Abstract
Blastobotrys proliferans is an ascomycetous yeast never previously reported as a human pathogen. Here we report a case of peritonitis due to Blastobotrys proliferans in a 46-year-old man undergoing peritoneal dialysis.
CASE REPORT
A 46-year-old Melanesian man undergoing continuous ambulatory peritoneal dialysis (CAPD) had a past medical history that included chronic bronchitis due to cigarette smoking and a certain state of malnutrition. He had been on CAPD for the past 3 years and had already had three episodes of peritonitis (two due to Staphylococcus aureus and one to Klebsiella pneumonia), from all of which he had recovered with adapted antibiotherapies.
On 3 January 2007, he was admitted to the community clinics in Lifou Island (northeast of the New Caledonia main island, Grande Terre) for abdominal pain and cloudy peritoneal dialysis bags, which clinically presumed peritonitis. The peritoneal dialysate fluid white blood cell count at admission confirmed peritonitis with more than 100/mm3 (90% polymorphonuclear neutrophils), and a Gram stain revealed numerous gram-positive cocci. The patient was started on a peritonitis protocol consisting of vancomycin (2 g/5 days) together with ceftazidime (2 g/day), both intraperitoneally. Ceftazidime was stopped when S. aureus was identified in cultures from the initial dialysate.
In spite of antibiotherapy, the patient continued to deteriorate with persistent abdominal pain. He was referred to the New Caledonia Territorial Hospital on 11 January 2007. Repeated sampling of the dialysate fluid showed persistent elevation of white blood cell count at 220/mm3 (35% neutrophils, 34% lymphocytes, and 17% eosinophils) and the presence of numerous yeasts and hyphae growing on Sabouraud agar in less than 24 h. The isolate was sent to the French National Reference Center for Mycoses due to an unusual microscopical aspect (rare segmented hyphae and unusual size and shape of conidia) and lack of identification using carbon assimilation patterns (ID32C; bioMérieux, Marcy-l'Etoile, France).
It was then decided to remove the Tenckoff catheter and to start antifungal therapy with oral fluconazole (100 mg/day), which resulted in a quick clinical improvement and the resolution of the patient's symptoms. However, treatment was switched to amphotericin B (3 mg/kg of body weight/day) for 3 weeks after antifungal drug susceptibility testing results (ATB-Fungus3; bioMerieux) showed decreased susceptibility to all of the antifungals tested, except amphotericin B. MICs of amphotericin B (0.5 μg/ml), flucytosine (8 μg/ml), fluconazole (64 μg/ml), voriconazole (2 μg/ml), posaconazole (0.5 μg/ml), and caspofungin (>8 μg/ml) were determined according to the EUCAST microdilution broth reference method (28). The inflammatory markers then returned to normal levels. The clinical symptoms resolved completely with a follow-up of 4 months.
Identification of the species Blastobotrys proliferans Marvanová was done based on the carbon assimilation pattern (ID32C and 50CH; bioMérieux) and microscopic morphology after slide culture in 2% malt agar medium after 6 days at 25°C using the keys established by de Hoog and colleagues(8, 9). Sequences were determined for the internal transcribed spacer 1 (ITS1)-5.8S-ITS2 regions (GenBank accession no. EF584542) and the D1/D2 variable region of the ribosomal DNA gene (GenBank accession no. EF58451) using universal primers V9D/LS266 (7, 21) and NL1/NL4 (24). Identification of the ascomycetous yeast B. proliferans was confirmed by comparison of the D1-D2 region nucleotidic sequence with those published in GenBank (accession no. U40098 and DQ442684) (15-17), with 99% similarity over 590 bp.
Discussion.
Peritonitis remains a common complication of peritoneal dialysis and occurs at an overall average rate of one episode every 29 months (32). The most common etiology is bacterial peritonitis, with S. aureus being the most frequently implicated species. However, in New Caledonia, the frequency of peritonitis is higher, due to poor housing conditions, reaching the rate of one episode every 16 to 20 months. Fungal peritonitis is a less frequent (4 to 6% of all peritonitis in this context) (1) but a more severe complication, requiring Tenckoff catheter removal and a switch to definitive hemodialysis. A history of antibiotherapy for bacterial peritonitis within the 4 weeks preceding fungal peritonitis is often but not systematically reported (29). Risk factors also identified for development of fungal peritonitis include recent bacterial peritonitis (3) and lupus (13, 30). Our patient's history thus conforms to these reports. Outcome of fungal peritonitis appears to be more favorable in children (33) and in patients with residual renal function (18).
