Products Company (Hangzhou city, Zhejiang province, China), and indirect IgM kits used in Anhui province were from Hainan Chemical Products Company (Haikou city, Hainan province, China). All of the serologic tests for rubella IgM reported here were done by provincial laboratory staffs. All IgM results reported here were confirmed at the National Institute for Viral Disease Control and Prevention using an indirect test kit (Dade Behring, Mannheim, Germany).
RT-PCR.
Viral RNA from throat swabs was extracted using Tri-Reagent LS for liquid samples (Molecular Research Center, Cincinnati, OH) according to the manufacturer's protocol. RT-PCR was performed using Superscript II One-Step RT-PCR (Invitrogen, Carlsbad, CA), according to the manufacturer's recommendations, to amplify a 185-nucleotide region in the E1 coding region (nucleotides 8807 to 8991) using a forward primer (RV11 [5′-CAA CAC GCC GCA CGG ACA AC-3′]) and a reverse primer (RV12 [5′-CCA CAA GCC GCG AGC AGT CA-3′]). After the RT step for 30 min at 55°C and denaturation for 5 min at 95°C, the reaction mixtures were incubated for 35 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min, followed by 72°C for 5 min. These primers and cycling conditions were based on a previously reported RT-PCR system that gave a DNA product from rubella virus and not from 16 other RNA viruses (3). The system used here gave no product when tested with Sindbis virus-infected cells. Sindbis virus is an Alphavirus in the family Togaviridae that is related to rubella virus. Negative controls (five reactions with mixtures containing water instead of template) were included with each set of clinical specimens. If any control was contaminated, all results from the run were discarded. The positive control was RNA from a rubella virus laboratory strain (Therien). The PCR products were resolved on a 2% agarose gel and visualized by ethidium bromide staining.
Hybridization of RT-PCR products.
To perform Southern hybridization, DNA in the agarose gel was denatured using 1.5 M NaCl-0.5 M NaOH followed by neutralization using 1.5 M NaCl-0.5 M Tris (pH 7.5), and the DNA was transferred onto a nylon membrane by standard blotting techniques. Hybridization was done using agarose gel-purified, digoxigenin (DIG)-labeled probe in DIG Easy Hyb for 3 h at 44°C (Roche Molecular Biochemicals, Mannheim, Germany). The 143-bp DNA probe was made by RT-PCR using a forward primer (RV13 [5′-CTC GAG GTC CAG GTC CYG CC-3′]) and a reverse primer (RV14 [5′-GAA TGG CGT TGG CAA ACC GG-3′]), DIG-labeled dUTPs (Roche Molecular Biochemicals, Mannheim, Germany), and Therien RNA. These primers were part of a nested set reported previously (3). Detection of the PCR products after hybridization was done using horseradish peroxidase-conjugated anti-DIG antibody followed by chemiluminescence with the detection of emitted light on film. The method of RT-PCR plus hybridization described here gave no signal with Sindbis virus-infected cells.
Protocol for culture plus RT-PCR.
Vero cells in 35-mm plates were inoculated with material from throat swabs according to standard methods (2). Briefly, cells inoculated with clinical material were incubated at 35°C for 7 days. The culture medium was harvested and used to inoculate fresh cells for two additional 7-day passages. Total RNA was extracted from the cells at each passage of the culture using Tri reagent (Molecular Research Center, Cincinnati, OH). The detection of rubella virus-specific RNA by RT-PCR was done as described above, without hybridization. Negative results after each of three passages were required for a specimen to be considered negative.
Mock-infected Vero cells were always carried through this protocol with each set of clinical specimens as a negative control. The positive control was RNA from Therien-infected Vero cells, which were cultured separately from clinical specimens to avoid contamination during multiple passages in tissue culture.
IFA protocol.
IFA was done using cells grown in a monolayer and inoculated with supernatant from the first culture passage. At 3 days postinoculation, cells were fixed with 2% paraformaldehyde on ice and permeabilized with 100% methanol. The presence of virus was determined by detection of viral structural proteins in cells using rubella-specific mouse monoclonal antibodies to the E1 (monoclonal antibody 1-6), E2 (monoclonal antibody 26-24), and C (monoclonal antibody 2-36) proteins (Viral Antigens, Memphis, TN) and AlexaFluor 488-conjugated goat anti-mouse secondary antibody (Molecular Probes, Portland, OR). The cell nuclei were counterstained with propidium iodide, and the result was examined by using a fluorescence microscope. The use of this low-background IFA protocol was necessary since rubella virus replicates to relatively low levels compared to levels of other viruses (e.g., measles virus). Since this assay was done with the supernatant from the first passage, this method is considered to be based on the detection of the presence of virus at the second passage of the clinical specimen.
