Abstract
We evaluated the accuracy of serologic capsule typing by analyzing capsule genes and related markers among invasive Haemophilus influenzae isolates before and after the introduction of H. influenzae serotype b (Hib) conjugate vaccines. Three hundred and sixty invasive H. influenzae isolates were collected as part of Active Bacterial Core surveillance within the Georgia Emerging Infections Program between 1 January 1989 and 31 July 1998. All isolates were biotyped, serotyped by slide agglutination serotyping (SAST), and evaluated using PCR capsule typing. Nontypeable H. influenzae (NTHi) isolates were probed with Hib cap-gene-containing plasmid pUO38 and with IS1016; a subset was examined with phosphoglucose isomerase (pgi) genotyping and pulsed-field gel electrophoresis (PFGE). Discrepancies between SAST and PCR capsule typing were found for 64/360 (17.5%) of the isolates; 48 encapsulated by SAST were NTHi by PCR, 8 NTHi by SAST were encapsulated by PCR, 6 encapsulated by SAST were a different capsule type by PCR, and 2 encapsulated by SAST were capsule-deficient Hib variants (Hib-minus). None of the PCR-confirmed NTHi isolates demonstrated homology with residual capsule gene sequences; 19/201 (9.5%) had evidence of IS1016, an insertion element associated with division I H. influenzae capsule serotypes. The majority of IS1016-positive NTHi were biotypes I and V and showed some genetic relatedness by PFGE. In conclusion, PCR capsule typing was more accurate than SAST and Hib-minus variants were rare. IS1016 was present in 9.5% of NTHi isolates, suggesting that this subset may be more closely related to encapsulated organisms. A better understanding of NTHi may contribute to vaccine development.
Haemophilus influenzae is a gram-negative, exclusively human pathogen responsible for a wide variety of respiratory infections and potentially life-threatening invasive diseases such as meningitis (45). H. influenzae may be encapsulated (typeable) with one of six polysaccharide capsules, designated serotypes a through f (36), or nonencapsulated (nontypeable). H. influenzae pathogenicity varies depending on the presence or absence of capsule and the specific capsule type (46). Prior to routine infant use of H. influenzae serotype b (Hib) conjugate vaccines in the early 1990s, Hib was responsible for the majority of invasive H. influenzae disease in young children and up to 50% of adult H. influenzae disease in the United States (14, 50, 51). In the Hib conjugate vaccine era, nontypeable H. influenzae (NTHi) isolates have become the most common cause of invasive H. influenzae disease in all age groups (Active Bacterial Core Surveillance Report, Emerging Infections Network, Haemophilus influenzae; http://www.cdc.gov/ncidod/dbmd/abcs/survreports.htm) (9).
Encapsulated and nonencapsulated H. influenzae isolates can be distinguished using either standard slide agglutination serotyping (SAST) (36) or molecular capsule typing by PCR (12). Encapsulated H. influenzae isolates contain genes for the production of their respective polysaccharide capsules within the cap locus (28, 37). The cap locus for all H. influenzae serotypes consists of three functionally defined regions: 1, 2, and 3 (27, 28, 37). Regions 1 and 3 are common to all six capsule types and contain genes necessary for the processing and transport of the capsular material (bexABCD and hcsAB) (26, 37, 43). Region 2 genes are involved in capsule biosynthesis and are unique to each of the six capsule types (48).
Several studies have demonstrated molecular capsule typing methods to be more sensitive and specific than SAST (7, 12, 29). PCR capsule typing uses multiple primer sets that recognize sequences in the bexA gene in region 1 and capsule-specific (types a to f) genes in region 2 of the cap locus. Strains containing capsule-specific genes (region 2) may be phenotypically and serologically nontypeable if critical elements of the gene cluster such as the capsule export gene bexA (region 1) or transport genes hcsA and hcsB (region 3) are missing (18, 25, 32, 43). The nucleotide sequences of the highly conserved housekeeping gene for phosphoglucose isomerase (pgi, one of the seven housekeeping loci used in multilocus sequence typing [MLST]) are conserved within capsule types, and this additional molecular scheme has been suggested as a surrogate for capsule typing (2).
In Hib division I strains, the cap locus is flanked by direct repeats of an insertion element, IS1016, and is frequently amplified (8, 20, 27). Genetic rearrangement of a duplicated Hib cap locus by recombination can result in the loss of the bexA gene, producing a capsule-negative phenotype known as Hib-minus (18, 27). Such a variant would be nonencapsulated, and therefore serologically nontypeable, but would retain all other characteristics of a Hib strain. Although IS1016 can be found in division II strains, in this case it is not associated with the cap genes (27). In general, clinical isolates of NTHi do not harbor IS1016 (27). However, St. Geme III et al. found approximately 11% of NTHi pharyngeal carriage isolates to be positive for IS1016 (41).
In this study, we used a PCR-based capsule typing method to evaluate the accuracy of SAST among 360 invasive H. influenzae isolates collected as part of an active population-based surveillance system between 1989 and 1998. NTHi isolates were further evaluated for residual capsule or capsule-associated sequences, including IS1016, by using Southern blot hybridization with the entire cap gene locus. Characteristics of IS1016-positive NTHi were compared with IS1016-negative NTHi to assess relatedness to each other and to encapsulated strains.
