Abstract
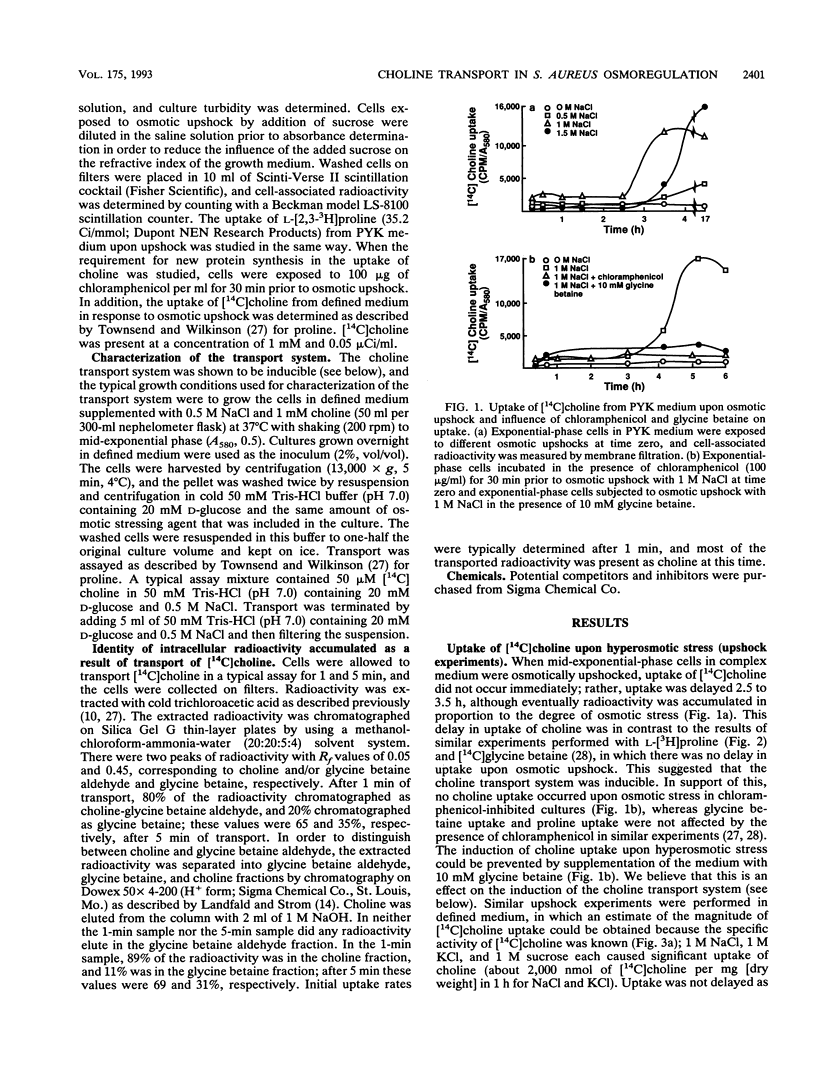
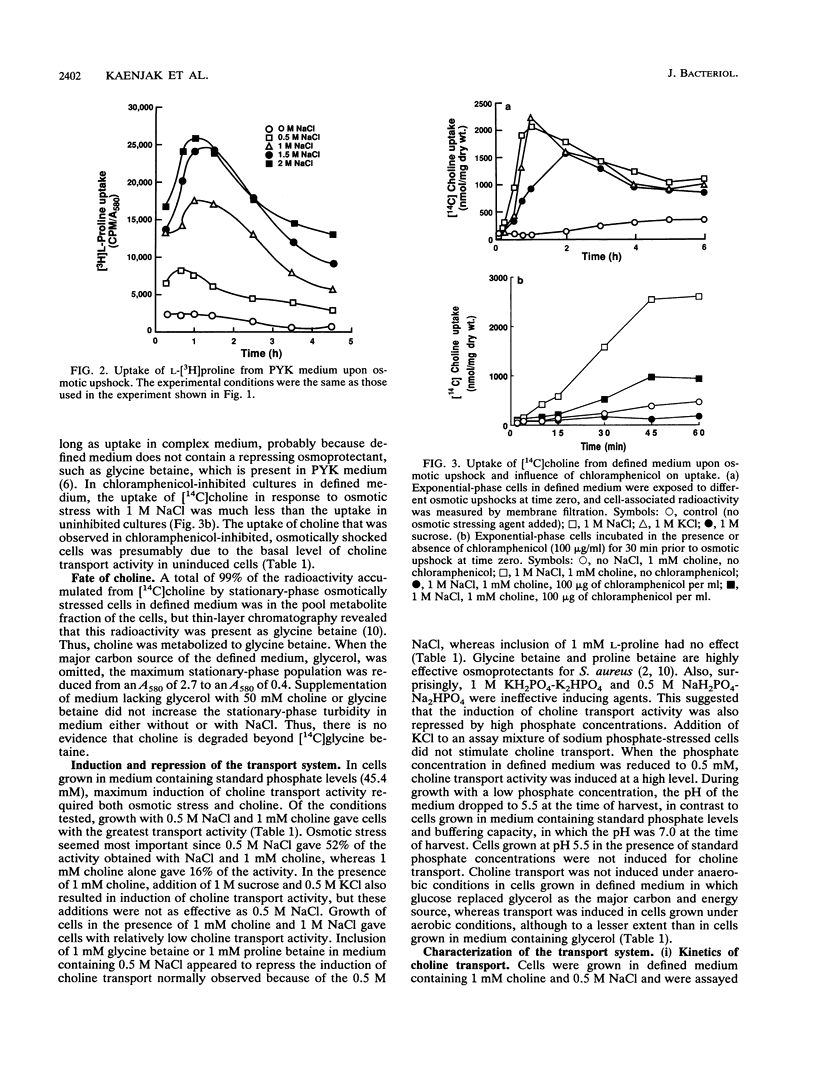
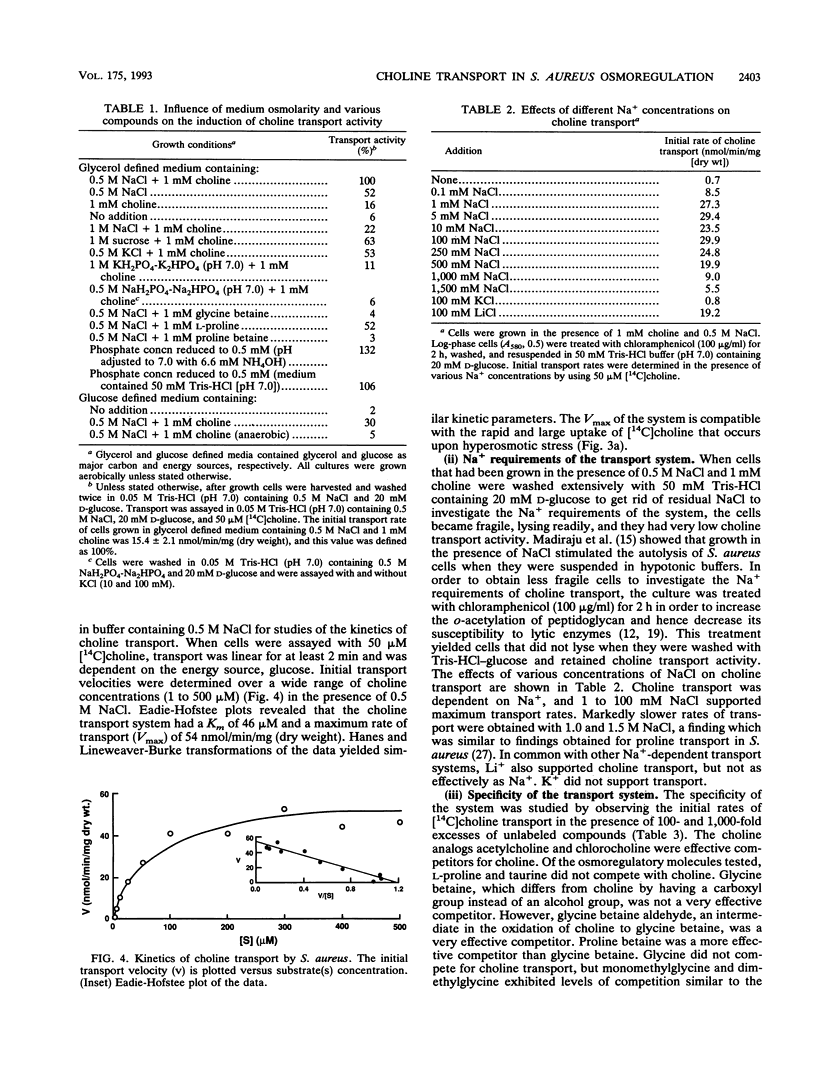
Uptake of [14C]choline upon hyperosmotic stress of exponential-phase Staphylococcus aureus cultures in a complex medium occurred after a delay of 2.5 to 3.5 h. This uptake could be prevented by chloramphenicol, suggesting that it occurred via an inducible transport system. Radioactivity from [14C]choline was accumulated as [14C]glycine betaine. However, neither choline nor glycine betaine could act as the major carbon and energy source for the organism, suggesting that choline was not metabolized beyond glycine betaine. Assay of choline transport activity in cells grown under different conditions in defined media revealed that osmotic stress was mainly responsible for the induction, but choline gave a further increase in induction. The system was not induced in anaerobically grown cells. Choline transport activity was repressed by glycine betaine and proline betaine, suggesting that these compounds are corepressors. Choline transport activity was not induced in cells osmotically stressed by 1 M potassium phosphate or 0.5 M sodium phosphate, but was induced in cells grown in low-phosphate medium in the absence of osmotic stress. This suggests that there is a connection between the phosphate and osmotic stress regulons. Choline transport was energy and Na+ dependent and had a Km of 46 microM and a maximum rate of transport (Vmax) of 54 nmol/min/mg (dry weight). The results of competition studies suggested that N-methyl and an alcohol group or aldehyde groups at the ends of the molecule were important in its recognition by the system. Glycine betaine was not a highly effective competitor, suggesting that its transport system and the choline transport system were distinct from each