Abstract
Enterotoxigenic Escherichia coli (ETEC) is one of the main causes of childhood diarrhea in developing countries and in travelers. However, this pathogen has often not been reported in surveys of diarrheal pathogens, due to lack of simple standardized methods to detect ETEC in many laboratories. ETEC expresses one or both of two different enterotoxin subtypes: heat-stable toxins, a heat-labile toxin (LT), and more than 22 different colonization factors (CFs) that mediate adherence to the intestinal cell wall. Here we compare established phenotypic and genotypic detection methods and newly developed PCR detection methods with respect to sensitivity, specificity, positive predictive value, and ease of performance. The methods include GM1-enzyme-linked immunosorbent assay and dot blot techniques using specific monoclonal antibodies (MAbs) for phenotypic detection of the toxins and CFs, respectively, as well as different PCR and DNA/DNA hybridization techniques, including new PCR assays, for genotypic identification of the toxin and CF genes, respectively. We found very good general agreement in results derived from genotypic and phenotypic methods. In a few strains, LT and CFs were identified genetically but not phenotypically. Based on our analyses, we recommend initial screening for ETEC in clinical samples by multiplex toxin gene PCR. Toxin-positive strains may then be analyzed by dot blot tests for detection of the CFs expressed on the bacterial surface and by PCR for determination of additional CFs for which MAbs are currently lacking as well as for strains that harbor silent CF genes.
Enterotoxigenic Escherichia coli (ETEC) is one of the main causes of morbidity and mortality in children under the age of five in developing countries and in travelers and soldiers positioned in these areas (3, 18, 21, 25). ETEC has, however, been grossly underestimated as the cause of diarrhea in many studies, mainly because few laboratories have suitable methods in place for ETEC diagnostics (18). Hence, it is of great importance to introduce simple and reliable methods for diagnosis of ETEC in all laboratories that are involved in identifying microbes associated with diarrhea. This study was undertaken to identify ETEC diagnostic methods that are suitable for more widespread introduction into research and clinical laboratories.
ETEC expresses one or both of two types of toxin: heat-stable toxin (ST) and heat-labile toxin (LT), both of which induce diarrhea. The ST toxin is encoded by two different genes, estA and st1, both of which code for small peptides (19 and 18 amino acids) with similar functions and gene sequences. The translated toxins are called STh (estA), originally found in ETEC isolated from humans, and STp (st1), found in ETEC isolated from pigs. The ST genes may be expressed alone or in combination with the LT genes eltA and eltB. Since eltAB may also be expressed alone, there are seven possible toxin combinations that can be expressed in individual ETEC strains: STh, STp, STh/LT, STp/LT, LT, and less commonly, STh/STp and LT/STh/STp.
ETEC may also express several different colonization factors (CFs) that bind to the intestinal cell lining and mediate bacterial adhesion to the intestinal cells (6). Since ETEC strains express one or more of over 22 different ETEC colonization factors, some of which are well characterized and others less so (1, 5, 6, 7, 8, 16), there is a large variation in virulence factor profiles in clinical isolates of ETEC.
The detection and characterization of clinical ETEC isolates have been accomplished by a variety of phenotypic and genotypic methods. These include phenotypic assays for toxins and CFs based on recognition of monoclonal antibodies (MAbs) (2, 9, 10, 17, 23, 24, 26) and genotypic methods based on either DNA/DNA hybridization (22), PCR, or real-time PCR techniques (19, 27).
In the present study, we compared the performances of different methods for detection of enterotoxins and CFs. A modified GM1-enzyme-linked immunosorbent assay (ELISA) for analysis of the ST and LT toxins along with gene probes for DNA/DNA hybridization (22) and a new multiplex PCR for simultaneous detection of the LT gene eltB and the ST variants st1 (STp) and estA (STh) were compared. We also compared a new set of PCR assays with MAb dot blot analyses for detection of different CFs. These studies were performed to establish reliable methods that could be used in different laboratories all over the world to detect ETEC. Identification of ETEC in clinical samples worldwide will provide important information for the development of successful vaccines against ETEC, such as identification of the most common virulence factors that are capable of inducing protective immunity to the pathogen and identification of the most common combinations of ETEC toxins and CFs in different geographic locations and over time.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Three ETEC strain collections were used. They included (i) a well-studied reference strain collection containing strains that express known combinations of toxins and CFs, used for setting up the methods (Table 1); (ii) a collection of 126 strains isolated from soldiers with diarrhea in Israel which had previously been indicated to be ETEC positive, used for testing the sensitivity and specificity of the two genotypic methods, DNA/DNA hybridization and PCR, for comparison to the sensitivity and specificity of the phenotypic methods used as gold standards, GM1-ELISA and dot blot tests; and (iii) an E. coli strain collection from Egypt that includes several ETEC strains, used to analyze E. coli strains for genotypic and phenotypic expression of ETEC toxins and CFs and to determine the sensitivity, specificity, and positive predictive value (PPV) of the multiple-toxin PCR and CF PCR assays compared to those of the phenotypic methods. The reference strain collection was kept at −70°C in our department. The Israeli and Egyptian strains were sent to Sweden as individual colonies in deep agar, and after being cultured on blood agar to test for purity, the colony stocks were kept at −70°C in Luria Bertani (LB) medium containing 15% glycerol. Just prior to testing, one loop of bacteria from the frozen stock cultures was spread onto a colonization factor antigen (CFA) agar plate containing bile salts (2% agar, 1% Casamino Acids [Difco, Becton Dickinson, Sparks, MD], 0.15% yeast extract [Difco, Becton Dickinson, Sparks, MD], 0.15% bile salts [Oxoid, Hampshire, United Kingdom], 0.41 mM MgSO4, 0.04 mM MnCl2 [pH 7.4.]) and grown overnight at 37°C. Three colonies were individually picked from each plate, using sterile toothpicks, and inoculated onto GM1-coated ELISA plates containing supplemented LB broth. The same toothpicks were used to inoculate each colony onto a new CFA agar plate for further studies. After being confirmed as toxin positive by GM1-ELISA, one of the three plated colonies from the CFA plate (referred to as plate 1 in the following sections) was selected and tested for CFs by dot blot tests and used for DNA extraction and analysis by DNA/DNA hybridization (22). The described procedure was performed to ensure that the same original clone was used for all analyses. Strains tested for the colonization factor CS21(Longus) were grown on blood agar plates before the dot blot test was performed. Strains expressing the CFs CFA/I, CS1-6, CS8, CS12, CS14, and CS17 were initially grown on CFA agar plates both with and without added bile salts. Phenotypic expression levels were compared, and since addition of bile salts did not affect the expression of CFA/I, CS1 to CS4, and CS6, which are independent of bile for surface expression, we subsequently grew all strains on bile salt-containing plates before performing phenotypic tests of CF profiles.