MATERIALS AND METHODS
Bacterial strains and growth.
Three hundred and sixty invasive Haemophilus influenzae isolates were collected as part of the Active Bacterial Core surveillance of the Georgia Emerging Infections Program from January 1989 through July 1998. For some of the analysis, isolates collected from 1989 through 1992 were designated “prevaccine” (including a transition year after the 1991 licensure for infant use) and those collected from 1993 through 1998 were designated “postvaccine.” Bacteria were grown on Chocolate II solid media or in brain heart infusion broth supplemented with 10 μg/ml hemin and 2 μg/ml NAD (Becton Dickinson Microbiology Systems, Cockeysville, MD). Isolates were routinely serotyped by the SAST method at the Centers for Disease Control and Prevention (CDC) with either CDC or Difco H. influenzae serotype-specific rabbit antisera (BD Biosciences, Franklin Lakes, NJ) (29) and stored at −70°C for further characterization.
PCR typing and SAST.
Single-colony bacterial lysates were prepared as follows. A single colony was picked, suspended in 50 μl of sterile water, and lysed at 95°C for 5 min. Each lysate was frozen at −20°C and used as a template in PCRs targeting all six capsule-specific (cap) genes and the capsule export gene (bexA) using Promega (Madison, WI) reagents and oligonucleotide DNA primers as described by Falla et al. (12). Oligonucleotide DNA primers were synthesized by the Emory University Microchemical Facility or Sigma-Genosys (The Woodlands, TX). Amplification for capsules a, b, d, e, and f and bexA genes was performed in a total reaction volume of 50 μl with 5 μl of template, 4 mM Mg2+, and 20 pmol of primer. An annealing temperature of 55°C was required for optimal amplification of capsule genes a, b, d, and f and bexA; amplification for the type e-specific capsule gene required an annealing temperature of 45°C. Amplification for type c-specific capsule genes was performed in a total reaction volume of 50 μl with 2 μl of template, 2.5 mM Mg2+, and 10 pmol of primer at an annealing temperature of 45°C. PCR parameters were as follows: 30 s at 94°C, 30 s at appropriate annealing temperature, 1 min at 72°C, for 35 cycles, 10 min at 72°C, and 4°C hold. PCR products were analyzed on a 1% agarose gel (Bio-Rad, Hercules, CA) containing 0.1 μg/ml ethidium bromide (Sigma, St. Louis, MO). Isolates containing PCR products for both a cap-specific gene (a, b, c, d, e, or f) and the bexA gene were designated the specific capsule type; those containing a cap gene but not bexA were designated capsule-deficient variants or capsule-minus strains (e.g., Hib-minus). Isolates lacking both bexA and any of the cap genes were considered NTHi. In the case of discrepancies between previous SAST and PCR capsule typing, H. influenzae isolates were reserotyped in a blinded fashion using SAST and antisera from the CDC (Atlanta, GA) and Difco Laboratories (Detroit, MI) as described by the manufacturers. H. influenzae strains 9006 (capsule type a), 9007 (capsule type c), 9008 (capsule type d), 8142 (capsule type e), and 700222 (capsule type f) from the American Type Culture Collection (Manassas, VA) were used as positive controls. H. influenzae clinical isolate 1007 was used as the capsule type b control (30, 37). H. influenzae Rd KW20 was used as a nonencapsulated control and reference strain (15).
Southern hybridization.
Chromosomal DNA was extracted from NTHi strains (as determined by PCR assay) and capsule a to f control strains as previously described (5). Ten micrograms of H. influenzae chromosomal DNA was digested to completion with EcoRI (New England Biolabs, Beverly, MA) overnight at 37°C and separated by 0.7% agarose (Bio-Rad, Hercules, SC) gel electrophoresis and transferred to nylon membranes (Osmonics, Westborough, MA). Membranes were prehybridized and then hybridized with digoxigenin (DIG)-labeled pUO38 (25), pBR322 (4), or IS1016 (described below), and hybridized DNA was detected according to the manufacturer's protocol (Roche Diagnostics, Indianapolis, IN). Plasmid probes pUO38 and pBR322 were prepared using a DIG-nick translation mix (Roche Diagnostics, Indianapolis, IN). IS1016 probes were prepared using a PCR DIG probe synthesis kit (Roche Diagnostics, Indianapolis, IN) and a Gene Amp PCR system 9700 (Applied Biosystems, Foster City, CA). A complete IS1016 probe was generated by PCR using plasmid pUO38 containing IS1016 and primers IS1016 (bp 45 to 69) (5′ GCAAGTGCACTAGTCTATAAAAATG 3′) and IS1016 (bp 685 to 709) (5′ CTCCATTTTCGCAATGTTTTAAGC 3′); a truncated IS1016 probe was generated using primers HI1018 (bp 83 to 105) (5′ CAGCGGCTGATTTACTCGATATC 3′) and HI1018 (bp 451 to 429) (5′ GATTGATTCGTTCGTGGTGAAAT 3′) (15). Since a complete IS1016 element may or may not be present, both probes were used in Southern blots.