Candida species are the most common fungi isolated (6, 26, 27). Peritonitis due to various filamentous fungi is also reported. Aspergillus spp. are responsible for a severe form of peritonitis, frequently lethal, and require prompt removal of the Tenckoff catheter while starting intravenous amphotericin B (2, 22, 23). Zygomycetes remain an uncommon cause of peritonitis associated with a high mortality rate of 57% (23). Other filamentous fungi and yeasts are even less frequently reported (Fusarium, Trichoderma, Penicillium, Paecilomyces, Curvularia, Acremonium, Rhodotorula, and Trichosporon) (4, 5, 10-12, 14, 19, 20, 25, 31). To our knowledge, B. proliferans has never been reported as a cause of infection in humans or animals, even though strain CBS 293.84 stored at the Centraal Bureau voor Schimmelcultures (Utrecht, The Netherlands) is indicated as recovered from a “cystic lesion of ankle in a man.” The reservoir of B. proliferans is unknown.
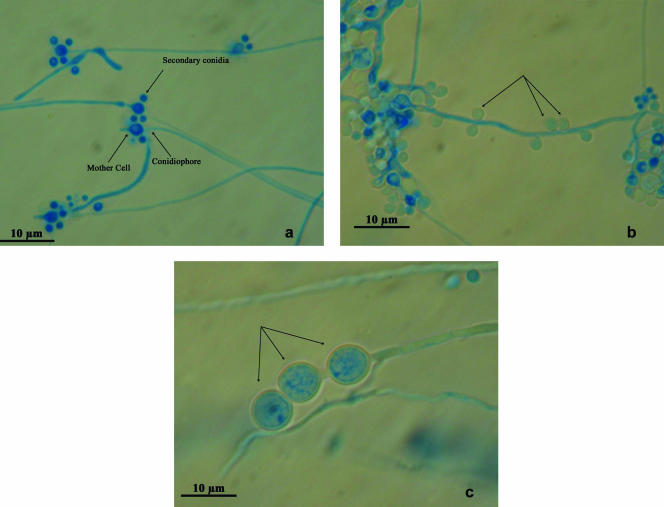
Until recently, the dimorphic genus Blastobotrys was treated as a hyphomycete, close to the ascomycetous genus Sporothrix. What distinguishes both genera is conidiogenesis. In fact, Blastobotrys species have distinct mother cells (primary conidia) and secondary conidia, whereas in Sporothrix species there is no visible differentiation between conidia of the first and second orders. The species B. proliferans has conidiophores bearing pear-shaped mother cells containing a conspicuous body (Fig. 1a). The mother cells are single, each crowned with secondary conidia. Globose, lateral conidia (Fig. 1b) and hyaline, thick-walled, terminal and intercalary chlamydospores (Fig. 1c) are present. Blastobotrys proliferans is different from all other Blastobotrys species by its proliferating mother cells and the refractive bodies in those mother cells (8). It grows with most carbon sources, does not assimilate nitrate, and ferments glucose. Growth in the presence of melibiose, raffinose, and at 37°C is characteristic of B. proliferans isolates in the genus.
FIG. 1.
Blastobotrys proliferans from a culture on 2% malt agar examined with Nomarski interphase contrast (×100). (a) Conidiophores bearing the mother cell with refractive body and secondary conidia. (b) Lateral conidia (arrows). (c) Chlamydospores (arrows).
For the purpose of phylogenetic analysis, Kurtzman and Robnett (17) have reexamined the relationship between Blastobotryx, Arxula, Sympodiomyces, and several Candida species with a multigene analysis. They have demonstrated that Blastobotrys, Arxula, Sympodiomyces, and some Candida species were members of the same clade. The multigene sequence analysis showed also Trichomonascus to represent the ascosporic state of this clade. Finally, Blastobotrys spp. are considered as anamorphic members of the Saccharomycetales treated under the yeasts, while Sporothrix belongs to the Ophiostomatales.
Of note, despite the decreased in vitro susceptibility of the isolate to fluconazole assessed by two techniques, clinical improvement was observed rapidly after the introduction of oral fluconazole. This corroborates the usual lack of correlation between in vitro susceptibility testing results and clinical efficacy. However, it does not mean that fluconazole should be the first choice for the treatment of fungal peritonitis due to uncommon species.
Conclusion.
Clinical features of fungal peritonitis are not different from those of bacterial peritonitis but are less frequent. Persistence of clinical or biological abnormalities despite adequate antibiotherapy for bacterial peritonitis should prompt new sampling and suspicion of fungal peritonitis. Identification of the pathogen is always required to adapt the treatment. Infections due to uncommon fungi are most frequently seen in immunocompromised patients but are also an emerging threat in those with end-stage renal failure. Whether B. proliferans represents a new source of human infection is unknown.
Footnotes
Published ahead of print on 15 August 2007.