Protocol for replicon-based detection of infectious rubella virus.
A cell line was established using BHK cells expressing C*, which has two stop codons and an extra G residue immediately after the initiation codon for C, by using pCI-Neo (Promega, Madison, WI); a second start codon is presumably used, resulting in C* lacking the N-terminal 8 amino acids (5). Both replicons and rubella virus replicate better in cells expressing C* than in BHK cells (M.-H. Chen, unpublished results).
The rubella virus replicon (RUBdsPAC/GFP400) in the C*-expressing BHK cell line contained duplicated rubella virus intergenic regions, where RUB represents rubella virus, ds represents duplicate regions containing the promoter for subgenomic RNA, and PAC represents puromycin resistance). Intergenic regions are thought to contain the promoter for rubella virus subgenomic RNA synthesis; this replicon also contained the nonstructural protein coding region and the 3′-terminal 400 nucleotides of the rubella genome. A puromycin resistance gene was inserted after the first subgenomic promoter, and the gene encoding GFP was inserted after the second subgenomic promoter. Replicons are not packaged in BHK cells expressing C* due to the lack of E1 and E2 proteins.
The presence of helper virus (in this case, virus from the clinical samples) was determined by transferring the supernatant from the replicon cell culture (C*_RUBdsPAC/GFP400), which had been inoculated with clinical specimens, onto uninfected Vero cells. If infectious virus was present in the clinical specimen, the replicons were packaged and transferred onto the uninfected Vero cells, and the expression of the reporter gene (GFP gene) was visible in these Vero cells.
To detect infectious virus in the clinical specimens, clinical specimens were inoculated onto replicon cells and incubated for 5 to 7 days. The replicon cultures were centrifuged, and the supernatants were transferred onto uninfected Vero cells. At about 24 to 48 h postinfection, if infectious virus was present in clinical samples, expression of the GFP gene could be observed in the Vero cells with a fluorescence microscope. The negative control in these assays was mock-infected cells, which always gave no fluorescence. Sindbis virus-infected cells were repeatedly tested with this assay and never gave any fluorescent cells. Because replicon-based detection of rubella virus requires additional passage in Vero cells to examine GFP expression, replicon results are considered to be at the second passage of virus in tissue culture.
RESULTS
Twenty-two throat swab specimens were analyzed in this study (Table 1). The 22 samples were from Henan and Anhui provinces, all were clinically diagnosed as rubella, and 17 were laboratory confirmed by IgM. Test results using the four methods are summarized in Table 2. By RT-PCR alone, 68% of the specimens (15/22) tested positive. The sensitivity of direct RT-PCR was much improved after hybridization; six more samples became positive (91% of the clinical cases), including two specimens with negative serological results (Table 1).
TABLE 2.
Comparison of sensitivities of techniques for detecting infectious rubella virus or viral RNA using specimens from Anhui and Henan
| Techniquea | No. of patients with clinical rubellab | No. of positive samples | % Positive samplesc |
|---|---|---|---|
| RT-PCR | 22 | 15 | 68 |
| RT-PCR + hybridization | 22 | 21 | 91 |
| IFA | 22 | 17 | 77 |
| Culture + RT-PCR passage 1 | 22 | 17 | 77 |
| Total after passage 2 | 22 | 17 | 77 |
| Total after passage 3 | 22 | 17 | 77 |
| Replicon cells | 22 | 17 | 77 |
See Table 1 for results for individual specimens.
Three patients were rubella IgM negative, and two patients were not tested for IgM.
Percentage of clinical rubella cases that tested positive.
Approximately 77% (17/22) of clinically defined rubella cases were positive using any of the three techniques using culture. Using RT-PCR plus hybridization as the standard, 81% (17/21) of the positive specimens were identified using culture techniques. The results from all three culture-based methods agreed with one another. None of the 22 specimens was negative by the method of culture plus RT-PCR after the first passage and positive after the second or third passage (Table 1).