IS1016 chromosomal location.
To determine whether a retained IS1016 element was cap locus associated in the chromosomes of the NTHi strains, PCR using primers outside of HI1018 (GenBank accession no. U32782; bp 7618 to 8191), the complete copy of IS1016 remaining in Rd after the excision of the cap locus, was performed. Primers flanking HI1018, IS1016-L1 and IS1016-R2, were used in a PCR as previously described (34).
pgi alleles.
PCR for the housekeeping gene pgi (phosphoglucose isomerase) was carried out using the primers and methods described by Meats et al. (31). PCR products were sequenced by Lark Technologies (Houston, TX), and the MLST website (www.mlst.net) was used to determine pgi allele number when available.
PFGE.
Pulsed-field gel electrophoresis (PFGE) was performed as described by PulseNet, The National Molecular Subtyping Network for Foodbourne Disease Surveillance of the CDC (Atlanta, GA) (44). The protocol for the pathogen Escherichia coli O157:H7 was used (http://www.cdc.gov/pulsenet/protocols.htm). Gels were prepared and run in 0.5× TAE (Tris-acetate-EDTA) on CHEF-DR III (Bio-Rad, Hercules, CA) with an initial switch time of 1 s, a final switch time of 26 s, at 6 V with an included angle of 120° for 25 h. Gels were photographed and digitized with a Gel Doc XR system (Bio-Rad, Hercules, CA) and saved as a TIFF file for analysis with BioNumerics software (Applied Maths, Kortrijk, Belgium). Staphylococcus aureus NCTC 8325 was included as a reference standard. Cluster analysis was performed, and percent similarities were displayed on a dendrogram derived from the unweighted-pair group method of arithmetic averages (UPGMA) and using Dice coefficients with band position tolerance and optimization set at 1.5% (BioNumerics 4.5; Applied Maths, Kortrijk, Belgium).
Biotyping.
Biochemical assays to determine biotype were performed at either the CDC or the Georgia Public Health Laboratory (Atlanta, GA) using the method of Kilian (24) and API 20E biochemical test strips purchased from bioMerieux (St. Louis, MO) (21).
Statistics.
The positive predictive value (PPV) of SAST was determined by dividing the number of true positives by the number of true positives plus the number of false positives, using the PCR capsule typing result as the true measure of capsule type. Negative predictive value (NPV) of SAST was determined by dividing the number of true negatives by the number of true negatives plus the number of false negatives. For PFGE analysis, the cluster cutoff method of BioNumerics was used as a statistical tool to define relevant clusters. The cutoff maximizes the ratio of between-group to within-group variance in similarity values.
RESULTS
From January 1989 through July 1998, 360 invasive Haemophilus influenzae isolates from the metropolitan Atlanta area were collected as part of the Active Bacterial Core surveillance of the Georgia Emerging Infections Program. Clinical isolates were obtained from normally sterile sites such as blood and cerebrospinal fluid. Twenty cases occurred in neonates (<1 month of age), 99 cases in 1 month to 4 year olds, 16 cases in 5 to 17 year olds, and 225 cases in adults 18 years or older.
Overview of SAST versus PCR capsule typing.
We performed PCR capsule typing using primers specific for the types a to f cap genes and bexA genes (12) on all 360 isolates and compared the results with the routinely performed SAST. One hundred and sixty-eight isolates were reported to be nontypeable, and 192 were reported to be encapsulated by SAST. Of the 168 SAST NTHi, 160 were confirmed to be NTHi and 8 were found to be encapsulated by PCR capsule typing. Of the 192 isolates originally serotyped as encapsulated by SAST, 48 (19 initially reported to be type a, 22 type b, 6 type e, and 1 type f) were determined to be NTHi by PCR capsule typing, 6 were a different capsule type by PCR, and 2 were Hib-minus variants.
Proportion of NTHi found to be encapsulated.
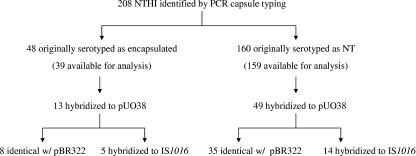
One hundred and sixty-eight invasive H. influenzae isolates were reported to be nontypeable by SAST. One hundred and twenty-eight (76.2%) of these isolates were collected from adults 18 years of age and older (Table 1). Eight serologically NTHi isolates were found to contain PCR products consistent with an encapsulated genotype (Table 2). Six of the eight isolates contained the bexA export gene and the type b-specific capsule gene and were confirmed to be Hib—including three children (1 month to 4 years) and three adults (≥18 years of age). The other two isolates contained the type f-specific capsule gene and the bexA export gene according to PCR (Table 2). Among the total 360 H. influenzae isolates initially serotyped by SAST, 208 were found to lack both capsule-specific and bexA genes by PCR and were designated NTHi (Fig. 1 and Table 2). Capsule typing by PCR increased the proportion of all invasive cases attributable to NTHi from 46.8% (by SAST) to 58.5% (Table 1), a difference of 11.7%. The PPV of SAST was 95% and the NPV of SAST was 75% for diagnosis of NTHi.