REFERENCES
- 1.Bibashi, E., D. Memmos, E. Kokolina, D. Tsakiris, D. Sofianou, and M. Papadimitriou. 2003. Fungal peritonitis complicating peritoneal dialysis during an 11-year period: report of 46 cases. Clin. Infect. Dis. 36:927-931. [DOI] [PubMed] [Google Scholar]
- 2.Bonfante, L., F. Nalesso, M. Cara, A. Antonello, A. Malagoli, G. Pastori, M. Guizzo, A. D'Angelo, and G. Gambaro. 2005. Aspergillus fumigatus peritonitis in ambulatory peritoneal dialysis: a case report and notes on the therapeutic approach. Nephrology 10:270-273. [DOI] [PubMed] [Google Scholar]
- 3.Bren, A. 1998. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Eur. J. Clin. Microbiol. Infect. Dis. 17:839-843. [DOI] [PubMed] [Google Scholar]
- 4.Canon, H. L., S. C. Buckingham, R. J. Wyatt, and D. P. Jones. 2001. Fungal peritonitis caused by Curvularia species in a child undergoing peritoneal dialysis. Pediatr. Nephrol. 16:35-37. [DOI] [PubMed] [Google Scholar]
- 5.Chang, H. R., K. H. Shu, C. H. Cheng, M. J. Wu, C. H. Chen, and J. D. Lian. 2000. Peritoneal-dialysis-associated penicillium peritonitis. Am. J. Nephrol. 20:250-252. [DOI] [PubMed] [Google Scholar]
- 6.Das, R., E. Vaux, L. Barker, and R. Naik. 2006. Fungal peritonitis complicating peritoneal dialysis: report of 18 cases and analysis of outcomes. Adv. Perit. Dial. 22:55-59. [PubMed] [Google Scholar]
- 7.de Hoog, G. S., and A. H. Gerrits van den Ende. 1998. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41:183-189. [DOI] [PubMed] [Google Scholar]
- 8.de Hoog, G. S., A. H. Rantio-Lehtimaki, and M. T. Smith. 1985. Blastobotrys, Sporothrix and Trichosporiella: generic delimitation, new species, and a Stephanoascus teleomorph. Antonie Leeuwenhoek 51:79-109. [DOI] [PubMed] [Google Scholar]
- 9.de Hoog, G. S., and M. T. Smith. 1998. Blastobotrys von Klopotek, p. 443-448. In C. P. Kurtzman and J. W. Fell (ed.), The yeasts, a taxonomic study, 4th ed. Elsevier Science B.V., Amsterdam, The Netherlands.
- 10.de Zoysa, J. R., M. Searle, K. L. Lynn, and R. A. Robson. 2001. Successful treatment of CAPD peritonitis caused by Rhodotorula mucilaginosa. Perit. Dial. Int. 21:627-628. [PubMed] [Google Scholar]
- 11.Esel, D., A. N. Koc, C. Utas, N. Karaca, and N. Bozdemir. 2003. Fatal peritonitis due to Trichoderma sp. in a patient undergoing continuous ambulatory peritoneal dialysis. Mycoses 46:71-73. [DOI] [PubMed] [Google Scholar]
- 12.Huang, J. W., T. S. Chu, M. S. Wu, Y. S. Peng, and B. S. Hsieh. 2000. Visible Penicillium spp. colonization plaques on a Tenckhoff catheter without resultant peritonitis in a peritoneal dialysis patient. Nephrol. Dial. Transplant. 15:1872-1873. [DOI] [PubMed] [Google Scholar]
- 13.Huang, J. W., K. Y. Hung, K. D. Wu, Y. S. Peng, T. J. Tsai, and B. S. Hsieh. 2000. Clinical features of and risk factors for fungal peritonitis in peritoneal dialysis patients. J. Formos. Med. Assoc. 99:544-548. [PubMed] [Google Scholar]
- 14.Keceli, S., I. Yegenaga, N. Dagdelen, B. Mutlu, H. Uckardes, and A. Willke. 2005. Case report: peritonitis by Penicillium spp. in a patient undergoing continuous ambulatory peritoneal dialysis. Int. Urol. Nephrol. 37:129-131. [DOI] [PubMed] [Google Scholar]
- 15.Kurtzman, C. P., and C. J. Robnett. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 35:1216-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtzman, C. P., and C. J. Robnett. 1995. Molecular relationships among hyphal ascomycetous yeasts and yeastlike taxa. Can. J. Bot. 73:824-830. [Google Scholar]
- 17.Kurtzman, C. P., and C. J. Robnett. 2007. Multigene phylogenetic analysis of the Trichomonascus, Wickerhamiella and Zygoascus yeast clades, and the proposal of Sugiyamaella gen. nov. and 14 new species combinations. FEMS Yeast Res. 7:141-151. [DOI] [PubMed] [Google Scholar]