TABLE 1.
Reference strains and primer sequences used for ETEC toxins and CFs
| Reference straina | Virulence factor | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) | Product size (bp)b |
|---|---|---|---|---|
| 286 C2 | LT | ACGGCGTTACTATCCTCTC | TGGTCTCGGTCAGATATGTG | 273 |
| VX67356 | STp | TCTTTCCCCTCTTTTAGTCAG | ACAGGCAGGATTACAACAAAG | 166 |
| ST64111 | STh | AGTGGTCCTGAAAGCATG | TACAAGCAGGATTACAACAC | 64 |
| 258909-3 | CFA/I | GCTTATTCTCCCGCATCAAA | ACTTGTCCTCCCCATGACAC | 170 |
| 60R936 | CS1 | TCCGTTCGGCTAAGTCAGTT | CCGCACATTTCCTGTGTTCT | 243 |
| 58R957 | CS2 | CTGAGCACAGCTGCAACAT | TAGTTTGCTGGGTGCTTCCT | 118 |
| E19446 | CS3 | CTAGCTTTGCCACCACCATT | GGCAACTGACTCCCATTTGT | 100 |
| E11881/9 | CS4 | ACCTGCGGCAAGTCGTTT | TCTGCAGGTTCAAAAGTCACA | 198 |
| E17018/A | CS5 | TCCGCTCCCGTTACTCAG | GAAAAGCGTTCACACTGTTTATATT | 226 |
| E11881/14 | CS6 | CTGTGAATCCAGTTTCGGGT | CAGGAACTTCCGGAGTGGTA | 152 |
| E29101A | CS7 | CGCCGGTTACACGTAGTGAT | CCATTTAAAGTGATTGCGACTT | 154 |
| E34420A | CS8 | ATCCGGATTATCAAGCTCCA | GAAGATGTTATTGCACCACCAA | 166 |
| 350C1A | CS12 | CCAGTCTATGCCAGGTTGCT | TGTGGGGTCACAGTTTACCA | 137 |
| NTb | CS13 | GGGACTGCCACAATGAATTT | CAGCACCACCTGCTGATTTA | 178 |
| E7476A | CS14 | TTTGCAACCGACATCTACCA | CCGGATGTAGTTGCTCCAAT | 162 |
| NT | CS15 | CGAAATTGGACAAGCGATG | TCCAGCAGGGATATTATTCG | 130 |
| E20738A | CS17 | GGAGACGCTGAATACAACTGA | CTCAGGCGCAGTTCCTTGT | 130 |
| CS18 | CS18 | AACCAGCACCGGTGATAAAG | CTGGCTGGCCATTTAAGGTA | 131 |
| E20738A, CS19 | CS17/19 | CGGTGCGTTTAACACAGCTA | TCGATACACTCGCATTCGTT | 195 |
| CS20 Ws 7179A | CS20 | AGGTATCCAAATCCGCACTG | CATCAGCCAGCACATAGGAA | 114 |
| E9034A | CS21(Longus) | CCAGATTTTGTGGACCCATT | GTTAAAGCACCGCCAATAGC | 158 |
| NTb | CS22 | ATTGGACAAGCGTCCAACAC | TTCCAGCAGGGATATTATCATTTT | 127 |
All strains were from the strain collection of the Department of Microbiology and Immunology, Institute of Biomedicine, Göteborg University, Sweden.
NT, not tested in this study; bp, base pair.
Toxin GM1-ELISA.