TABLE 1.
Distributions of H. influenzae capsule types by age group as determined by SAST and PCR
| Distribution | Capsule type
|
|||||
|---|---|---|---|---|---|---|
| a | b | e | f | NTa | b−b | |
| SAST | ||||||
| Neonatal | 7 | 13 | N/Ac | |||
| 1 mo-4 yr | 3 | 68 | 3 | 8 | 17 | N/A |
| 5 yr-17 yr | 5 | 1 | 10 | N/A | ||
| >17 yr | 20 | 33 | 14 | 30 | 128 | N/A |
| % of total | 6.4% | 31.5% | 4.7% | 10.8% | 46.6% | N/A |
| Total | 23 | 113 | 17 | 39 | 168 | 0 |
| PCR | ||||||
| Neonatal | 1 | 19 | 0 | |||
| 1 mo-4 yr | 67 | 9 | 21 | 2 | ||
| 5 yr-17 yr | 5 | 1 | 10 | 0 | ||
| >17 yr | 1 | 23 | 11 | 32 | 158 | 0 |
| % of total | 0.25% | 26% | 3% | 12% | 58% | 0.5% |
| Total | 1 | 95 | 11 | 43 | 208 | 2 |
NT, nontypeable.
Capsule-negative Hib variant.
N/A, not applicable.
TABLE 2.
Comparison of H. influenzae SAST and PCR capsule typinga
| Serotype by SAST | Capsule type by PCR
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | NTb | b−c | Total | |
| a | 1 | 3 | 19 | 23 | |||||
| b | 86 | 3 | 22 | 2 | 113 | ||||
| c | 0 | ||||||||
| d | 0 | ||||||||
| e | 11 | 6 | 17 | ||||||
| f | 38 | 1 | 39 | ||||||
| NT | 6 | 2 | 160 | 0 | 168 | ||||
| Total | 1 | 95 | 0 | 0 | 11 | 43 | 208 | 2 | 360 |
Row totals indicate original capsule type by serology; column totals indicate capsule types identified by PCR.
NT, nontypeable.
Capsule-negative Hib variant.
FIG. 1.
Summary of capsule gene analysis on NTHi strains by Southern hybridization with probes pUO38, pBR322, and IS1016.
Capsule type b.
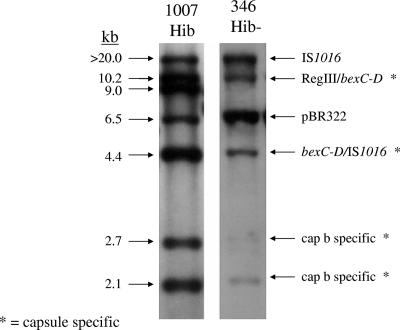
Twenty-two of the 113 (19.5%) isolates that were originally serotyped as Hib were found to lack PCR products for the bexA gene and any of the six capsule-specific genes and were designated NTHi (Table 2). Among these 22 isolates, 6 were from neonates (≤1 month), 2 from children (1 month to 4 years) and 14 from adults (≥18 years). Three isolates originally serotyped as Hib were found to contain the type f-specific capsule gene and bexA by PCR capsule typing and were designated capsule type f: 1 from a neonate (≤1 month), 1 from a child (1 month to 4 years), and 1 from an adult (≥18 years). Two isolates, GA834 (blood isolate from a 36-month-old child in 1990) and GA346 (cerebrospinal fluid isolate from a 6-month-old infant in 1989), originally reported to be Hib by SAST were found to be Hib-minus variants by molecular analysis. Hib-minus variant GA834 was detected by PCR capsule typing; GA346 was missed by the initial PCR, but capsule genes were detected by Southern blotting with pUO38 (Fig. 2); repeat PCR capsule typing confirmed the Hib-minus genotype. While the majority (20/27, 74%) of the isolates misclassified as Hib were collected in the prevaccine era (1989 to 1992) (Table 3), the rate of false-positive Hib designations by SAST was higher in the postvaccine era (44% post- versus 20% prevaccine). The overall PPV of SAST for Hib was 76%; the NPV was 96%.
FIG. 2.
Southern hybridization of EcoRI-digested chromosomal DNA from Hib strain 1007 and Hib-minus strain GA346 probed with DIG-labeled pUO38. GA346 contains the major hybridizing bands corresponding to the Hib cap locus (20, 10.2, 4.4, 2.7, and 2.1 kb) and is missing the 9-kb central bridge fragment.
TABLE 3.
Chronological distribution of H. influenzae capsule type
| Capsule type | Distribution as determined by:
|
|||
|---|---|---|---|---|
| Serology
|
PCR analysis
|
|||
| Prevaccinea | Postvaccine | Prevaccine | Postvaccine | |
| a | 6 | 17 | 0 | 1 |
| b | 97 | 16 | 84 | 11 |
| e | 4 | 13 | 1 | 10 |
| f | 8 | 31 | 10 | 33 |
| NTb | 31 | 137 | 50 | 158 |
| b−c | N/Ad | N/A | 2 | 0 |
Prevaccine, 1989 to 1992; postvaccine, 1993 to 1998.
