Abstract
The diagnosis of acute otitis media (AOM) is often difficult, depending heavily on the experience and skills of the examiner. However, it is important to identify episodes of AOM that involve the risk of complications and to treat these episodes appropriately. The present study was performed in order to evaluate the use of a rapid antigen assay for Streptococcus pneumoniae, the Binax NOW test, as a diagnostic tool in patients with severe AOM and associated complications. The study included 70 patients with 74 episodes of AOM, 18 of them with complications. Cultures, Binax NOW tests, and a PCR assay were performed on nasopharyngeal secretions, middle ear fluid, and in some cases mastoid bone, cerebrospinal fluid, and urine. According to culture and PCR of the middle ear fluid, 30 (41%) of the episodes were caused by S. pneumoniae. The Binax NOW test was positive in 24 of these episodes (80%). It identified pneumococcal AOM independent of antibiotic treatment, and it was easily adapted to bone tissue. The test yielded sensitivity, specificity, and positive and negative predictive values for middle ear specimens of 85%, 100%, 100%, and 89%, respectively. The corresponding positive and negative values for predicting the bacterial etiology with nasopharyngeal secretions were 51% and 75%. This study showed that the Binax NOW test is a useful diagnostic tool for patients with severe AOM with or without complications.
Acute purulent otitis media (AOM) is the most common bacterial infection diagnosed among children. By 3 years of age, up to 85% of all children have experienced at least one episode of AOM (40), and 15 to 20% of the children suffer from recurrent episodes (1, 7).
The most frequent microbiological cause of AOM is Streptococcus pneumoniae (26 to 48%), followed by Haemophilus influenzae (15 to 41%), Moraxella catarrhalis (23 to 25%), and Streptococcus pyogenes (4 to 8%) (2, 3, 20, 27). S. pneumoniae and S. pyogenes are the two most virulent pathogens of the middle ear, and they are the leading causes of complications such as mastoiditis, labyrinthitis, and meningitis (15, 22, 23, 28, 38).
AOM is the single most common reason for prescribing antibiotics to children (12, 24). The antibiotic load and selective pressure in the youngest age groups are consequently high, and clinical problems with antibiotic resistance have emerged (5, 41). The routine use of antimicrobial drugs in AOM treatment has been reevaluated in recent years, and watchful waiting has become an alternative (34). Watchful waiting is, however, not without risk. A safer approach is to use stricter diagnostic criteria for AOM, to improve the diagnostic tools, and to treat only those patients who are the least likely to recover spontaneously.
The diagnosis of AOM, especially in young children, is often difficult and inadequate (4, 12, 33, 42). Previous studies have shown that physicians are uncertain of the diagnosis in 40% of cases (31), and general practitioners are more likely to diagnose AOM than are otorhinolaryngologists (4). Furthermore, about 49 to 88% of patients with extracranial and intracranial complications have received or are under antibiotic treatment prior to admission (14-16, 21, 23, 28, 38), which decreases the chances of identifying the causative agent with conventional culture methods. In situations such as this, a simple test to identify S. pneumoniae, the pathogen with the lowest spontaneous recovery rate and the highest complication frequency, would be valuable.
A rapid urinary antigen test, the Binax NOW test, was recently introduced to the market. This test has been shown to be a useful tool for identifying severe pneumococcal infections in adults (25, 37). The aim of the present study was to evaluate the ability of the Binax NOW test to detect S. pneumoniae in patients with severe AOM and associated complications.
MATERIALS AND METHODS
The study was performed as a prospective multicenter study including four otorhinolaryngology centers in the south of Sweden. The study was approved by the ethics committee of Lund University.
Patients.