Representative results from the direct RT-PCR assay before (Fig. 1A) and after (Fig. 1B) Southern hybridization, from the IFA assay (Fig. 2A), and from the replicon-based assay (Fig. 2B) are shown. In the current study, negative specimens such as RVTS35 had no fluorescent cells, and the positive specimens such as RVTS20 had many fluorescent cells. However, since the background in the replicon-based assay is low, even a single fluorescent cell would have been considered a positive result.
FIG. 1.
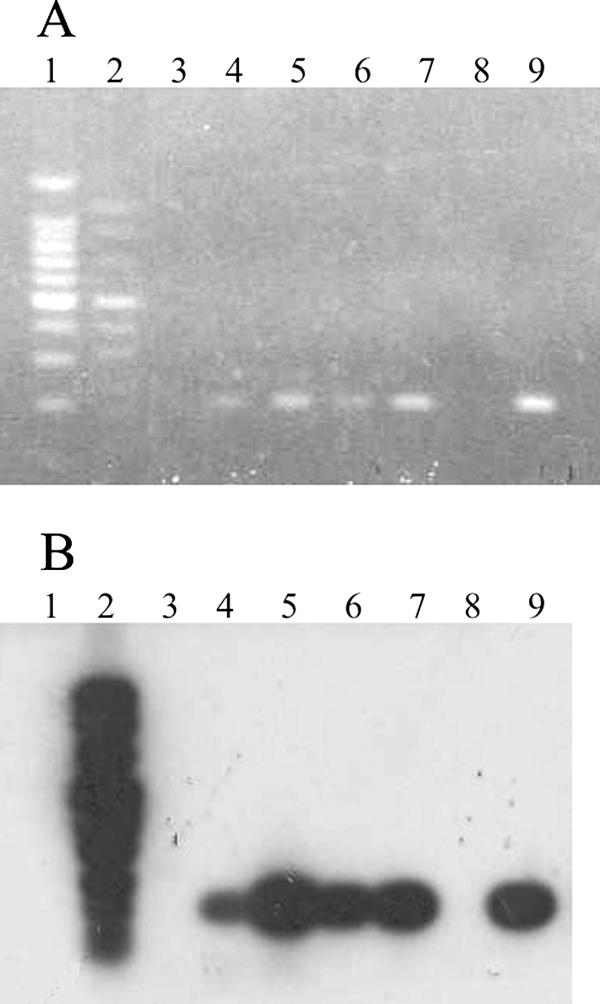
Representative results for the protocol of RT-PCR plus hybridization. A shows the RT-PCR products from four throat swabs after electrophoresis on an agarose gel and staining with ethidium bromide, and B shows results after hybridization. The samples in each lane are as follows: lane 1, marker; lane 2, DIG marker; lane 3, H2O; lane 4, RVTS25; lane 5, RVTS36; lane 6, RVTS37; lane 7, RVTS38; lane 8, empty; lane 9, positive control (rubella virus [Therien] RNA). Additional laboratory controls run with throat swabs were all negative (data not shown).
FIG. 2.

Representative results from IFA protocol and replicon protocol. (A) Vero cells infected with the supernatant from the first passage of clinical specimens were fixed at 3 days postinfection. The presence of virus was determined by the detection of viral structural proteins using rubella virus-specific mouse monoclonal antibodies and AlexaFluor 488-conjugated secondary antibody (green). The nuclei were stained using propidium iodide (red) as a counterstain. (B) GFP expressed from replicons was detected in Vero cells only when the infectious virus was present in the specimen (RVTS20) but not in the absence of infectious virus (RVTS35).
Description of results for selected individual specimens.
Among the 13 specimens from Henan province, although 3 of them were IgM negative (RVTS17, RVTS19, and RVTS23) and 2 of them (RVTS 24 and RVTS 25) were not tested for IgM, 10 were positive by direct RT-PCR without Southern hybridization, and all (13/13) were positive after hybridization (Table 1). The three methods that required tissue culture gave the same positive and negative specimens; four that were negative by culture plus RT-PCR (RVTS17, RVTS22, RVTS23, and RVTS24) were also negative by the IFA and replicon-based tests. Thus, compared with RT-PCR plus hybridization, these culture-based methods each detected about 69% (9/13) of the positive specimens from Henan. Among the three specimens that were RT-PCR negative before Southern hybridization, RVTS17, RVTS22, and RVTS29, only one was positive using the three viral culture techniques (RVTS29), suggesting that RT-PCR plus hybridization is more sensitive than culture for detecting rubella cases.