NT, nontypeable.
Capsule-negative Hib variant.
N/A, not applicable.
Capsule type f.
Of the 39 SAST type f strains, 38 (97%) were confirmed to be capsule type f by PCR capsule typing, thus showing a high level of agreement between SAST and PCR for capsule type f. Only one isolate, from an adult patient, was found to lack both the bexA gene and any capsule-specific genes by PCR and was designated NTHi (Table 2). Capsule type f strains, although not as common as Hib, were the second most common capsule serotype associated with invasive disease in the prevaccine era and the most common capsule serotype in the postvaccine era, comprising 15% of the total number of invasive H. influenzae strains collected from 1993 to 1998 (Table 3). The PPV of SAST for capsule type f was 97%; the NPV was 98.4%.
Capsule type e.
Among the seventeen strains initially characterized by SAST as type e, 35% (6/17) were found to be NTHi by PCR capsule typing: 3 from children (1 month to 4 years) and 3 from adults (≥18 years) (Table 2). Based on PCR capsule typing, the proportion of invasive H. influenzae disease attributable to capsule type e decreased from 4.7% to 3% (Table 1). The PPV of SAST for capsule type e was 65%; the NPV was 100%.
Capsule type a.
Capsule type a strains were found to have the highest discrepancy between SAST and PCR capsule typing. Twenty-three capsule type a strains were initially reported by SAST. However, only one strain was found to contain both the capsule type a-specific capsule gene and the bexA gene by PCR (Table 2). Nineteen isolates failed to demonstrate PCR products for any of the six type-specific capsule genes or the bexA gene primer sets and were designated NTHi. Three isolates were found to be Hib by PCR (one from a 14-month-old infant and two from adults). The PPV of SAST for capsule type a was 4.3%; the NPV was 100%.
Confirmatory serotyping.
When the PCR results did not coincide with the original serotype, isolates were reserotyped in a blinded fashion. All reserotyping results were in agreement with the PCR capsule typing. For example, a strain that was originally serotyped as nontypeable but found to contain both a bexA and a capB gene product by PCR was found to be Hib by the repeat SAST.
Southern analysis of NTHi.
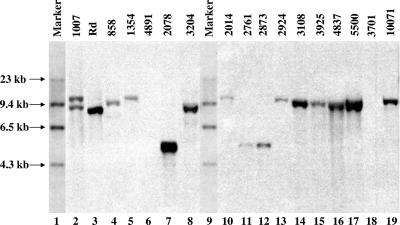
Southern blot hybridization with pUO38 was performed on PCR-confirmed NTHi isolates to look for residual capsule-specific DNA not detected by the PCR primers. Two hundred one of the 208 PCR-confirmed NTHi isolates were available for testing (42 of the 48 originally reported as encapsulated by SAST and 159 of the 160 found to be NTHi by both SAST and PCR capsule typing). The plasmid pUO38 has been described previously (25) and contains a complete cap locus from a division I Hib strain, including a copy of the insertion sequence IS1016. Under high stringency conditions, 62 of 201 NTHi isolates hybridized to pUO38, including 13 originally serotyped as encapsulated and 49 identified as NTHi by both SAST and PCR capsule typing (Fig. 1). To determine whether hybridization to pUO38 was related to the presence of cap-specific DNA and/or IS1016, or a consequence of shared sequences found in pBR322 (the vector backbone for pUO38), the 62 strains that hybridized to pUO38 were probed with DIG-labeled pBR322 and IS1016. Forty-three of the 62 isolates showed identical patterns with pUO38 and pBR322, i.e., one band at approximately 6.5 kb. The hybridization to pBR322 in all 43 strains could be accounted for by hybridization to the beta-lactamase (bla) gene from the vector backbone and each was found to be ampicillin resistant when tested (data not shown). Nineteen strains hybridized to IS1016 and had the same pattern as with pUO38, a single band ranging between ∼5 and 10 kb. A schematic summary of the Southern blot survey of NTHi isolates is shown in Fig. 1. A representative sample of Southern blot results from IS1016-positive and IS1016-negative NTHi strains is shown in Fig. 3.
FIG. 3.
Southern hybridization analysis of representative isolates demonstrating hybridization with IS1016. Chromosomal DNA was digested with EcoRI from Hib 1007 (lane 2), Rd (lane 3), GA858 (lane 4), GA1354 (lane 5), GA4891 (lane 6), GA2078 (lane 7), GA3204 (lane 8), GA2014 (lane 10), GA2761 (lane 11), GA2873 (lane 12)ç GA2924 (lane 13), GA3108 (lane 14), GA3925 (lane 15), GA4837 (lane 16), GA5500 (lane 17), GA3701 (lane 18), and GA10071 (lane 19). Lanes 1 and 9, DIG-labeled DNA molecular weight marker II (Roche Diagnostics GmbH). DNA was probed with IS1016 that was PCR DIG labeled using a kit from Roche Diagnostics GmbH. Molecular size markers are noted at the left. Lanes 6 and 18 are NTHi strains without IS1016.