AOM patients with spontaneous perforation of the tympanic membrane or in need of myringotomy were invited to participate in the study. There were no restrictions concerning age or previous or current antibiotic treatment, but patients with transmyringeal ventilation tubes or underlying complicating diseases were excluded. Informed consent was obtained from the patients and, where appropriate, their parents. Medical history, including information on the number of days since the onset of symptoms, fever, previous contacts with medical health care, and ongoing antibiotic treatment, was collected. The diagnostic criteria for AOM were an acute onset of symptoms combined with either (i) otomicroscopic findings of an abnormal tympanic membrane with decreased or vanished mobility and opaque fluid behind the tympanic membrane or (ii) perforated tympanic membrane and purulent secretion in the external auditory canal. An otomicroscope was used for diagnosis at all times. Myringotomy was performed according to previous recommendations (32). A local anesthetic (phenol) was used with adults, whereas most children were generally anesthetized.
Controls.
Twenty healthy control patients, 19 children and one adult, were included in the study. The majority of these patients were participants in a vaccine study at the Department of Oto-Rhino-Laryngology at Lund University Hospital.
Bacterial cultures.
Bacterial samples were collected from all middle ears as well as from the nasopharynx by using swabs. The samples were cultured on blood and chocolate agar plates and were incubated for 48 h in a moist environment with 5% CO2. Bacteria were identified using standard laboratory procedures (18, 19, 35), and isolates were frozen at −70°C. Samples of mastoid bone tissue or cerebrospinal fluid were, in addition, inoculated in brain heart infusion broth (Difco Laboratories, Detroit, MI). All urine samples positive by the Binax NOW test were cultured in order to control any cross-reactivity.
Pneumococcal antigen test.
The Binax NOW test (Binax, Inc., Portland, ME) is, in brief, a rapid in vitro immunochromatographic assay that detects pneumococcal antigen in urine. The test kit contains a nitrocellulose membrane onto which rabbit antipneumococcal antibody is attached. The test sample is mixed with reaction buffer and then placed on the test membrane. If the test sample contains pneumococcal antigen, a pink/purple line will appear within 15 min. The antigen against which the Binax NOW test reacts is a cell wall polysaccharide on S. pneumoniae, which is present in all clinically relevant strains. In this study, middle ear fluid, nasopharyngeal secretions, and cerebrospinal fluid were tested as previously described (10, 11, 36). The Binax NOW test was also adapted for bone tissue by placing the tissue in a tube with phosphate-buffered saline (1 ml) and vortexing it for 30 s. The sample was thereafter mixed with the reaction buffer as described above. All samples were tested after the culture procedure had been carried out. The collection of urine samples from the patients was optional, and urine test results were therefore not available for all patients. After the Binax NOW testing, the swabs were put in a lysing buffer (Roche Diagnostics Corporation, Indianapolis, IN) and frozen at −20°C for further analysis with the PCR technique if necessary.
PCR assay.
A real-time PCR assay was performed on some middle ear samples exhibiting discrepant results between cultures and Binax NOW tests. The PCR assay was performed as described earlier (6), with some modifications. Briefly, bacterial DNA was extracted by using a MagNAPure LC DNA isolation kit III with a MagNAPure LC machine (Roche) according to the manufacturer's instructions. Real-time PCR amplifications were performed in 20-μl reaction volumes containing FastStart DNA master hybridization probes (Roche), 30 nM probe, 500 nM primers, 5 mM MgCl2, and 5 μl of DNA extract. The nucleotide sequence of the forward primer for the pneumolysin gene was 5′-TGCAGAGCGTCCTTTGGTCTAT-3′, the sequence for the reverse primer was 5′-CTCTTACTCGTGGTTTCCAACTTGA-3′ (DNA Technology, Aarhus, Denmark), and the sequence of the 6-carboxyfluorescein-labeled probe was 5′-TGGCGCCCATAAGCAACACTCGAA-3′ (TIB Molbiol, Berlin, Germany). The DNA was amplified and detected with a LightCycler system by using the following cycling parameters: heating at 95°C for 10 min, followed by 40 cycles of a two-stage temperature profile of 95°C for 15 s and 60°C for 1 min. If no increase in fluorescent signal was observed after 40 cycles, the sample was regarded as negative. Positive (S. pneumoniae ATCC 49619) and negative (distilled H2O) controls were included at every extraction and at every PCR run.