Ganglioside-GM1-ELISA and inhibition GM1-ELISA to detect LT and ST, respectively, were performed essentially as previously described (23, 24), with some modifications. ELISA microtiter wells were coated with GM1 (0.5 μg/ml in phosphate-buffered saline [PBS] at room temperature overnight), and the E. coli strains to be tested were grown in 100 μl of LB broth supplemented with lincomycin (45 μg/ml) and glucose (2.5 mg/ml) in GM1-coated microtiter wells at 37°C overnight. Released LT from the bacteria samples bound to the solid-phase GM1 and was detected by means of an anti-LT MAb incubated for 90 min at room temperature. Detection of bound MAbs was performed, using goat anti-mouse immunoglobulin G horseradish peroxidase followed by incubation for 90 min, and finally, H2O2 and ortho phenylenediamine were added as substrates in order to visualize the results. For detection of ST using an inhibition ELISA, half of the bacterial suspension (50 μl) grown in the microtiter plate wells described above was transferred to a new GM1 plate freshly coated with a covalently linked ST choleratoxin & subunit conjugate. Thereafter, an anti-ST MAb that competitively binds either free ST in the bacterial supernatant or solid-phase-bound ST choleratoxin & subunit was immediately added, and the mixture was incubated at room temperature for 90 min. Binding of the MAb was detected as described for the LT GM1-ELISA. In contrast to the LT GM1-ELISA, high concentrations of ST in the medium resulted in reduced absorbance after detection in the ST inhibition ELISA.
MAb dot blot assays.
Dot blot tests to detect CFs expressed on the bacteria were performed by applying approximately 4 × 106 bacteria (2 μl at an optical density at 600 nm of 1.6 in PBS) to a nitrocellulose membrane. The dried membrane was blocked with bovine serum albumin-PBS for 20 min followed by the addition of the CF MAb diluted in 0.1% bovine serum albumin-PBS-0.05% Tween 20. The membrane was incubated for 2 h in a humid chamber and washed with PBS-0.05% Tween. Goat anti-mouse immunoglobulin G horseradish peroxidase conjugate solution was added to the membrane, followed by incubation for 2 h, and the bound MAb was detected by addition of 4-chloro-1-naphthol chromogen and H2O2. All incubations were performed at room temperature.
A positive reaction was visualized as a dark blue- or gray-stained dot on the surface of the strip. The MAbs against CFA/I, CS1 to CS6, CS8, CS12, CS14, and CS17 were developed in-house at our department (9, 10, 26). The MAbs against CS19 were kindly provided by Sami Farid Khalil (NAMRU-3, Cairo, Egypt), and the MAb for CS21(Longus) was kindly provided by Firdausi Qadri (ICDDR,B Dhaka, Bangladesh).
DNA/DNA probe hybridization assay.
Bacteria from the selected colony spread on CFA agar plate 1 were analyzed for DNA probe hybridization. Transformed E. coli strains were kindly provided by H. Sommerfelt and H. Steinsland, and plasmids were extracted from these strains and used to generate digoxigenin-labeled PCR probes used for hybridization, as previously described in detail (22).
DNA extraction and PCR.
For PCR assays, a loop of bacteria from the selected colony spread on CFA agar plate 1 was mixed in Milli-Q water (500 μl) and boiled for 10 min. The remaining pellet was quickly spun down, and the supernatant was diluted 1:500 in Milli-Q water and used in the subsequent PCR steps to amplify the specific gene sequence of each CF or toxin. PCR primers were designed for detection of CFA/I, CS1, CS2, CS3, CS4, CS5, CS6, CS7, CS8, CS12, CS13, CS14, CS15, CS17, CS18, CS19, CS20, CS21, and CS22 (Table 1). The CF primers were designed from unique subunit sequences of the specific CFs, except for the CS17/CS19 primers that recognize both CS17 and CS19, since those related CFs have very similar sequences. The specificity of the primers was tested by both BLAST search and PCR amplification, using the reference strain collection (Table 1). Specific amplification of the desired gene product was confirmed for all the analyzed reference strains, using both DNA purified by a DNeasy kit (QIAGEN, Hilden, Germany) and DNA prepared by rapid boiling. The primers for CS13, CS15, and CS20 were not tested for amplification with the reference strain collection since we lacked reference strains for those CFs. Strains (from the different strain collections) that express CFA/I, CS2, CS3, CS6, CS7, CS8 CS12, CS14, CS17, and CS19 were analyzed.
PCR was performed in 96-well plates. For each 25 μl PCR mixture, 10 μl of boiled bacterial DNA (1/500 dilution, 10 to 100 ng) was mixed with 15 μl of Master mix containing a final concentration of 1× PCR buffer, 2.0 mM MgCl2 (Sigma Aldrich, St. Louis, MO), 200 nM deoxynucleoside triphosphate (dNTP; Roche, Mannheim, Germany), 200 nM of each primer, and 1 U Taq polymerase (Sigma Aldrich, St. Louis, MO). The 96-well plates were sealed with plastic films and amplified in a PCR thermocycler by an initial denaturation step at 94°C for 5 min followed by 40 cycles at 94°C (30 s), 52°C (30 s), and 72°C (30 s). Final elongation was performed at 72°C for 5 min. All CF primers were amplified by using the same PCR conditions and amplified PCR products of the correct lengths. The PCR products were separated in 2% agarose gel, stained by ethidium bromide, and visualized under UV light.
Multiplex-toxin PCR.
For multiplex-toxin PCR, the conditions were the same as described above except that the dNTPs were increased to 350 nM per reaction and the three toxin primer sets were added together to each reaction (200 nM each). Annealing was performed at 52 to 54°C, depending on the local conditions, as described below. The multiplex reaction mixtures were separated in 3% agarose gel and stained as described above.
