Galactomannan (GM) detection by the Platelia Aspergillus (PA) enzyme immunoassay (Bio-Rad, France) is a test widely used for the early diagnosis of invasive aspergillosis (IA) in hematooncological patients. False-positive results for this test were reported by several authors, associated mainly with the use of the beta-lactam antibiotics piperacillin-tazobactam and amoxicillin-clavulanic acid (1, 4). Recently, the intravenous hydration fluid PLASMA-LYTE (Baxter) was identified as the cause of false-positive results with the PA test when it was used for bronchoalveolar lavage (3). However, regarding the serum concentration following intravenous administration, the authors speculated that it would be below the detection limit of the PA assay (due to dilution in the intravascular compartment).
The PA test has been used in our department since May 2003 for regular screening (twice a week) of patients with hematological malignancy who are at risk for IA. Between January 2005 and March 2007, a total of 1,137 patients were tested with the PA assay, and 172 of them had more than two positive blood samples (optical density index above 0.5 within 7 days). Fifty-five (32%) positive patients had proven or probable IA, according to the European Organization for Research and Treatment of Cancer/Mycoses Study Group criteria (2). For 117 (68%) patients, the test results were finally assessed as false positives. A gradual increase in the number of false-positive results among patients tested for GM in sera was observed during the year 2006 and at the beginning of 2007. False positivity due to the use of beta-lactam antibiotics was excluded, as all batches of piperacillin-tazobactam and amoxicillin-clavulanic acid used at our department have been tested for the presence of GM since July 2005. Only negative batches (PA assay optical density index of <0.5) were administered to our patients.
After the report about the possible influence of PLASMA-LYTE on PA assay results had been published, we tested the four different batches of PLASMA-LYTE solution available at our institution. All of these were highly GM positive, with a mean optical density index of 7.73 (range, 7.15 to 8.53).
Therefore, we decided to perform a study with healthy volunteers to confirm our hypothesis that the intravenous administration of PLASMA-LYTE can also lead to false-positive results with the PA assay when serum is tested. Four lots (1,000 ml each) from two different batches were given to four healthy volunteers in 1-h infusions. Blood samples for GM detection using the PA test were obtained by separate venipunctures before administration, then immediately after the end of infusion, and then after 5, 7, 10, 24, and 34 h. In all healthy subjects, PLASMA-LYTE administration led to positive PA assay results that persisted for 24 h after infusion (Table 1).
TABLE 1.
Results of the Platelia Aspergillus test after PLASMA-LYTE infusion in healthy volunteers
| Samples used | Optical density index
|
||
|---|---|---|---|
| Mean | Min | Max | |
| PLASMA-LYTE lots | 7.78 | 7.67 | 7.88 |
| Blood sampled before infusion | 0.11 | 0.09 | 0.13 |
| Blood sampled after infusion (h) | |||
| 0 | 2.74 | 2.52 | 3.1 |
| 5 | 1.52 | 1.04 | 1.81 |
| 7 | 1.37 | 0.84 | 1.71 |
| 10 | 0.92 | 0.56 | 1.06 |
| 24 | 0.53 | 0.28 | 0.66 |
| 34 | 0.40 | 0.18 | 0.57 |
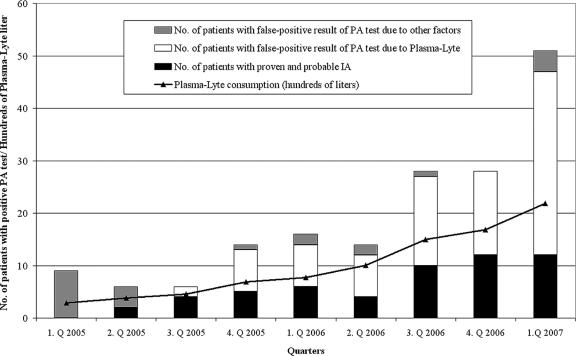
The validation of PLASMA-LYTE as the cause of false positivity in healthy volunteers made us reevaluate the clinical and medical histories of all patients with false-positive results with the PA test in order to trace the possible source of GM contamination. When we focused on PLASMA-LYTE, which has been used in our hematooncological department since October 2004, we found that the administration of this solution could account for 80% of false-positive results with the PA test for our patients tested between January 2005 and March 2007 and, more precisely, 90% of those for patients given only negative batches of beta-lactam antibiotics (July 2005 to March 2007). Moreover, an increase in the number of false-positive results correlated well with the increased consumption of the PLASMA-LYTE solution. Data concerning false and true PA test positivity, the cause of false positivity, and PLASMA-LYTE consumption are summarized in Fig. 1.
FIG. 1.
Number of patients who tested positive with the Platelia Aspergillus test (column depth) and the cause of positivity in relation to PLASMA-LYTE consumption. Q, quarter.
To the best of our knowledge, this is the first report of false-positive results with the PA test, using PLASMA-LYTE as an intravenous hydration and testing the serum used. PLASMA-LYTE solution has been retrospectively identified as the most probable cause of a false-positive result with the PA assay in over 80% of samples from patients without clinical evidence of invasive aspergillosis. This observation has also been confirmed with healthy volunteers. Our findings are very important for routine practice, and such information could avoid the unnecessary preemptive use of mold-active antifungal drugs.
Acknowledgments
This work was supported by a research grant from the Ministry of Health, Czech Republic, IGA NR 8452-3/2005, and partly by CELL, the Czech Leukemia Study Group for Life.
Footnotes
Published ahead of print on 1 August 2007.
REFERENCES
- 1.Adam, O., A. Auperin, F. Wilquin, J. H. Bourhis, B. Gachot, and E. Chachaty. 2004. Treatment with piperacillin-tazobactam and false-positive Aspergillus galactomannan antigen test results for patients with hematological malignancies. Clin. Infect. Dis. 38:917-920. [DOI] [PubMed] [Google Scholar]
- 2.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 3.Hage, C. A., J. M. Reynolds, M. Durkin, L. J. Wheat, and K. S. Knox. 2007. Plasmalyte as a cause of false-positive results for Aspergillus galactomannan in bronchoalveolar lavage fluid. J. Clin. Microbiol. 45:676-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viscoli, C., M. Machetti, P. Cappellano, B. Bucci, P. Bruzzi, M. T. Van Lint, and A. Bacigalupo. 2004. False-positive galactomannan platelia Aspergillus test results for patients receiving piperacillin-tazobactam. Clin. Infect. Dis. 38:913-916. [DOI] [PubMed] [Google Scholar]