RESULTS
Study population.
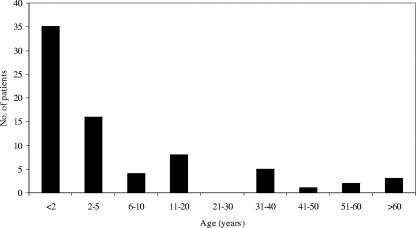
Seventy subjects, with a total of 74 episodes of AOM, were enrolled in the study. Twenty-six (37%) of the patients were female, and 44 (63%) were male. The mean and median ages were 10.6 years (±17.4) and 2.2 years (6 weeks to 69 years), respectively (Fig. 1). In 35 of the episodes (47%), the patient was younger than 2 years.
FIG. 1.
Age distribution of the study population.
Myringotomy was performed in 30 episodes (41%), and spontaneous perforations occurred in the remaining 44 episodes (59%). Bilateral AOM was present in 23 episodes (31%). AOM with serious complications was present in 18 cases (24%); in 12 of these cases, the patient was under 2 years of age. The patients had fever in at least 32 of the episodes (43%); in this group, the median age was 1.9 years (6 weeks to 57 years). Approximately 42% of the patients had received recently (within 3 days) or were receiving antibiotics when the cultures were obtained.
Middle ear fluid.
Primary AOM pathogens (S. pneumoniae, H. influenzae, M. catarrhalis, and S. pyogenes) were isolated in cultures from 31 (42%) of the middle ear samples. In three of the samples (4%), more than one AOM pathogen was present. The median age in relation to the pathogen present in the middle ear was 1.6 years for patients with S. pneumoniae, 1.5 years for patients with H. influenzae, 0.5 years for patients with M. catarrhalis, 4.3 years for patients with S. pyogenes, 4.5 years for patients without primary AOM pathogens (skin flora or gram-negative rods), and 1.9 years for the group in which no bacterial growth in the middle ear was recorded.
According to culture and PCR, 30 (41%) of the AOM episodes were caused by S. pneumoniae. With the Binax NOW test, four additional pneumococcal episodes were recorded. Table 1 gives the characteristics of the samples in which pneumococci were present.
TABLE 1.
Summary of Binax NOW results in relation to findings of S. pneumoniae in the middle ear
| Middle ear finding | No. of episodes | No. of patients with antibiotic treatment/total no. of patients | No. of positive Binax NOW tests/total no. of tests (% positive)
|
||
|---|---|---|---|---|---|
| Middle ear | Nasopharynx | Urine | |||
| S. pneumoniae alone (culture positive) | 19 | 4/19 | 16/18 (89) | 14/17 (82) | 8/15 (53) |
| S. pneumoniae plus other pathogen (culture positive) | 3 | 0/3 | 3/3 (100) | 2/2 (100) | 0/1 (0) |
| S. pneumoniae (PCR positive, culture negative) | 8 | 7/8 | 5/8 (62) | 3/6 (50) | 1/4 (25) |
| No S. pneumoniae (culture and/or PCR negative) | 44 | 20/44 | 4/43 (9)a | 18/40 (45) | 7/29 (24) |
In three of the four cases, S. pneumoniae was detected either in bone tissues (culture, n = 1; PCR, n = 1) or in secretions from the nasopharynx (culture, n = 1). In the final case, the specimens from the mastoid bone, the middle ear, and the nasopharynx were all Binax NOW positive.
H. influenzae grew in middle ear fluid in nine episodes (12%), M. catarrhalis in three episodes (4%), and S. pyogenes in two episodes (3%). In 42 (57%) of the episodes, the cultures yielded no growth or growth of nonprimary AOM pathogens. Fifty-two percent of the patients in this group were already under antibiotic treatment when the samples were collected, compared with 22% in the group with middle ear cultures positive for primary AOM pathogens.