Statistical analysis.
Sensitivity, specificity, and positive predictive values were determined, using GM1-ELISA and dot blot techniques as the gold standards. Two-sided 95% confidence intervals corrected for continuity were determined (11).
RESULTS
Comparison of phenotypic and genotypic methods for detection of ST and LT using GM1-ELISA, DNA/DNA hybridization, and PCR.
Initial screening for ETEC in diarrhea samples should focus on specific and accurate detection of ST and LT. To compare phenotypic and genotypic methods, the GM1-ELISA method was compared with DNA/DNA hybridization (22) and PCR amplification (4) for detection of ETEC toxins and their respective genes. A collection of reference strains (Table 1) and 127 samples from an Israeli strain collection were tested for the presence of ST and LT by GM1-ELISA (Table 2) and compared with results obtained from testing the strains for St1 (STp), estA (STh), and eltB (LT) by DNA/DNA hybridization and PCR (see Table 4), using the primers described in Table 1. Analyses of the reference strains by the different methods to determine their capacities to detect the different toxins indicated that each method was specific and accurate for detection of the respective toxin or gene, and no false positive or negative results were obtained with any method. Analyses of the 126 Israeli strains with the GM1-ELISA, DNA/DNA hybridization, and PCR methods indicated a good level of agreement between the phenotypic and genotypic methods, although the DNA/DNA hybridization technique had lower levels of sensitivity and specificity than the PCR methods, which showed the highest level of sensitivity (Table 2). The results for ST expression of the GM1-ELISA were compared with the genotypic expression levels of both estA and st1, since our GM1-ELISA does not distinguish between the two toxins STh and STp. However, the two genotypic methods, PCR and DNA/DNA hybridization, distinguished between the ST gene variants estA and st1, and these methods always identified the same ST-encoding gene in each strain. Consequently, the phenotypic and genotypic methods were specific for each toxin and generated similar results in most strains regardless of the method used. However, a slightly higher level of agreement was obtained for the results of the ELISA and PCR than for the results of the ELISA and DNA/DNA hybridization.
TABLE 2.
Sensitivity and specificity of the two genotypic methods compared to those of GM1-ELISA in identifying toxins from strains of the Israeli ETEC strain collection
| Method | Toxin | Level (%) sensitivity | 95% CI | No. of strains positive/no. of strains positive by GM1-ELISA | Level (%) specificity | 95% CI | No. of strains negative/no. of strains negative by GM1-ELISA |
|---|---|---|---|---|---|---|---|
| PCR | ST | 98 | 86-100 | 43/44 | 99 | 92-100 | 81/82 |
| LT | 100 | 84-100 | 26/26 | 98 | 92-100 | 98/100 | |
| DNA/DNA hybridization | ST | 88 | 74-96 | 39/44 | 95 | 87-98 | 78/82 |
| LT | 92 | 73-99 | 24/26 | 97 | 91-99 | 97/100 |
TABLE 4.
Detection of CFs in the Israeli ETEC strain collection by dot blot, PCR, and DNA probe hybridization
| CF | No. of strains positive by indicated method (no. of strains positive by both phenotypic and indicated genotypic methods):
|
||
|---|---|---|---|
| Dot blot | PCR | Probe hybridization | |
| CFA/Ia | 3 | 3 (3) | 3 (3) |
| CS2b | 10 | 11 (10) | 10 (10) |
| CS3b | 10 | 11 (10) | NTb |
| CS4a | 3 (0) | ||
| CS5 | 12 | 12 (12) | 12 (12) |
| CS6 | 26 | 26 (26) | 25 (25) |
| CS7 | 11 (0)c | ||
| CS8 | 4 | 4 (4) | 8 (3)d |
The DNA/DNA hybridization probe for CFA/I cross-hybridized with CS4.
One genotypically positive and phenotypically negative CS2/CS3 strain was detected by PCR. CS3 was not tested (NT) by DNA/DNA hybridization.
The assays for the closely related CFs CS5 and CS7 cross-reacted during DNA/DNA hybridization. PCR distinguished between CS5 and CS7 and detected only CS5 in this strain collection.
The CS8 DNA/DNA hybridization assay identified five strains as positive for CS8 that were not recognized by either dot blot or PCR for CS8 and missed one strain positive by dot blot and PCR.
Multiplex PCR for detection of toxin genes.
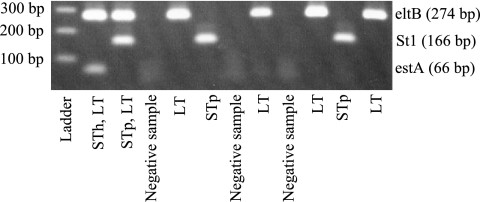
Based on our finding that the PCR-based method had a higher level of sensitivity than the DNA/DNA hybridization method and was easier to perform, we favor PCR for genotypic detection of ETEC toxins. However, since amplification of a single PCR product is not time efficient, we evaluated the possibility of pooling the STh, STp, and LT primer pairs in one reaction mixture, using a mixture of the toxin reference strains as the template (Fig. 1). By using this multiplex PCR, we were able to detect all three products by increasing the amount of dNTPs to 350 nM and the PCR cycling to 40 cycles.