other. Choline transport was highly susceptible to a variety of inhibitors, which may be related to the greater dependence on respiratory metabolism of cells grown in the presence of high NaC1 concentrations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abee T., Palmen R., Hellingwerf K. J., Konings W. N. Osmoregulation in Rhodobacter sphaeroides. J Bacteriol. 1990 Jan;172(1):149–154. doi: 10.1128/jb.172.1.149-154.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen P. A., Kaasen I., Styrvold O. B., Boulnois G., Strøm A. R. Molecular cloning, physical mapping and expression of the bet genes governing the osmoregulatory choline-glycine betaine pathway of Escherichia coli. J Gen Microbiol. 1988 Jun;134(6):1737–1746. doi: 10.1099/00221287-134-6-1737. [DOI] [PubMed] [Google Scholar]
- Bae J. H., Miller K. J. Identification of two proline transport systems in Staphylococcus aureus and their possible roles in osmoregulation. Appl Environ Microbiol. 1992 Feb;58(2):471–475. doi: 10.1128/aem.58.2.471-475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber E. J., Wilkinson B. J. Sodium-dependent uptake of taurine in encapsulated Staphylococcus aureus strain M. Biochim Biophys Acta. 1984 Mar 14;770(2):127–135. doi: 10.1016/0005-2736(84)90121-4. [DOI] [PubMed] [Google Scholar]
- Eshoo M. W. lac fusion analysis of the bet genes of Escherichia coli: regulation by osmolarity, temperature, oxygen, choline, and glycine betaine. J Bacteriol. 1988 Nov;170(11):5208–5215. doi: 10.1128/jb.170.11.5208-5215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouesbet G., Abaibou H., Wu L. F., Mandrand-Berthelot M. A., Blanco C. Osmotic repression of anaerobic metabolic systems in Escherichia coli. J Bacteriol. 1993 Jan;175(1):214–221. doi: 10.1128/jb.175.1.214-221.