Of the 28 Binax NOW-positive samples, 24 were culture or PCR positive. The remaining four had findings of pneumococci at other sites (e.g., mastoid bone or nasopharynx). When the results of the Binax NOW test were compared with the results of the middle ear cultures and the PCR assay, the sensitivity of the test was 85%, the specificity was 100%, and the positive and negative predictive values were 100% and 89%, respectively.
Nasopharyngeal secretion.
Primary AOM pathogens were isolated in cultures from the nasopharynx in 48 of the 74 AOM episodes (65%): S. pneumoniae in 33, H. influenzae in 24, M. catarrhalis in 26, and S. pyogenes in 3. Four additional pneumococcal cases were found with PCR and one with the Binax NOW test.
Growth of multiple pathogens was more common in the nasopharynx than in the middle ear (39% of the cases). M. catarrhalis was the most common copathogen. Table 2 provides further information about the correlation between the microbiological findings in the nasopharynx and in the middle ear.
TABLE 2.
Microbiological findings in the nasopharynx in relation to findings in the middle ear
| Nasopharyngeal finding | No. of episodes | No. of cases with identical finding in the middle ear | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| S. pneumoniae | 36a | 21a | 64 | 85 |
| H. influenzae | 23 | 8 | 35 | 100 |
| S. pyogenes | 3 | 2 | 67 | 100 |
| M. catarrhalis | 26 | 1 | 4 | 95 |
| Mixed pathogens | 32 | 1 | NDb | ND |
| Positive Binax NOW test | 37 | 19a | 51 | 75 |
Presence of S. pneumoniae according to culture and/or PCR.
ND, not done.
The Binax NOW test was positive in 37 of the nasopharyngeal samples; 33 of these samples were culture or PCR positive for S. pneumoniae, three showed false positives with the Binax NOW test, and the final one was not examined with PCR. S. pyogenes or alpha-hemolytic streptococci were isolated from the three false-positive samples. A false-positive test result was also recorded for a culture-negative urine sample from one of these patients but not in the two middle ear samples containing S. pyogenes. To exclude a possible cross-reaction, the frozen isolates were recultured and new tests were performed with Binax NOW. The pure cultures were negative by the test. False-negative results were found in three cases; the middle ear fluid was also falsely negative in two of these cases. This yielded a sensitivity of 92% and a specificity of 89% for the Binax NOW test compared with nasopharyngeal cultures and PCR. The positive and negative predictive values were 92% and 89%, respectively.
Urine.
In 16 (22%) of the episodes, urine samples were positive by the Binax NOW test. However, due to the study design, urine tests were not available in 25 of the cases (34%). Among the 16 positive cases were five subjects with mastoiditis, one with labyrinthitis, and six with AOM combined with fever and an affected general state of health. Nine of the 16 were positive for S. pneumoniae in middle ear fluid as judged by culture or PCR, and 14 of 16 had pneumococci in the nasopharynx. In 11 episodes, the Binax NOW test showed no pneumococcal antigen in the urine sample despite findings of pneumococci in the middle ear fluid.
Patients with complications.
Sixteen patients developed 18 episodes of severe complications: mastoiditis (n = 13, including one patient with three episodes over a period of 1.5 years), labyrinthitis (n = 3), and meningitis (n = 2).
Mastoidectomy was performed in 7 of the 13 mastoiditis episodes. All of these patients were under antibiotic treatment when the samples were collected. Eleven of the 13 mastoiditis episodes were of pneumococcal origin. Of the two patients with meningitis, one was infected with S. pneumoniae and the other with H. influenzae type b. All three patients with labyrinthitis had pneumococcal infections. Combining the three methods used in the study, S. pneumoniae was identified as the causative agent in 15 of the 18 episodes with complications (83%). The Binax NOW test was able to identify the bacterium in 14 (78%) of these cases, and the test results were accessible within hours.