FIG. 1.
Multiplex-toxin PCR performed on a sample of ETEC strains phenotypically positive for the respective toxin.
A strain collection from Egypt consisting of 81 E. coli strains, both toxin positive and toxin negative, that had been isolated from children diagnosed with diarrhea was used for comparison of the abilities of the GM1-ELISA and multiplex-PCR methods to identify ETEC toxins. A single PCR for each of the toxin genes was performed on the same samples to verify the accuracy of the multiplex PCR. The multiplex PCR for detection of toxin genes was as efficient as single-gene PCR, and the results obtained by the two methods showed complete concordance. The multiplex PCR and the toxin ELISA results agreed for all ST and LT/ST ETEC strains tested. However, three LT-only ETEC strains were found to be eltB positive by multiplex PCR but LT negative by GM1-ELISA (Table 3). It is possible that these strains contain silent genes or produce low LT levels that are below the detection limit of the phenotypic method. Consequently, multiplex PCR is a fast and sensitive method to detect ETEC in clinical specimens and allows detection of the corresponding genes in all phenotypically positive strains (100% sensitivity), while the PPV for detecting the toxin in genotypically positive strains is 88% (95% confidence interval = 67% to 98%).
TABLE 3.
Sensitivity, specificity, and PPV of GM1-ELISA and multiplex PCR for ST versus estA and/or st1 and LT versus eltB in identifying toxins from strains of the Egyptian E. coli strain collectiona
| Toxin | Level (%) of sensitivity | 95% CI | No. of strains positive by multiplex PCR/no. of strains positive by GM1-ELISA | Level (%) of specificity | 95% CI | No. of strains negative by multiplex PCR/no. of strains negative by GM1-ELISA | PPV | 95% CI | No. of strains positive by GM1-ELISA/no. of strains positive by multiplex PCR |
|---|---|---|---|---|---|---|---|---|---|
| ST | 100 | 79-100 | 19/19 | 100 | 93-100 | 62/62 | 100 | 79-100 | 19/19 |
| LT | 100 | 80-100 | 21/21 | 95 | 85-99 | 57/60 | 88 | 67-98 | 21/24 |
Multiplex PCR and GM1- ELISA gave identical results for all ST-only strains (n = 7) and ST/LT strains (n = 12) and for 9/12 LT-only strains. The gene for eltB was detected in three strains that were negative for both ST and LT by GM1-ELISA.
Phenotypic and genotypic methods to detect CFs.
The Israeli strain collection was used to analyze ETEC CFs by the phenotypic and genotypic methods. For the phenotypic analysis, a dot blot analysis based on MAbs specific for CFA/I, CS1, CS2, CS3, CS4, CS5, CS6, CS8, CS12, CS14, CS17, CS19, and CS21 was used. For the genotypic expression of CFs, all strains were analyzed by dot blotting for all available MAbs and (for most of the CFs) by DNA/DNA hybridization and our new set of PCR assays for 19 CFs. Comparisons of strains for CFs by dot blot analysis and PCR generally showed a good level of agreement (Table 4), whereas in some cases, the DNA/DNA hybridization method showed cross-hybridization between related CFs (Table 4). This was observed for the closely related CFs CS5 and CS7 and for CFA/I and CS4, which share homologous sequence regions. We were able, however, to design specific PCR assays for these genes. CS17 and CS19 cross-hybridized during DNA/DNA hybridization and cross-reacted immunologically in dot blot tests. The PCR designed to amplify CS19 was found to detect both CS17 and CS19, but we were able to design PCR primers that specifically amplified only CS17. Since PCR was more specific than DNA/DNA hybridization for detection of some of the CFs, the 28 E. coli strains from the Egyptian collection which were positive in the toxin ELISA were analyzed for the presence of CFs by dot blot and PCR. As shown in Table 5, we found silent genes coding for CS6 and CS19, but all phenotypically positive strains in this strain collection were found to have the corresponding gene.
TABLE 5.
Detection of CFs by dot blot and PCR for the Egyptian strain collectiona
| CF | No. of positive strains determined by indicated method:
|
No. of positive strains determined by both dot blot and PCR: | |
|---|---|---|---|
| Dot blot | PCR | ||
| CFA/I | 1 | 1 | 1 |
| CS2 | 1 | 1 | 1 |
| CS3 | 4 | 4 | 4 |
| CS6b | 5 | 7 | 5 |
| CS7 | 2 | 2 | 2 |
| CS12 | 4 | 4 | 4 |
| CS14 | 2 | 2 | 2 |
| CS17 | 1 | 1 | 1 |
| CS19b | 0 | 2 | 0 |
The PCR assays for CS6 and CS19 (i.e., positive for CS17/CS19 but negative for CS17) each detected two strains that were phenotype negative.
DISCUSSION
There are a variety of different genotypic and phenotypic methods available for detection of ETEC toxins and colonization factors in clinical isolates. In this study, we performed an extensive comparison of previously used and newly established methods in order to determine the sensitivity, specificity, and PPV for each of the tested methods. This was done by testing strain collections for different virulence factors with different genotypic and phenotypic methods. We confirmed that the genotypic and phenotypic detection methods gave results that were mainly in agreement, although some discrepancies between the different methods, which may have both biological and methodological explanations, were observed.
