Abstract
Intertriginous toe webs harboring cellulitis-causing bacteria constitute a risk factor for lower-limb cellulitis. Molecular typing of Streptococcus pyogenes and S. dysgalactiae subsp. equisimilis isolates from blood and toe webs of two cellulitis patients revealed identical strains for each species. This finding supports the role of toe webs as a potential site of entry for cellulitis pathogens.
CASE REPORT
The patients described below were included in a case-control study on the risk factors for lower-limb cellulitis that took place at Landspítali University Hospital, Reykjavík, Iceland (4). The study was approved by the hospital's ethics committee, and participants signed an informed consent statement. Collection of clinical data and microbiological methods for cultures of blood, skin, and nail specimens have been described previously (4).
Patients 1 and 2 were a 75-year-old woman and a 76-year-old man, respectively, both with a <24-h history of a first episode of cellulitis on one leg, a temperature of >39°C, chills, and a white blood cell count of >16,000/μl. Neither patient had received antibiotics prior to admission. The medical histories and comorbid conditions in both cases included obesity (body mass indexes of 33 and 38, respectively), diabetes mellitus type 2, coronary artery disease, atrial fibrillation, and chronic edema of the infected leg. In addition, patient 1 reported a previous femoral-popliteal bypass on both limbs, and patient 2 had had a saphenectomy of the infected limb. A physical examination of the foot on the cellulitis-afflicted limb revealed onychodystrophic toenails and toe web intertrigo, with scaling and erythema in both cases; an interdigital skin erosion also was noted for patient 1. No other skin abrasions or ulcers were found on the infected limb of either patient. Mycological examinations of scrapings from nails, toe webs, and the sole of the cellulitis-infected limb yielded Trichophyton rubrum for patient 2, but the results were negative for patient 1. Blood cultures revealed Streptococcus pyogenes in one bottle out of eight from patient 1, and a toe web swab yielded heavy growth of the same species. Likewise, S. dysgalactiae subsp. equisimilis was isolated from four blood bottles and a toe web swab from patient 2. Both patients were successfully treated with penicillin derivatives and were discharged 9 and 19 days, respectively, after admission.
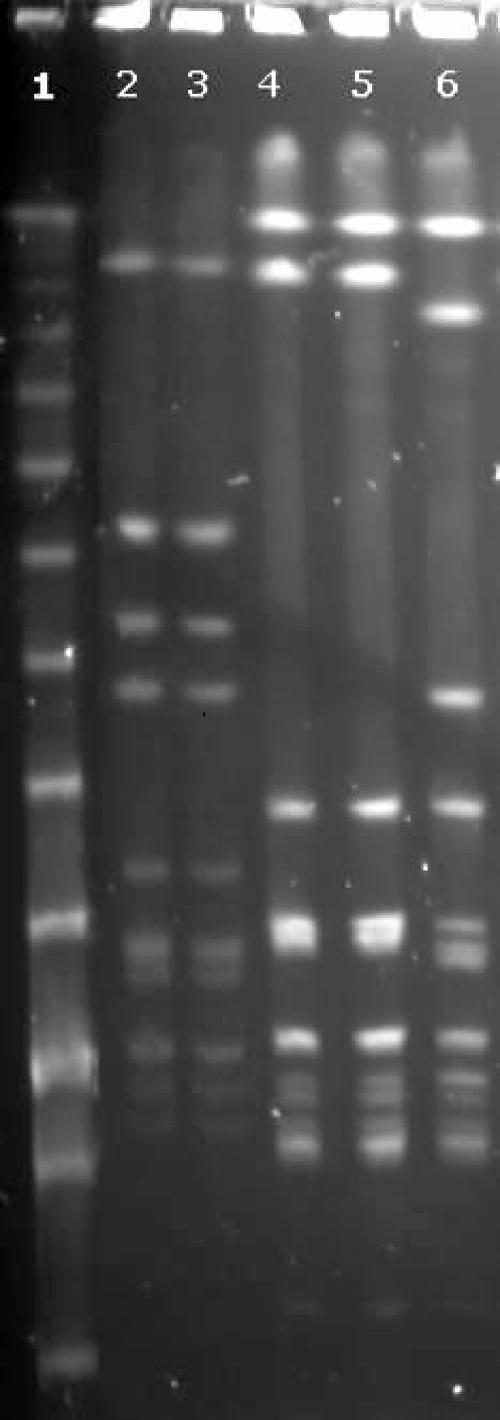
The streptococcal isolates from blood and toe web specimens were stored at −80°C in tryptose broth with 20% glycerol until further testing. They subsequently were typed by macrorestriction of the genomic DNA by SmaI, followed by pulsed-field gel electrophoresis (PFGE); this method has been shown to have a high discriminatory power for the typing of pyogenic streptococci (3). Bacterial cell harvesting, preparation, digestion of DNA, and PFGE were performed as described by Sá-Leão (10), except that PIV buffer (1 M NaCl, 10 mM Tris HCl) was used instead of phosphate buffer for the preparation of cells and agarose plugs, and a lambda ladder PFG marker (New England Biolabs) was used as the molecular weight standard. A reference strain of S. dysgalactiae subsp. equisimilis, ATCC 12394, was included in the PFGE for comparison with patient strains of the same species. A ChemiImager System 5500 (Alpha Innotech) was used for photographing the agarose gel. Visual examination of the PFGE patterns, using currently accepted criteria (12), revealed identical restriction profiles for blood and toe web isolates in each case (Fig. 1).