Controls.
Ten out of 20 healthy controls carried S. pneumoniae in the nasopharynx according to culture results. Urine samples from 6 of the 10 pneumococcal carriers yielded positive Binax NOW tests. Three controls with no nasopharyngeal growth had false-positive Binax NOW urine tests; two of these urine samples showed growth of enterococci and streptococci, which were all Binax NOW negative when tested as pure cultures. Due to these test results, the study was augmented by another 40 patients with urogenital infections caused by gram-positive cocci (Enterococcus faecalis, n = 12; Enterococcus faecium, n = 5; S. pyogenes, n = 2; beta-hemolytic streptococcus group G, n = 3; Streptococcus agalactiae, n = 8; other types of streptococci, n = 6; and mixed cultures of gram-positive cocci, n = 4). Three of the urine samples were positive by the Binax NOW test. There was growth of E. faecalis in one of these samples and mixes of gram-positive cocci, including S. agalactiae and alpha-hemolytic streptococci, in two. The alpha-hemolytic streptococci were later identified as S. pneumoniae.
DISCUSSION
This study evaluated the usefulness of the Binax NOW test for detecting S. pneumoniae in patients with severe AOM and associated complications. The test allowed rapid selection of patients with pneumococcal infections, and it served as a support when choosing the initial treatment.
The Binax NOW test was originally developed as a urine test. However, studies have been performed on nasopharyngeal secretions (10), middle ear effusions (11), cerebrospinal fluids (36), bronchoalveolar lavage fluids (17), pleural fluids (30), and blood cultures (29). The present study included the first three of these sample types, and the test was further successfully adapted to bone tissue. The Binax NOW test identified the majority of culture-positive samples, independent of sample type. Furthermore, it was able to identify pneumococci in samples from treated patients with negative cultures. The only other method available in these cases is the PCR assay. Although sensitive, the PCR technique is time-consuming, especially compared with the Binax NOW test. It also has the disadvantage of being more expensive, requiring specially trained staff.
Culture of middle ear fluid is recommended for accurate diagnosis of AOM. In practice such material is not always available, and so nasopharyngeal secretions are sometimes used for bacteriological documentation. Several earlier studies have shown a rather weak correlation between the results of nasopharyngeal and middle ear fluid cultures, with positive predictive values of about 50% at best (9, 13). The absence of S. pneumoniae in the nasopharynx has, however, a higher negative predictive value for recovery of the organism in the middle ear fluid of patients with AOM (9, 13). A negative Binax NOW test may therefore be more useful than a positive one in clinical practice.
In this study, the correlation was relatively high for cultures yielding growth of S. pneumoniae and S. pyogenes. In contrast, while the two gram-negative bacteria M. catarrhalis and H. influenzae were often isolated from the nasopharynx, they were relatively seldom present in the middle ear fluid in the investigated patients. These findings may have been due to the choice of study population. However, the results also indicate that the leading otitis pathogens should probably not be considered as a single entity but as four bacterial species with quite different capacities to cause middle ear infections.
The use of urine samples as a diagnostic tool in severe AOM was not without its problems. Pneumococcal antigen was detected in some of the most severely ill patients, but the majority of patients with pneumococcal AOM remained Binax NOW negative in the urine. Furthermore, a large proportion of the healthy control patients with pneumococci in the nasopharynx were Binax NOW positive in the urine, and there were also cross-reactions with other gram-positive bacteria. Previous studies of patients with invasive pneumococcal disease have shown that the Binax NOW test used as a urine test is more reliable in adults than in children (8, 26, 37). In patients with severe AOM, the urine test appeared to be of limited value. This was independent of age.