Identification of ETEC in clinical and environmental samples is best performed by detecting the ST and LT toxins. We compared GM1-ELISA, DNA/DNA hybridization, PCR, and multiplex PCR to evaluate whether these methods gave similar results. Combined GM1-ELISA for detection of ST and LT from the same bacterial culture was used as the gold standard, since it has been used in our laboratory and in many other laboratories for several years for the screening of ETEC from clinical isolates (2, 12, 14, 15, 17, 20, 21). When comparing the results of the phenotypic methods for identifying ETEC toxins with those of the genotypic methods, we found the gene in the absence of the toxin by both PCR and DNA/DNA hybridization in one strain from the Israeli strain collection that was positive for estA and two strains that were positive for eltB. In addition, the eltB gene was detected by both single-gene PCR and multiplex PCR in three phenotypically negative strains from the Egyptian strain collection. Consequently, the possibility that either the eltB gene is present as a silent gene or, alternatively, that the phenotypic expression level of LT in certain strains may be very low might have to be considered (in epidemiological studies, for example), since its expression might have been lost during culture but present in the initial clinical isolate.
Our results confirm that the multiplex PCR for toxin detection developed in this study was as efficient as the combination of single-gene PCR for eltB, estA, and st1. The multiplex-PCR had high levels of sensitivity and specificity and high PPVs, especially for detection of the ST toxins and, in addition, took less time to perform. Hence, this method may be considered to be a suitable approach for ETEC detection in laboratories that have the necessary facilities and that are planning to initiate ETEC diagnostics and/or surveillance.
Although detection of the toxins is enough to verify the presence of ETEC, toxin-positive strains may be further analyzed for the presence of CFs, particularly in epidemiological or vaccine studies, by dot blot tests using specific MAbs, DNA/DNA hybridization, or PCR. E. coli strains were grown on CFA agar containing bile salts, since phenotypic expression of CS5, CS7, CS8, CS14, and CS17 requires bile in the medium. Comparisons of phenotypic expression levels with and without bile salts were made for those CFs that do not require bile salts for phenotypic expression, i.e., CFA/I, CS1 to CS4, and CS6. All tested CFs were almost unaffected by the presence of bile. Based on these results, we cultured all strains on CFA containing bile salts prior to the dot blot and DNA extraction procedures. However, it should be noted that detection of CS21(Longus) is possible only after growth on blood agar plates and thus needs to be tested separately.
When comparing the results for dot blot tests, DNA/DNA hybridization, and PCR of the Israeli strain collection, we found a good level of agreement among the results, although cross-hybridizations between related CFs were observed for the DNA/DNA hybridization technique in some cases (22). The corresponding gene was almost always detected in phenotypically positive strains with both genotypic methods, and consequently, both genotypic assays seemed to have a high level of sensitivity for detecting the gene in strains with the corresponding phenotypic expression. On the other hand, very few strains that were positive by the genotypic methods were phenotypically negative, although the DNA probe for CS8 detected five strains that were negative by dot blot tests using all available MAbs and negative by all PCR assays. Whether these strains contain a yet-unknown CF with a relationship to CS8 remains to be determined.
When comparing the two genotypic methods used in this study, we observed different advantages and disadvantages: for instance, DNA/DNA hybridization may cause misinterpretation due to cross-hybridization between closely related CFs, while mutations at single-nucleotide positions may cause the failure of PCR amplification if the complementary primer binding sequence is affected. However, due to the higher sensitivity and specificity of PCR, we used only PCR to compare genotypic and phenotypic expression levels of CFs in the Egyptian strain collection. Also, for these strains we found that the corresponding gene in all instances was found in phenotypically positive strains and that the few discrepancies observed between the results for the phenotypic and genotypic methods were due to the presence of the gene and the absence of phenotypic expression of the respective CF. For instance, two Egyptian STp/LT strains were negative by the dot blot tests and when tested for CS17 by PCR but were positive by the CS17/CS19 PCR; these strains probably either contained silenced CS19 genes or had very low levels of surface expression of CS19. In addition, two Egyptian LT-only strains were negative for phenotypic expression of any CFs by dot blot tests but had the CS6 gene. The absence of phenotypic expression of CS6 in LT-only-expressing ETEC strains that harbor the CssA to CssD genes coding for CS6 has been described before (7), and we have recently shown that this feature may have a genetic explanation (12).
The probability of phenotypic silencing may increase even more during storage and recultivation, either due to loss of regulatory genes or posttranscriptional or posttranslational events. The relevance of gene silencing in vivo is unclear, but genotypic methods may detect silenced genes, and the possibility that these genes have been expressed during infection must be taken into account.
In summary, for identification of ETEC we recommend initial analysis of E. coli strains by a multiplex-toxin PCR, due to the high levels of sensitivity and specificity of this method and its ability to detect both expressed and putatively silent genes, which ensures that no ETEC strains are missed. This may be done for strains grown on MacConkey agar or a related medium that allows differentiation of lactose-fermenting E. coli from other bacteria present in the stool. The multiplex PCR was designed to work well with DNA obtained by rapid boiling of the bacteria. In order to ensure detection of ETEC, if present, we recommend testing a mixture of several individual colonies (10, 11, 13-16) or a loop of bacteria from an area of confluent growth from an agar plate that selects for E. coli growth.