- 18.Liu, Y. L., C. C. Huang, and M. T. Kao. 2006. Residual renal function predicts outcome of fungal peritonitis in peritoneal dialysis patients. Perit. Dial. Int. 26:407-409. [PubMed] [Google Scholar]
- 19.Madariaga, M. G., A. Tenorio, and L. Proia. 2003. Trichosporon inkin peritonitis treated with caspofungin. J. Clin. Microbiol. 41:5827-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzano-Gayosso, P., F. Hernandez-Hernandez, L. J. Mendez-Tovar, J. Gonzalez-Monroy, and R. Lopez-Martinez. 2003. Fungal peritonitis in 15 patients on continuous ambulatory peritoneal dialysis (CAPD). Mycoses 46:425-429. [DOI] [PubMed] [Google Scholar]
- 21.Masclaux, F., E. Gueho, G. S. de Hoog, and R. Christen. 1995. Phylogenetic relationships of human-pathogenic Cladosporium (Xylohypha) species inferred from partial LS rRNA sequences. J. Med. Vet. Mycol. 33:327-338. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto, N., H. Shiraga, K. Takahashi, K. Kikuchi, and K. Ito. 2002. Successful treatment of Aspergillus peritonitis in a peritoneal dialysis patient. Pediatr. Nephrol. 17:243-245. [DOI] [PubMed] [Google Scholar]
- 23.Nannini, E. C., N. I. Paphitou, and L. Ostrosky-Zeichner. 2003. Peritonitis due to Aspergillus and zygomycetes in patients undergoing peritoneal dialysis: report of 2 cases and review of the literature. Diagn. Microbiol. Infect. Dis. 46:49-54. [DOI] [PubMed] [Google Scholar]
- 24.O'Donnell, K. 1993. Fusarium and its near relatives, p. 225-233. In D. R. Reynolds and J. W. Taylor (ed.), The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, United Kingdom.
- 25.Pimentel, J. D., K. Mahadevan, A. Woodgyer, L. Sigler, C. Gibas, O. C. Harris, M. Lupino, and E. Athan. 2005. Peritonitis due to Curvularia inaequalis in an elderly patient undergoing peritoneal dialysis and a review of six cases of peritonitis associated with other Curvularia spp. J. Clin. Microbiol. 43:4288-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad, N., and A. Gupta. 2005. Fungal peritonitis in peritoneal dialysis patients. Perit. Dial. Int. 25:207-222. [PubMed] [Google Scholar]
- 27.Raaijmakers, R., C. Schroder, L. Monnens, E. Cornelissen, and A. Warris. 2007. Fungal peritonitis in children on peritoneal dialysis. Pediatr. Nephrol. 22:288-293. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Tudela, J. L., F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, D. Denning, J. P. Donnelly, B. Dupont, W. Fegeler, C. Moore, M. Richardson, and P. E. Verweij. 2003. Method for the determination of minimum innhibitory concentration (MIC) by broth dilution of fermentative yeast. Clin. Microbiol. Infect. 9:1-8.12691538 [Google Scholar]
- 29.Rosa, N. G., S. Silva, J. A. Lopes, P. Branco, E. de Almeida, C. Ribeiro, F. Abreu, J. Barbas, and M. M. Prata. 2007. Fungal peritonitis in peritoneal dialysis patients: is previous antibiotic therapy an essential condition? Mycoses 50:79-81. [DOI] [PubMed] [Google Scholar]
- 30.Schattner, A., A. Kagan, and O. Zimhony. 2006. Aspergillus peritonitis in a lupus patient on chronic peritoneal dialysis. Rheumatol. Int. 26:762-764. [DOI] [PubMed] [Google Scholar]
- 31.Vachharajani, T. J., F. Zaman, S. Latif, R. Penn, and K. D. Abreo. 2005. Curvularia geniculata fungal peritonitis: a case report with review of literature. Int. Urol. Nephrol. 37:781-784. [DOI] [PubMed] [Google Scholar]
- 32.Verger, C., J. P. Ryckelynck, M. Duman, G. Veniez, T. Lobbedez, E. Boulanger, and O. Moranne. 2006. French peritoneal dialysis registry (RDPLF): outline and main results. Kidney Int. Suppl. November:S12-S20. [DOI] [PubMed]
- 33.Warady, B. A., M. Bashir, and L. A. Donaldson. 2000. Fungal peritonitis in children receiving peritoneal dialysis: a report of the NAPRTCS. Kidney Int. 58:384-389. [DOI] [PubMed] [Google Scholar]