The Binax NOW test has other limitations. Some patients in the present study yielded negative tests in spite of pneumococcal growth in the nasopharynx and the middle ear. The reason for this remains to be elucidated. The Binax NOW test also failed to identify a case of pneumococcal meningitis. The causative agent was detected by PCR alone, suggesting a low bacterial count; the manufacturer specifies a detection limit of about 105 CFU/ml in cerebrospinal fluid. Furthermore, urine samples can remain positive for weeks after a pneumococcal infection (39), and the test will be affected if the patient has recently been vaccinated with a pneumococcal vaccine.
In summary, the Binax NOW test increased the diagnostic yield for pneumococcal AOM and its complications. With this test, health care professionals are provided with a rapid adjunct diagnostic tool to clinical findings.
Acknowledgments
The study was supported by grants from Malmö Health Services District Foundations and Ronald McDonald's Children Foundation.
We thank Binax, Inc., for donating the antigen detection kits.
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Alho, O. P. 1997. How common is recurrent acute otitis media? Acta Otolaryngol. Suppl. 529:8-10. [DOI] [PubMed] [Google Scholar]
- 2.Arguedas, A., R. Dagan, C. Soley, C. Loaiza, K. Knudsen, N. Porat, A. Perez, E. Brilla, and M. L. Herrera. 2003. Microbiology of otitis media in Costa Rican children, 1999 through 2001. Pediatr. Infect. Dis. J. 22:1063-1068. [DOI] [PubMed] [Google Scholar]
- 3.Block, S. L., J. Hedrick, C. J. Harrison, R. Tyler, A. Smith, R. Findlay, and E. Keegan. 2004. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr. Infect. Dis. J. 23:829-833. [DOI] [PubMed] [Google Scholar]
- 4.Blomgren, K., and A. Pitkaranta. 2003. Is it possible to diagnose acute otitis media accurately in primary health care? Fam. Pract. 20:524-527. [DOI] [PubMed] [Google Scholar]
- 5.Brown, S. D., and M. J. Rybak. 2004. Antimicrobial susceptibility of Streptococcus pneumoniae, Streptococcus pyogenes and Haemophilus influenzae collected from patients across the USA, in 2001-2002, as part of the PROTEKT US study. J. Antimicrob. Chemother. 54(Suppl. 1):i7-i15. [DOI] [PubMed] [Google Scholar]
- 6.Corless, C. E., M. Guiver, R. Borrow, V. Edwards-Jones, A. J. Fox, and E. B. Kaczmarski. 2001. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J. Clin. Microbiol. 39:1553-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly, K. A., J. E. Brown, B. R. Lindgren, M. H. Meland, C. T. Le, and G. S. Giebink. 1999. Epidemiology of otitis media onset by six months of age. Pediatrics 103:1158-1166. [DOI] [PubMed] [Google Scholar]
- 8.Dowell, S. F., R. L. Garman, G. Liu, O. S. Levine, and Y. H. Yang. 2001. Evaluation of Binax NOW, an assay for the detection of pneumococcal antigen in urine samples, performed among pediatric patients. Clin. Infect. Dis. 32:824-825. [DOI] [PubMed] [Google Scholar]
- 9.Eldan, M., E. Leibovitz, L. Piglansky, S. Raiz, J. Press, P. Yagupsky, A. Leiberman, and R. Dagan. 2000. Predictive value of pneumococcal nasopharyngeal cultures for the assessment of nonresponsive acute otitis media in children. Pediatr. Infect. Dis. J. 19:298-303. [DOI] [PubMed] [Google Scholar]