When we established the multiplex-toxin PCR, we particularly aimed to develop a robust PCR optimized for use with DNA prepared by the rapid-boil technique to allow examination of large strain collections without the need for labor-intensive DNA preparations and for use in less-well-equipped laboratories, e.g., in developing countries. The assay was found to be robust, allowed for accurate identification of the three toxin genes with DNA prepared by simple boiling of the bacteria, and allowed rapid identification (less than 24 h) of ETEC from E. coli strains derived from cultures of diarrheal stool samples when the method was tested at ICDDR,B in Dhaka, Bangladesh (Å. Sjöling, F. Qadri et al., unpublished data). It would also be possible to extract DNA directly from diarrheal stool samples and test for the presence of ETEC by the multiplex-PCR method. However, this approach would require DNA extraction with a commercial kit to avoid PCR inhibitory factors present in the stool.
Although the optimal annealing temperature for the multiplex-toxin PCR was 52°C in our laboratory, we found that increasing the annealing temperature to 54°C may provide more accurate results during work in tropical temperatures such as those in Dhaka. The reason for this is probably that a more stringent annealing temperature is required to ensure specific amplification of each gene when the reactions are set up in room temperatures exceeding 30°C. Hence, optimization of the multiplex PCR may be necessary in certain cases, and we recommend either increasing or decreasing the annealing temperature.
This study was performed to provide an optimized protocol for ETEC detection and surveillance as well as to provide descriptions for several genotypic and phenotypic methods for detection of both toxins and CFs. At present, we have access to MAbs against ST and LT as well as MAbs against 13 different CFs. The primer sequences and assays for the multiplex-toxin PCR (3 toxins) and 19 different CFs are described in this study. Our intention is that this paper will facilitate the implementation of methods for identification and characterization of ETEC in clinical diarrheal samples. The choice of method for any specific laboratory is dependent on equipment, resources, and time constraints, but we suggest initial analysis of E.coli colonies from clinical isolates by using multiplex-toxin PCR. Subsequent analyses for ETEC CFs could be performed on one to two toxin-positive colonies from each original isolate, using either dot blot tests or PCR.
Acknowledgments
We acknowledge Firdausi Qadri (ICDDR,B, Dhaka, Bangladesh) for testing the multiplex PCR and for MAbs, Sami Farid Khalil (Namru-3, Cairo, Egypt) for providing MAbs, Halvor Sommerfelt (Bergen, Norway) for providing strains for DNA hybridization and suggestions for statistical analysis, and Kerstin Andersson for excellent technical assistance.
This work was supported by the Swedish Agency for Research and Economic Cooperation (Sida-SAREC); the Swedish Research Council, grant no. 6X-09084; the Knut and Alice Wallenberg Foundation through its support of the Gothenborg University Vaccine Research Institute (GUVAX); and the World Health Organization. Å.S. acknowledges the Swedish Society for Medical Research (SSMF) for support.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Anantha, R. P., A. L. McVeigh, L. H. Lee, M. K. Agnew, F. J. Cassels, D. A. Scott, T. S. Whittam, and S. J. Savarino. 2004. Evolutionary and functional relationships of colonization factor antigen I and other class 5 adhesive fimbriae of enterotoxigenic Escherichia coli. Infect. Immun. 72:7190-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binsztein, N., M. J. Jouve, G. I. Viboud, L. Lopez Moral, M. Rivas, I. Orskov, C. Ahren, and A.-M. Svennerholm. 1991. Colonization factors of enterotoxigenic Escherichia coli isolated from children with diarrhea in Argentina. J. Clin. Microbiol. 29:1893-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, R. E. 1990. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12(Suppl. 1):S73-S79. [DOI] [PubMed] [Google Scholar]
- 4.Bölin, I., G. Wiklund, F. Qadri, O. Torres, L. A. Burgeois, S. Savarino, and A.-M. Svennerholm. 2006. Enterotoxigenic Escherichia coli with STh and STp genotypes is associated with diarrhea both in children in endemic areas and in travelers. J. Clin. Microbiol. 44:3872-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleckenstein, J. M., K. Roy, J. F. Fischer, and M. Burkitt. 2006. Identification of a two-partner secretion locus of enterotoxigenic Escherichia coli. Infect. Immun. 74:2245-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaastra, W., and A.-M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 11:444-452. [DOI] [PubMed] [Google Scholar]