Chromosomal location of IS1016.
The nonencapsulated H. influenzae Rd strain (formerly capsular serotype d) contains one complete copy of IS1016 found in section 97 at chromosomal coordinates 1082514 to 1081941 on the negative strand of the genome (15). The IS1016 in section 97 is a remnant of the complete deletion of the cap d locus. In experiments not shown, we were unable to find evidence that the IS1016 elements in the PCR-confirmed NTHi strains were located in section 97.
pgi alleles.
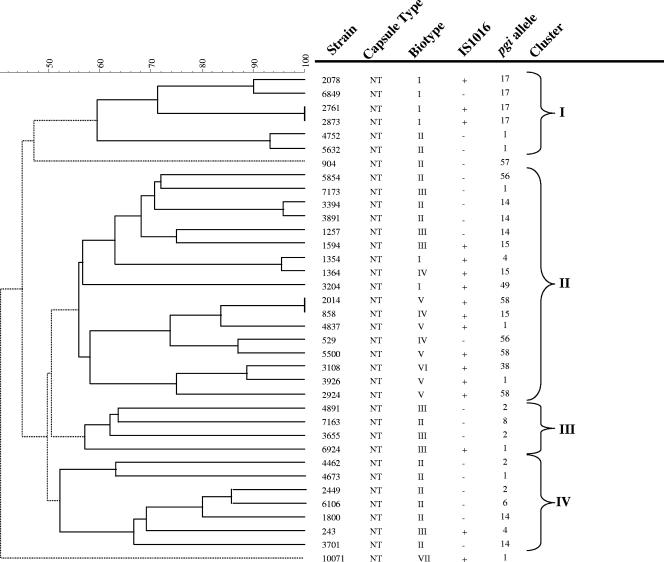
Since phosphoglucose isomerase (pgi) genotypes are highly conserved within capsular groups of H. influenzae and have been suggested as a surrogate method for serotyping (2), we evaluated the pgi genotypes of 18 IS1016-positive (one isolate unavailable) and 19 IS1016-negative NTHi isolates. The pgi allele sequences were compared with the H. influenzae alleles as reported on www.mlst.net. The IS1016-positive and IS1016-negative NTHi strains had pgi alleles associated primarily with nontypeable strains (Fig. 4).
FIG. 4.
Evolutionary analysis of invasive NTHi strains. Dendrogram generated by BioNumerics software showing the genetic relationships between seventeen IS1016-positive and nineteen IS1016-negative NTHi strains. Dendrogram was constructed by cluster analysis of PFGE patterns (data not shown) obtained after digestion of chromosomal DNA with SmaI enzyme, using UPGMA. Strain number, capsule type by PCR, biotype, presence or absence of IS1016, and pgi alleles are shown. Four different clusters (I to IV) of patterned branches were identified by the cluster cutoff method of BioNumerics software. A group of solid lines branching from a dashed line root represent a cluster. Solid lines in the dendrogram link entries in a cluster while dashed lines link different clusters. NT, nontypeable.
Biotypes.
The biotypes of PCR-confirmed NTHi isolates with and without IS1016 are shown in Table 4. Among the invasive NTHi isolates, 28/208 (13.5%) were biotype I, 103/208 (49.5%) biotype II, 48/208 (23%) biotype III, 18/208 (8.6%) biotype IV, 7/208 (3%) biotype V, and less than 1% biotypes VI or VII. Although six different biotypes were noted among IS1016-positive NTHi, more than half were biotypes I and V (11/19, 58%) (Fig. 4). It was notable that the otherwise uncommon biotype V (3% of NTHi) was strongly associated with the presence of IS1016 and accounted for 6 of 19 (31.5%) of the IS1016-positive NTHi. IS1016 was present in 100% of the biotype V isolates available for testing (6/7 were tested for IS1016) compared to a prevalence of 9.5% in the overall collection of NTHi tested for IS1016. Another significant finding was the complete absence of IS1016 positivity among biotype II NTHi.
TABLE 4.
Biotypes of PCR-confirmed NTHi isolates and association of IS1016
| Isolate | Distribution of biotype:
|
||||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | |
| NTHi with IS1016 | 5 | 0 | 3 | 3 | 6 | 1 | 1 |
| NTHi without IS1016 | 20 | 103 | 45 | 12 | 0 | 1 | 1 |
| NTHi IS1016 N/Aa | 3 | 0 | 0 | 3 | 1 | 0 | 0 |
| Total | 28 | 103 | 48 | 18 | 7 | 2 | 2 |
IS1016 status unknown.
PFGE.