- 10.Faden, H., M. Heimerl, G. Goodman, P. Winkelstein, and C. Varma. 2002. New technique (the NOW test) for rapid detection of Streptococcus pneumoniae in the nasopharynx. J. Clin. Microbiol. 40:4748-4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faden, H., C. Poje, M. Pizzuto, M. Nagy, and L. Brodsky. 2002. A new technique (the NOW test) for the detection of Streptococcus pneumoniae in the effusions of otitis media. J. Laryngol. Otol. 116:499-501. [DOI] [PubMed] [Google Scholar]
- 12.Froom, J., L. Culpepper, P. Grob, A. Bartelds, P. Bowers, C. Bridges-Webb, I. Grava-Gubins, L. Green, J. Lion, B. Somaini, et al. 1990. Diagnosis and antibiotic treatment of acute otitis media: report from International Primary Care Network. BMJ 300:582-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehanno, P., G. Lenoir, B. Barry, J. Bons, I. Boucot, and P. Berche. 1996. Evaluation of nasopharyngeal cultures for bacteriologic assessment of acute otitis media in children. Pediatr. Infect. Dis. J. 15:329-332. [DOI] [PubMed] [Google Scholar]
- 14.Go, C., J. M. Bernstein, A. L. de Jong, M. Sulek, and E. M. Friedman. 2000. Intracranial complications of acute mastoiditis. Int. J. Pediatr. Otorhinolaryngol. 52:143-148. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein, N. A., M. L. Casselbrant, C. D. Bluestone, and M. Kurs-Lasky. 1998. Intratemporal complications of acute otitis media in infants and children. Otolaryngol. Head Neck Surg. 119:444-454. [DOI] [PubMed] [Google Scholar]
- 16.Harley, E. H., T. Sdralis, and R. G. Berkowitz. 1997. Acute mastoiditis in children: a 12-year retrospective study. Otolaryngol. Head Neck Surg. 116:26-30. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs, J. A., E. E. Stobberingh, E. I. Cornelissen, and M. Drent. 2005. Detection of Streptococcus pneumoniae antigen in bronchoalveolar lavage fluid samples by a rapid immunochromatographic membrane assay. J. Clin. Microbiol. 43:4037-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janda, M. J., and J. S. Knapp. 2003. Neisseria and Moraxella catarrhalis, p. 585-608. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 19.Kilian, M. 2003. Haemophilus, p. 623-635. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 20.Kilpi, T., E. Herva, T. Kaijalainen, R. Syrjanen, and A. K. Takala. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654-662. [DOI] [PubMed] [Google Scholar]
- 21.Kvestad, E., K. J. Kvaerner, and I. W. Mair. 2000. Acute mastoiditis: predictors for surgery. Int. J. Pediatr. Otorhinolaryngol. 52:149-155. [DOI] [PubMed] [Google Scholar]
- 22.Linder, T. E., H. R. Briner, and T. Bischoff. 2000. Prevention of acute mastoiditis: fact or fiction? Int. J. Pediatr. Otorhinolaryngol. 56:129-134. [DOI] [PubMed] [Google Scholar]
- 23.Luntz, M., A. Brodsky, S. Nusem, J. Kronenberg, G. Keren, L. Migirov, D. Cohen, S. Zohar, A. Shapira, D. Ophir, G. Fishman, G. Rosen, V. Kisilevsky, I. Magamse, S. Zaaroura, H. Z. Joachims, and D. Goldenberg. 2001. Acute mastoiditis—the antibiotic era: a multicenter study. Int. J. Pediatr. Otorhinolaryngol. 57:1-9. [DOI] [PubMed] [Google Scholar]
- 24.McCaig, L. F., and J. M. Hughes. 1995. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA 273:214-219. [PubMed] [Google Scholar]
- 25.Murdoch, D. R., R. T. Laing, G. D. Mills, N. C. Karalus, G. I. Town, S. Mirrett, and L. B. Reller. 2001. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J. Clin. Microbiol. 39:3495-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuman, M. I., and M. B. Harper. 2003. Evaluation of a rapid urine antigen assay for the detection of invasive pneumococcal disease in children. Pediatrics 112:1279-1282. [DOI] [PubMed] [Google Scholar]