- 7.Grewal, H. M., A. Helander, A.-M. Svennerholm, M. K. Bhan, W. Gaastra, and H. Sommerfelt. 1994. Genotypic and phenotypic identification of coli surface antigen 6-positive enterotoxigenic Escherichia coli. J. Clin. Microbiol. 32:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grewal, H. M., H. Valvatne, M. K. Bhan, L. van Dijk, W. Gaastra, and H. Sommerfelt. 1997. A new putative fimbrial colonization factor, CS19, of human enterotoxigenic Escherichia coli. Infect. Immun. 65:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Vidal, Y., P. Klemm, and A.-M. Svennerholm. 1988. Monoclonal antibodies against different epitopes on colonization factor antigen I of enterotoxin-producing Escherichia coli. J. Clin. Microbiol. 10:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Vidal, Y., and A.-M. Svennerholm. 1990. Monoclonal antibodies against the different subcomponents of colonization factor antigen II of enterotoxigenic Escherichia coli. J. Clin. Microbiol. 28:1906-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newcombe, R. G. 1998. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 17:857-872. [DOI] [PubMed] [Google Scholar]
- 12.Nicklasson, M., Å. Sjöling, M. Lebens, J. Tobias, A. Janzon, L. Brive, and A.-M. Svennerholm. Mutations in the periplasmic chaperone leading to loss of surface expression of the colonization factor CS6 in enterotoxigenic Escherichia coli (ETEC) clinical isolates. Microb. Pathog., in press. [DOI] [PubMed]
- 13.Nirdnoy, W., O. Serichantalergs, A. Cravioto, C. LeBron, M. Wolf, C. W. Hoge, A.-M. Svennerholm, D. N. Taylor, and P. Echeverria. 1997. Distribution of colonization factor antigens among enterotoxigenic Escherichia coli strains isolated from patients with diarrhea in Nepal, Indonesia, Peru, and Thailand. J. Clin. Microbiol. 35:527-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paniagua, M., F. Espinoza, M. Ringman, E. Reizenstein, A.-M. Svennerholm, and H. Hallander. 1997. Analysis of incidence of infection with enterotoxigenic Escherichia coli in a prospective cohort study of infant diarrhea in Nicaragua. J. Clin. Microbiol. 35:1404-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peruski, L. F., Jr., B. A. Kay, R. A. El-Yazeed, S. H. El-Etr, A. Cravioto, T. F. Wierzba, M. Rao, N. El-Ghorab, H. Shaheen, S. B. Khalil, K. Kamal, M. O. Wasfy, A.-M. Svennerholm, J. D. Clemens, and S. J. Savarino. 1999. Phenotypic diversity of enterotoxigenic Escherichia coli strains from a community-based study of pediatric diarrhea in periurban Egypt. J. Clin. Microbiol. 37:2974-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pichel, M., N. Binsztein, and G. Viboud. 2000. CS22, a novel human enterotoxigenic Escherichia coli adhesin, is related to CS15. Infect. Immun. 68:3280-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qadri, F., S. K. Das, A. S. Faruque, G. J. Fuchs, M. J. Albert, R. B. Sack, and A.-M. Svennerholm. 2000. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J. Clin. Microbiol. 38:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qadri, F., A.-M. Svennerholm, A. S. Faruque, and R. B. Sack. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reischl, U., M. T. Youssef, H. Wolf, E. Hyytia-Trees, and N. A. Strockbine. 2004. Real-time fluorescence PCR assays for detection and characterization of heat-labile I and heat-stable I enterotoxin genes from enterotoxigenic Escherichia coli. J. Clin. Microbiol. 42:4092-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rockabrand, D. M., H. I. Shaheen, S. B. Khalil, L. F. Peruski, Jr., P. J. Rozmajzl, S. J. Savarino, M. R. Monteville, R. W. Frenck, A.-M. Svennerholm, S. D. Putnam, and J. W. Sanders. 2006. Enterotoxigenic Escherichia coli colonization factor types collected from 1997 to 2001 in US military personnel during operation Bright Star in northern Egypt. Diagn. Microbiol. Infect. Dis. 55:9-12. [DOI] [PubMed] [Google Scholar]
- 21.Shaheen, H. I., M. R. Rao, R. Abu Elyazeed, T. F. Wierzba, L. F. Peruski, Jr., S. Putnam, A. Navarro, B. Z. Morsy, A. Cravioto, J. D. Clemens, A.-M. Svennerholm, and S. J. Savarino. 2004. Phenotypic profiles of enterotoxigenic Escherichia coli associated with early childhood diarrhea in rural Egypt. J. Clin. Microbiol. 42:5588-5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinsland, H., P. Valentiner-Branth, H. M. Grewal, W. Gaastra, K. K. Molbak, and H. Sommerfelt. 2003. Development and evaluation of genotypic assays for the detection and characterization of enterotoxigenic Escherichia coli. Diagn. Microbiol. Infect. Dis. 45:97-105. [DOI] [PubMed] [Google Scholar]
- 23.Svennerholm, A.-M., and G. Wiklund. 1983. Rapid GM1-enzyme-linked immunosorbent assay with visual reading for identification of Escherichia coli heat-labile enterotoxin. J. Clin. Microbiol. 17:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svennerholm, A.-M., M. Wikström, M. Lindblad, and J. Holmgren. 1986. Monoclonal antibodies against Escherichia coli heat-stable toxin (STa) and their use in diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J. Clin. Microbiol. 24:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svennerholm, A.-M., and S. J. Savarino. 2004. Oral inactivated whole cell B subunit combination vaccine against enterotoxigenic Escherichia coli, p. 737-750. In M. M. Levine et al. (ed.), New generation vaccines, 3rd ed. Marcel Decker, New York, NY.
- 26.Viboud, G. I., N. Binsztein, and A.-M. Svennerholm. 1993. Characterization of monoclonal antibodies against putative colonization factors of enterotoxigenic Escherichia coli and their use in an epidemiological study. J. Clin. Microbiol. 31:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidal, R., M. Vidal, R. Lagos, M. Levine, and V. Prado. 2004. Multiplex PCR for diagnosis of enteric infections associated with diarrheagenic Escherichia coli. J. Clin. Microbiol. 42:1787-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]