Two patients (RVTS17 and RVTS23) were IgM negative and negative by techniques that included culture but positive by RT-PCR plus hybridization 1 day after rash onset. Many rubella cases were IgM negative and infectious virus positive at 1 day after the onset of rash (2). Thus, it is reasonable that these specimens initially contained infectious virus, which was inactivated during collection or transport. RVTS19 was positive by both direct RT-PCR and the three methods that included tissue culture. RVTS19 serum was IgM negative, although it was collected at 5 days after rash onset, when most rubella virus cases should be IgM positive. Thus, the IgM-negative result for this patient is suspect.
Five of nine specimens from Anhui province were positive by direct RT-PCR, and eight of nine were positive after Southern hybridization. All eight positive specimens were also positive using the three methods that required infectious virus for a positive result. RVTS35, the only sample that was negative by all four methods, was collected at 2 days after rash onset and was serologically positive. It is possible that the IgM result for this specimen is incorrect or that the degradation of virus and viral RNA in the specimen occurred after collection.
Viral isolates were obtained from both provinces by using the culture methods described above; the isolates were characterized further for molecular epidemiologic purposes (data not shown).
DISCUSSION
Laboratory techniques that detect rubella virus RNA or infectious rubella virions are important for supporting rubella virus control programs. Testing of specimens for rubella virus RNA or infectious virus can be used to confirm IgM serologically positive cases or, since as many as 50% of rubella cases are IgM negative on the day of rash onset, as the only laboratory data confirming a rubella virus case (2, 7). Confirmation of a positive serologic result is especially important when the number of rubella cases is low, such as when a country is on the verge of rubella elimination, since false-positive IgM results are expected to make up a large portion of suspected rubella cases under these circumstances. Furthermore, the identification of specimens that contain rubella virus RNA or infectious virus allows viral RNA from these specimens to be further characterized for molecular epidemiologic purposes, which can be an important component of rubella virus control programs (22).
In China, 331 subnational laboratories were established and a laboratory surveillance network was started in 2000. Considerable testing for rubella virus occurs in the surveillance network, partly because measles virus and rubella virus are frequently difficult to distinguish clinically. Thus, it is necessary for the network to establish sensitive, specific, and, above all, economical methods for rubella diagnosis. Samples from rural areas are sent to provincial laboratories, where basic testing by serologic methods and/or tissue culture methods is completed; positive samples are then transferred to the national laboratory at the China CDC for confirmation. Once the samples are confirmed by additional IgM testing or more advanced tests such as RT-PCR, the national laboratory is required to return the results to each provincial laboratory. One important means of confirming rubella virus infection is the detection of rubella virus RNA or infectious virus using throat swabs.
Only about 40% of rubella virus cases are IgM positive on the day of rash onset, rising to about 100% IgM positive by about 4 days after the onset of rash (2). However, about 90% of rubella patients are positive for virus in the throat by culture on the day of rash onset; this percentage of positive patients declines rapidly in the first week after rash onset. Therefore, two types of specimens are good to collect if unforeseen difficulties result in the degradation of one or the other type of specimen (e.g., poor storage of sera). As the results of this study show, collection of both serum and throat swab in the first few days after rash is prudent.
When the four methods used in this study were compared, direct RT-PCR plus hybridization correlated best with a clinical diagnosis of rubella virus infection. Because this method does not require the recovery of infectious virus from clinical specimens, it can be done in about 2 days. Since the high sensitivity of RT-PCR was increased by the addition of the hybridization step rather than a nested PCR, contamination by PCR products, which often occurs with the nested RT-PCR technique, was minimized. However, this technique is a complex procedure and requires good molecular reagents.
Compared with direct RT-PCR plus hybridization, the other three techniques, which used tissue culture methods to recover infectious virus, were less sensitive. This may be due primarily to the loss of infectivity of viruses during transport. The method using culture plus RT-PCR and the IFA method are rather complex procedures, require good molecular reagents, and are time-consuming, requiring 3 weeks to complete all passages. Protocols for both IFA and the replicon-based method require a fluorescent microscope. Among the techniques requiring tissue culture, the replicon-based method is particularly simple to do, requiring only tissue culture and microscopic techniques.