- 27.Palmu, A. A., E. Herva, H. Savolainen, P. Karma, P. H. Mäkelä, and T. M. Kilpi. 2004. Association of clinical signs and symptoms with bacterial findings in acute otitis media. Clin. Infect. Dis. 38:234-242. [DOI] [PubMed] [Google Scholar]
- 28.Petersen, C. G., T. Ovesen, and C. B. Pedersen. 1998. Acute mastoidectomy in a Danish county from 1977 to 1996 with focus on the bacteriology. Int. J. Pediatr. Otorhinolaryngol. 45:21-29. [DOI] [PubMed] [Google Scholar]
- 29.Petti, C. A., C. W. Woods, and L. B. Reller. 2005. Streptococcus pneumoniae antigen test using positive blood culture bottles as an alternative method to diagnose pneumococcal bacteremia. J. Clin. Microbiol. 43:2510-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ploton, C., A. M. Freydiere, Y. Benito, N. Bendridi, C. Mazzocchi, G. Bellon, and F. Vandenesch. 2006. Streptococcus pneumoniae thoracic empyema in children: rapid diagnosis by using the Binax NOW immunochromatographic membrane test in pleural fluids. Pathol. Biol. 54:498-501. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld, R. M. 2002. Diagnostic certainty for acute otitis media. Int. J. Pediatr. Otorhinolaryngol. 64:89-95. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld, R. M., and C. D. Bluestone. 2003. Evidence-based otitis media, 2nd ed. B. C. Decker, Hamilton, Ontario, Canada.
- 33.Rothman, R., T. Owens, and D. L. Simel. 2003. Does this child have acute otitis media? JAMA 290:1633-1640. [DOI] [PubMed] [Google Scholar]
- 34.Rovers, M. M., A. G. Schilder, G. A. Zielhuis, and R. M. Rosenfeld. 2004. Otitis media. Lancet 363:465-473. [DOI] [PubMed] [Google Scholar]
- 35.Ruoff, K. L., R. A. Whiley, and D. Beighton. 2003. Streptococcus, p. 405-421. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 36.Samra, Z., H. Shmuely, E. Nahum, D. Paghis, and J. Ben-Ari. 2003. Use of the NOW Streptococcus pneumoniae urinary antigen test in cerebrospinal fluid for rapid diagnosis of pneumococcal meningitis. Diagn. Microbiol. Infect. Dis. 45:237-240. [DOI] [PubMed] [Google Scholar]
- 37.Smith, M. D., P. Derrington, R. Evans, M. Creek, R. Morris, D. A. Dance, and K. Cartwright. 2003. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J. Clin. Microbiol. 41:2810-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spratley, J., H. Silveira, I. Alvarez, and M. Pais-Clemente. 2000. Acute mastoiditis in children: review of the current status. Int. J. Pediatr. Otorhinolaryngol. 56:33-40. [DOI] [PubMed] [Google Scholar]
- 39.Tateda, K., E. Kusano, T. Matsumoto, K. Kimura, K. Uchida, K. Nakata, and K. Yamaguchi. 2006. Semi-quantitative analysis of Streptococcus pneumoniae urinary antigen: kinetics of antigen titers and severity of diseases. Scand. J. Infect. Dis. 38:166-171. [DOI] [PubMed] [Google Scholar]
- 40.Teele, D. W., J. O. Klein, and B. A. Rosner. 1980. Epidemiology of otitis media in children. Ann. Otol. Rhinol. Laryngol. Suppl. 89:5-6. [DOI] [PubMed] [Google Scholar]
- 41.Thornsberry, C., and D. F. Sahm. 2000. Antimicrobial resistance in respiratory tract pathogens: results of an international surveillance study. Chemotherapy 46(Suppl. 1):15-23. [DOI] [PubMed] [Google Scholar]
- 42.Watson, R. L., S. F. Dowell, M. Jayaraman, H. Keyserling, M. Kolczak, and B. Schwartz. 1999. Antimicrobial use for pediatric upper respiratory infections: reported practice, actual practice, and parent beliefs. Pediatrics 104:1251-1257. [DOI] [PubMed] [Google Scholar]