We compared the fingerprints of genomic DNA from 17 IS1016-positive and 19 IS1016-negative NTHi. Thirty-four distinct restriction patterns were identified among the 36 strains available for PFGE, reflecting the expected genetic diversity among NTHi strains. However, some clustering with respect to the presence or absence of IS1016 and certain biotypes was noted (Fig. 4). Strains were grouped into four clusters using a dendrogram generated from PFGE patterns and cluster analysis combined with the cluster cutoff method. Clusters I and II contain the majority of IS1016-positive strains (14/17, 82%) (Fig. 4). In addition, 4/6 strains in cluster I are biotype I and pgi allele type 17. Cluster II contains all five biotype V strains examined by PFGE. Clusters I and II each contain a pair of strains that were genetically indistinguishable by PFGE. Clusters III and IV were composed predominantly of IS1016-negative NTHi (9/11, 82%) that were either biotype II or III.
DISCUSSION
In this study, we compared conventional capsule serotyping methods (SAST) for H. influenzae with a PCR capsule typing method that detects the bexA capsule export gene and capsule gene sequences specific to each of the six capsule types (a to f). Three hundred and sixty invasive H. influenzae isolates with serotype results available from routinely performed SAST were examined for capsule type by PCR. The overall rate of discordance was 17.5% (63/360). The most common error was the misidentification of NTHi as encapsulated strains by SAST. In addition, two Hib-minus variants were identified. While lacking any identified residual capsule genes, a distinct subgroup of PCR-confirmed NTHi isolates contained the capsule-associated IS1016 insertion element.
Accurate surveillance for H. influenzae disease in the post-Hib vaccine era is important for monitoring the effectiveness of Hib conjugate vaccines, including newer combination vaccine products that may alter immunogenicity (39). NTHi remains an important cause of invasive disease in adults and of local respiratory tract disease in both children and adults. Serious disease due to other capsule serotypes has been increasingly reported (1, 6, 16, 17, 22, 39, 47). Effective surveillance is dependent upon the ability to discriminate between Hib disease and that attributable to other capsular serotypes or NTHi.
The declining incidence of Hib disease raises concerns about the accuracy and PPV of serotyping methods. We found a significant increase in false-positive Hib designation by SAST in the postvaccine era, likely related to the low background rate of Hib diseases. Such false positives erroneously suggest vaccine failure, triggering potentially unnecessary public health and clinical responses. Specific areas of concern include the overall decline in the use of and laboratory expertise in H. influenzae serotyping, the misinterpretation of weak or slow agglutination as a positive result, and the subjectivity in reading an agglutination reaction. Although H. influenzae serotyping protocols should include individual serotype reactions from each of the six capsule types, many laboratories screen with polyclonal antiserum that fails to distinguish between individual serotypes. In addition, conventional serotyping methods fail to distinguish true NTHi from capsule-deficient variants.
Molecular capsule typing protocols using PCR have been shown to be more accurate than conventional serotyping (3, 29). LaClaire et al. reported a 40% discrepancy between SAST performed at state health departments and PCR capsule typing performed at the CDC, with the largest discrepancy being the overreporting of serotype b by state health department laboratories (29). Their findings emphasized a need for standardization to improve agreement and further suggested that molecular PCR capsule typing could serve as the method of choice to resolve SAST inconsistencies. Bokermann et al. also found PCR capsule typing to be a reliable method to resolve discrepancies between multiple slide agglutination methods. In their hands, initial screening with a Hib-specific antiserum correctly identified only 68% of invasive isolates and 46.5% of carriage isolates; SAST using all antisera in parallel improved the agreement rate; a polyvalent antiserum had the lowest discriminatory power (3). We found the PPV of SAST for capsule types other than type f to be suboptimal. PCR capsule typing is not without its limitations. Although relatively easy to perform and objective, PCR is not as rapid as SAST and requires molecular testing capacity not always available in clinical microbiology laboratories. No commercial kits are available for individual laboratory use, and a small sequence mismatch within the area of priming could lead to a false-negative result.
Overreporting of capsule types “e” and “a” were the most common errors noted in our comparison of SAST with PCR capsule typing. In both cases, most of the mismatched isolates were shown to be NTHi by PCR capsule typing. Individual user variation and/or unique technical issues in our diagnostic laboratory may have contributed to the very high discrepancy rate for capsule type a, since others have not reported such significant discrepancies. Carefully performed repeat SAST correlated with the PCR capsule typing result in all cases. Whatever the reasons, misidentification of NTHi strains as encapsulated strains adversely impacts the monitoring of H. influenzae disease in the postvaccine era. With a greater proportion of invasive disease now due to NTHi and non-Hib encapsulated strains (6, 9, 22, 47, 49), careful SAST determination of capsule type is essential and some form of monitoring of the accuracy of SAST by PCR capsule typing is warranted. Overall, we found that 19.5% of Hib and 31.6% of other capsular serotypes were reassigned to NTHi after PCR capsule typing, increasing the proportion of all invasive disease due to NTHi by 11.4% (Table 1). Although the analysis in this report does not include contemporary isolates, more than half of the isolates were collected after introduction of the conjugate vaccine into routine infant use, during a period of low Hib prevalence (Table 3).