GFP-expressing replicons used for the detection of rubella virus as described previously are cumbersome for a number of reasons, including the need to transfect previously infected Vero cells with replicon RNA (19). The technique used here relies on a stable cell line expressing a replicon and requires only standard virus passage and fluorescent microscopic techniques for the detection of infections virus (i.e., no RNA synthesis, no transfection, etc.). The stable cell method has been shown to be as accurate as the transfection-dependent replicon-based detection method (Chen, unpublished).
In summary, the protocol of RT-PCR plus hybridization was the most sensitive method and allowed the detection of rubella viruses in clinical samples directly. However, the other three methods also performed well, detecting most of the specimens positive by RT-PCR plus hybridization (81%). The three methods based on the growth of virus in tissue culture have the advantage of isolation of viruses, which can then be characterized in more detail (22, 28). The newly developed method utilizing GFP-expressing replicons performed well and required no laboratory techniques except tissue culture and simple fluorescent microscopy. Thus, this technique is considered to be the least difficult method to perform and could be automated. The techniques described here require only equipment that is commonly available, unlike some other techniques (e.g., real-time RT-PCR).
Acknowledgments
We thank our laboratory colleagues in the CDC Measles, Mumps, Rubella, and Herpesvirus Branch, particularly Shigetaka Katow for expert technical assistance.
We report no conflicts of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
Footnotes
Published ahead of print on 27 June 2007.
REFERENCES
- 1.Bao, J. S. 2003. Strategy and comparison of incidence of rubella in Luliang County in 2000-2001 disease. Chin. J. Public Health Manage. 19:137-138. [Google Scholar]
- 2.Bellini, W. J., and J. P. Icenogle. 2007. Measles and rubella viruses, p. 1378-1391. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 3.Bosma, T. J., K. M. Corbett, S. O'Shea, J. E. Banatvala, and J. M. Best. 1995. PCR for detection of rubella virus RNA in clinical samples. J. Clin. Microbiol. 33:1075-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottiger, M., and M. Forsgren. 1997. Twenty years' experience of rubella vaccination in Sweden: 10 years of selective vaccination (of 12-year-old girls and of women postpartum) and 13 years of a general two-dose vaccination. Vaccine 15:1538-1544. [DOI] [PubMed] [Google Scholar]
- 5.Chen, M. H., and J. P. Icenogle. 2004. Rubella virus capsid protein modulates viral genome replication and virus infectivity. J. Virol. 78:4314-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, N., Y. Bai, X. Hu, H. Pei, Y. Li, W. Zhang, X. Fan, P. Zhang, X. Zhou, Z. Chen, C. Li, P. He, and H. He. 2003. Prevalence of birth defects and rubella infection in pregnant women in Gansu, west China. A survey. J. Reprod. Med. 48:869-874. [PubMed] [Google Scholar]
- 7.Cooray, S., L. Warrener, and L. Jin. 2006. Improved RT-PCR for diagnosis and epidemiological surveillance of rubella. J. Clin. Virol. 35:73-80. [DOI] [PubMed] [Google Scholar]
- 8.Cutts, F. T., and E. Vynnycky. 1999. Modeling the incidence of congenital rubella syndrome in developing countries. Int. J. Epidemiol. 28:1176-1184. [DOI] [PubMed] [Google Scholar]
- 9.Danovaro-Holliday, M. C., C. W. LeBaron, C. Allensworth, R. Raymond, T. G. Borden, A. B. Murray, J. P. Icenogle, and S. E. Reef. 2000. A large rubella outbreak with spread from the workplace to the community. JAMA 284:2733-2739. [DOI] [PubMed] [Google Scholar]
- 10.Frey, T. K. 1994. Molecular biology of rubella virus. Adv. Virus Res. 44:69-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinman, A. R. 2003. Rubella and the Americas. Rev. Panam. Salud Publica 14:298-299. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 12.Katow, S. 2004. Molecular epidemiology of rubella virus in Asia: utility for reduction in the burden of diseases due to congenital rubella syndrome. Pediatr. Int. 46(2):207-213. [DOI] [PubMed] [Google Scholar]