FIG. 1.
PFGE patterns of SmaI digests of streptococcal strains from cellulitis patients. Lane 1, lambda ladder PFG marker; lanes 2 and 3, S. pyogenes from blood and toe web samples, respectively, from patient 1; lanes 4 and 5, S. dysgalactiae subsp. equisimilis from toe web and blood samples, respectively, from patient 2; and lane 6, S. dysgalactiae subsp. equisimilis ATCC 12394.
Cellulitis is a common inflammatory condition of the skin and subcutaneous tissue. The most frequent etiologic agents are S. pyogenes and Staphylococcus aureus, followed by non-group A beta-hemolytic streptococci and gram-negative bacilli (5, 7). Cellulitis is a medical emergency that often requires hospitalization due to the risk of septicemia and severe complications. The infection most commonly is located on the lower limbs (5). Risk factors include predisposing factors, such as obesity, lymphedema, and a previous history of cellulitis and saphenectomy, and sites of entry on the leg or foot (4, 6, 9).
Our patients had several recognized predisposing factors for lower-limb cellulitis, such as obesity, chronic leg edema, and a previous saphenectomy, but they had no apparent skin lesions, except for the toe web intertrigo. Although toe webs have long been suspected of providing a site of entry for cellulitis pathogens (1, 2), only two studies have included bacterial cultures of toe webs in the workup of case and control patients alike. Semel and Goldin isolated S. aureus and beta-hemolytic streptococci from the toe webs of 83% (20/24) of cellulitis cases, whereas they isolated them from only 23% (7/30) of controls (11). Similarly, the study from which the present report is derived showed that beta-hemolytic streptococci of groups A, B, C, and G and S. aureus were isolated from 48% of cases (48/100) but were isolated from only 5.5% of controls (11/200); multivariate analysis found the presence of the bacteria in toe webs to be the strongest independent risk factor for lower-limb cellulitis (odds ratio, 69.7; 95% confidence interval, 9.6 to 504.9) (4).
When cellulitis originates from the toe web, the bacterial isolates found in the bloodstream and the toe web should be identical. Our report is, to the best of our knowledge, the first one to reveal the molecular strain relationship between isolates from blood and a potential site of entry in patients with lower-limb cellulitis. The findings further support the hypothesis that toe webs can serve as a site of entry for cellulitis agents. Although it cannot be excluded that the toe web bacteria were the result rather than the cause of the cellulitis and bloodstream infection, it is an unlikely explanation. First, it has proven difficult to isolate the causative organisms from bacterial cellulits, and the infected area was not contiguous with the toe web in our cases. Second, samples obtained by swabbing the toe web usually are not bloody, despite a break in the cutaneous barrier, and also are infinitely smaller than the blood volume recommended for blood culture. A pathogen isolated from a toe web is therefore unlikely to be derived from the bloodstream.