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. E., Wilkinson B. J. Staphylococcus aureus osmoregulation: roles for choline, glycine betaine, proline, and taurine. J Bacteriol. 1992 Apr;174(8):2711–2716. doi: 10.1128/jb.174.8.2711-2716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Choline transport in Saccharomyces cerevisiae. J Bacteriol. 1980 Jul;143(1):176–181. doi: 10.1128/jb.143.1.176-181.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin C. M., Rudy J. Effect of NaCl-induced osmotic stress on intracellular concentrations of glycine betaine and potassium in Escherichia coli, Enterococcus faecalis, and staphylococci. J Lab Clin Med. 1991 Sep;118(3):217–224. [PubMed] [Google Scholar]
- Landfald B., Strøm A. R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986 Mar;165(3):849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiraju M. V., Brunner D. P., Wilkinson B. J. Effects of temperature, NaCl, and methicillin on penicillin-binding proteins, growth, peptidoglycan synthesis, and autolysis in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Nov;31(11):1727–1733. doi: 10.1128/aac.31.11.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S. E., Granett S., Jung J. U., Villarejo M. R. Osmotic regulation of PhoE porin synthesis in Escherichia coli. J Bacteriol. 1990 Sep;172(9):5501–5502. doi: 10.1128/jb.172.9.5501-5502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocard J. A., Bernard T., Smith L. T., Le Rudulier D. Characterization of three choline transport activities in Rhizobium meliloti: modulation by choline and osmotic stress. J Bacteriol. 1989 Jan;171(1):531–537. doi: 10.1128/jb.171.1.531-537.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinicke B., Blümel P., Giesbrecht P. Reduced degradability by lysozyme of staphylococcal cell walls after chloramphenicol treatment. Arch Microbiol. 1983 Aug;135(2):120–124. doi: 10.1007/BF00408020. [DOI] [PubMed] [Google Scholar]