- 13.Lanzieri, T. M., M. S. Parise, M. M. Siqueira, B. M. Fortaleza, T. C. Segatto, and D. R. Prevots. 2004. Incidence, clinical features and estimated costs of congenital rubella syndrome after a large rubella outbreak in Recife, Brazil, 1999-2000. Pediatr. Infect. Dis. J. 23:1116-1122. [PubMed] [Google Scholar]
- 14.Li, H., J. Y. Hu, L. N. Tao, and J. G. Zhang. 2005. Epidemiology characterizations and preventive strategies of CRS. Sh. J. Prev. Med. 17:72-74. (In Chinese.) [Google Scholar]
- 15.Malakmadze, N., L. A. Zimmerman, A. Uzicanin, L. Shteinke, V. M. Caceres, K. Kasymbekova, I. Sozina, J. W. Glasser, M. Joldubaeva, C. Aidyralieva, J. P. Icenogle, P. M. Strebel, and S. E. Reef. 2004. Development of a rubella vaccination strategy: contribution of a rubella susceptibility study of women of childbearing age in Kyrgyzstan, 2001. Clin. Infect. Dis. 38:1780-1783. [DOI] [PubMed] [Google Scholar]
- 16.Robertson, S. E., D. A. Featherstone, M. Gacic-Dobo, and B. S. Hersh. 2003. Rubella and congenital rubella syndrome: global update. Rev. Panam. Salud Publica 14:306-315. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 17.Su, S. B., and H. R. Guo. 2002. Seroprevalence of rubella among women of childbearing age in Taiwan after nationwide vaccination. Am. J. Trop. Med. Hyg. 67:549-553. [DOI] [PubMed] [Google Scholar]
- 18.Tzeng, W. P., M. H. Chen, C. A. Derdeyn, and T. K. Frey. 2001. Rubella virus DI RNAs and replicons: requirement for nonstructural proteins acting in cis for amplification by helper virus. Virology 289:63-73. [DOI] [PubMed] [Google Scholar]
- 19.Tzeng, W. P., Y. Zhou, J. Icenogle, and T. K. Frey. 2005. Novel replicon-based reporter gene assay for detection of rubella virus in clinical specimens. J. Clin. Microbiol. 43:879-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. 1996. Expanded Programme on Immunization (EPI) immunization schedules in the WHO Western Pacific Region, 1995. Wkly. Epidemiol. Rec. 71:133-137. [PubMed] [Google Scholar]
- 21.World Health Organization. 2000. Report of a meeting on preventing congenital rubella syndrome: immunization strategies, surveillance needs. World Health Organization, Geneva, Switzerland. http://www.who.int/vaccine-documents/DocsPDF00/www508.pdf.
- 22.World Health Organization. 2005. Standardization of the nomenclature for genetic characteristics of wild-type rubella viruses. Wkly. Epidemiol. Rec. 80:126-132. [PubMed] [Google Scholar]
- 23.Wu, Y. F. 2002. Epidemiological analysis of a rubella outbreak in 2000. Guangxi J. Prevent. Med. 8:121. [Google Scholar]
- 24.Xu, A., L. Song, C. Wang, A. Wang, Q. Xu, Z. Xiao, S. Wang, M. Li, S. Hao, and Z. Li. 2000. An observation of the immuno-persistence after inoculating with the domestic BRD II strain rubella vaccine among infants and young children. Chin. J. Epidemiol. 21:117-120. [PubMed] [Google Scholar]
- 25.Xu, F. G., C. X. Huang, and H. F. Yao. 1990. Study on forecasting local rubella outbreak in Hangzhou city proper by serologic method. Chin. J. Epidemiol. 11:20-23. [PubMed] [Google Scholar]
- 26.Yu, W. Z., S. J. Zhou, and W. K. He. 2002. Study on epidemiological characteristics and control measures of rubella in Anhui province. Chin. J. Vaccines Immuniz. 8:271-273. [Google Scholar]
- 27.Yuan, J. D., R. Z. Zhang, and S. R. Li. 1988. Epidemiological investigation of an outbreak of rubella in the urban area of Yan Tai city. Chin. J. Epidemiol. 9:210-213. [PubMed] [Google Scholar]
- 28.Zheng, D. P., T. K. Frey, J. Icenogle, S. Katow, E. S. Abernathy, K. J. Song, W. B. Xu, V. Yarulin, R. G. Desjatskova, Y. Aboudy, G. Enders, and M. Croxson. 2003. Global distribution of rubella virus genotypes. Emerg. Infect. Dis. 9:1523-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]