Heavy bacterial colonization with S. aureus, beta-hemolytic streptococci, and gram-negative rods is common in fungally infected and macerated toe webs (4, 8, 11). In the presence of breached skin, these bacteria can cause highly symptomatic infections in the toe web and potentially can spread to adjacent body sites and the bloodstream. Both patients in this report harbored known cellulitis bacteria in toe webs that revealed scaling and maceration or fissures. Our findings indicate that toe webs should be examined systematically in patients who have predisposing risk factors for lower-limb cellulitis, such as lymphedema or a previous history of cellulitis or saphenectomy. Prompt treatment of abnormal toe webs might prevent life-threatening infections, as was demonstrated by two cases for which the cessation of recurrent cellulitis was observed after treatment of tinea pedis (1).
Acknowledgments
This study was funded in part by grants from Landspítali University Hospital.
Footnotes
Published ahead of print on 3 July 2007.
REFERENCES
- 1.Baddour, L. M., and A. L. Bisno. 1984. Recurrent cellulitis after coronary bypass surgery. Association with superficial fungal infection in saphenous venectomy limbs. JAMA 251:1049-1052. [PubMed] [Google Scholar]
- 2.Baddour, L. M., and A. L. Bisno. 1985. Non-group A beta-hemolytic streptococcal cellulitis. Association with venous and lymphatic compromise. Am. J. Med. 79:155-159. [DOI] [PubMed] [Google Scholar]
- 3.Bert, F., C. Branger, and N. Lambert-Zechovsky. 1997. Pulsed-field gel electrophoresis is more discriminating than multilocus enzyme electrophoresis and random amplified polymorphic DNA analysis for typing pyogenic streptococci. Curr. Microbiol. 34:226-229. [DOI] [PubMed] [Google Scholar]
- 4.Björnsdóttir, S., M. Gottfredsson, A. S. Thórisdóttir, G. B. Gunnarsson, H. Ríkardsdóttir, M. Kristjánsson, and I. Hilmarsdóttir. 2005. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin. Infect. Dis. 41:1416-1422. [DOI] [PubMed] [Google Scholar]
- 5.Carratalà, J., B. Rosón, N. Fernández-Sabé, E. Shaw, O. del Rio, A. Rivera, and F. Gudiol. 2003. Factors associated with complications and mortality in adult patients hospitalized for infectious cellulitis. Eur. J. Clin. Microbiol. Infect. Dis. 22:151-157. [DOI] [PubMed] [Google Scholar]
- 6.Dupuy, A., H. Benchikhi, J. C. Roujeau, P. Bernard, L. Vaillant, O. Chosidow, B. Sassolas, J. C. Guillaume, J. J. Grob, and S. Bastuji-Garin. 1999. Risk factors for erysipelas of the leg (cellulitis): case-control study. BMJ 318:1591-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson, B., C. Jorup-Rönström, K. Karkkonen, A. C. Sjöblom, and S. E. Holm. 1996. Erysipelas: clinical and bacteriologic spectrum and serological aspects. Clin. Infect. Dis. 23:1091-1098. [DOI] [PubMed] [Google Scholar]
- 8.Leyden, J. J., and A. M. Kligman. 1978. Interdigital athlete's foot. The interaction of dermatophytes and resident bacteria. Arch. Dermatol. 114:1466-1472. [DOI] [PubMed] [Google Scholar]
- 9.Roujeau, J. C., B. Sigurgeirsson, H. C. Korting, H. Kerl, and C. Paul. 2004. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology 209:301-307. [DOI] [PubMed] [Google Scholar]
- 10.Sá-Leão, R., A. Tomasz, I. S. Sanches, S. Nunes, C. R. Alves, A. B. Avo, J. Saldanha, K. G. Kristinsson, and H. de Lencastre. 2000. Genetic diversity and clonal patterns among antibiotic-susceptible and -resistant Streptococcus pneumoniae colonizing children: day care centers as autonomous epidemiological units. J. Clin. Microbiol. 38:4137-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semel, J. D., and H. Goldin. 1996. Association of athlete's foot with cellulitis of the lower extremities: diagnostic value of bacterial cultures of ipsilateral interdigital space samples. Clin. Infect. Dis. 23:1162-1164. [DOI] [PubMed] [Google Scholar]
- 12.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]