- Rozwadowski K. L., Khachatourians G. G., Selvaraj G. Choline oxidase, a catabolic enzyme in Arthrobacter pascens, facilitates adaptation to osmotic stress in Escherichia coli. J Bacteriol. 1991 Jan;173(2):472–478. doi: 10.1128/jb.173.2.472-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvano M. A., Lisa T. A., Domenech C. E. Choline transport in Pseudomonas aeruginosa. Mol Cell Biochem. 1989 Jan 23;85(1):81–89. doi: 10.1007/BF00223517. [DOI] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R. Amino acid transport and staphylococcal membrane vesicles. Ann N Y Acad Sci. 1974 Jul 31;236(0):124–143. doi: 10.1111/j.1749-6632.1974.tb41487.x. [DOI] [PubMed] [Google Scholar]
- Shortridge V. D., Lazdunski A., Vasil M. L. Osmoprotectants and phosphate regulate expression of phospholipase C in Pseudomonas aeruginosa. Mol Microbiol. 1992 Apr;6(7):863–871. doi: 10.1111/j.1365-2958.1992.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Styrvold O. B., Falkenberg P., Landfald B., Eshoo M. W., Bjørnsen T., Strøm A. R. Selection, mapping, and characterization of osmoregulatory mutants of Escherichia coli blocked in the choline-glycine betaine pathway. J Bacteriol. 1986 Mar;165(3):856–863. doi: 10.1128/jb.165.3.856-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomochika K. Energy dependency on the salt-resistance of Staphylococcus aureus: Effects of various inhibitors on the growth in high salinity condition. Acta Med Okayama. 1975 Jun;29(3):171–182. [PubMed] [Google Scholar]
- Townsend D. E., Wilkinson B. J. Proline transport in Staphylococcus aureus: a high-affinity system and a low-affinity system involved in osmoregulation. J Bacteriol. 1992 Apr;174(8):2702–2710. doi: 10.1128/jb.174.8.2702-2710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