Once serotyping error has been eliminated, H. influenzae isolates that fail to express capsule may represent two distinct groups, nonencapsulated variants of encapsulated H. influenzae or true NTHi that are heterogeneous and genetically distinct from encapsulated strains. While SAST cannot differentiate between these two groups, molecular methods such as PCR capsule typing and Southern blot hybridization can identify capsule-deficient variants. In this study of 360 isolates from invasive H. influenzae disease collected systematically through active, population-based surveillance over more than 9 years, we identified only two (<1%) Hib-minus variants, as determined by the presence of Hib-specific capsule genes and absence of the bexA gene determined by PCR amplification. Others have identified Hib-minus variants using Southern blot hybridization and probing with pUO38. St. Geme III et al. found 1 (<1%) Hib-minus variant out of 123 NTHi pediatric pharyngeal carriage isolates (41), and Falla et al. found 3 Hib-minus variants out of 24 (12.5%) NTHi pediatric pharyngeal carriage isolates (13). The low rate of Hib-minus variants in our study may reflect the relative importance of capsule in the pathogenesis of invasive disease compared with mucosal colonization. It is also possible that one or both of our Hib-minus variants lost the ability to express capsules in vitro after isolation, since both isolates were initially reported to be Hib rather than NTHi by SAST.
Because recombination events can result in complete and incomplete duplications and deletions of genes within the capsule gene locus, we went further using Southern blot hybridization to examine the 201 PCR-confirmed NTHi strains for the presence of any cryptic capsule genes not found by PCR capsule typing. We found no residual capsule-specific DNA (using plasmid pUO38) but did identify IS1016 sequences that can be capsule associated in 9.5% of the NTHi isolates. Finding that none of the isolates in our collection of PCR-confirmed NTHi strains had residual capsule-specific genes is in contrast with previous reports. St. Geme III et al. found 24 out of 123 (20%) pediatric NTHi pharyngeal carriage isolates contained residual capsule genes when probed with pUO38 (41). Although all isolates reported by St. Geme III et al. and in our study were confirmed to be nontypeable, the anatomic site of isolation of the samples and the ages of the patients varied greatly. Our study consisted of invasive disease isolates from patients ranging in age from 0 to 103 years, the majority of whom were adults; the St. Geme III et al. report examined nasopharyngeal isolates from a homogenous Finnish pediatric population. O'Neill et al. found 5 of 28 invasive NTHi strains hybridized to pUO38, suggesting that as many as 18% of invasive NTHi strains may contain residual capsule DNA and/or IS1016 (35). However, it is possible that some of their strains were hybridizing to the pBR322 vector backbone. Since studies have shown that nonencapsulated H. influenzae isolates are more successful at colonization of the human pharynx and carriage is high in children (38), nonencapsulated variants of encapsulated strains may be more plentiful at mucosal surfaces. In fact, Hoiseth and Gilsdorf described two children with systemic Hib disease who, after 8 or 9 days of antibiotic treatment, had nonencapsulated pharyngeal isolates that were otherwise identical to the respective encapsulated invasive strains (19).
The presence in NTHi of IS1016, an element associated with the capsule gene locus in division I encapsulated H. influenzae strains, may be a marker for a subgroup of NTHi with unique features. The rate (9.5%) of IS1016-positive invasive NTHi in our study is similar to that seen in pharyngeal NTHi carriage in healthy children from two separate studies (23, 41) but slightly higher than that reported in a study of invasive NTHi disease in children and neonates (13). St. Geme III et al. found 14/123 (11%) pharyngeal NTHi isolates hybridized to IS1016 only at a single ∼9-kb EcoRI fragment (41), and Falla et al. found 1/21 (4.7%) invasive NTHi isolates hybridized to IS1016 at a single ∼5.6-kb EcoRI fragment (13). In the majority of our isolates, IS1016 hybridized to a ∼9-kb EcoRI fragment. The capsule locus in division I H. influenzae strains lies between direct repeats of IS1016 and has apparently been brought into the chromosome as a compound transposon (27). The H. influenzae strain Rd was originally a type d encapsulated H. influenzae strain and, within a laboratory environment, lost most of its virulence genes, including the entire cap d locus (52). A complete IS1016 element remains between base pairs 1082514 to 1081941 on the Rd genome, on a ∼9-kb EcoRI fragment (15, 37) where the capsule locus presumably resided (28). We were unable to show that any of the IS1016 elements in our NTHi isolates were in the same chromosomal location and further studies will be needed to determine the insertion sites, the completeness of the inserted copies, and the sequences.
A surprising number of the IS1016-positive NTHi strains were found to be biotype V, an otherwise very uncommon H. influenzae biotype. Preliminary results from our characterization of more-recent invasive H. influenzae isolates appear to confirm the strong association of biotype V NTHi with the presence of IS1016 (unpublished data). A highly virulent biotype V NTHi strain, R2866, has recently been characterized and its sequence completed (http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi) (10, 11, 33). This invasive NTHi, isolated from a child with meningitis, was unusually resistant to killing by normal human serum and contained residual IS1016 sequence (10).
The evolutionary relationship between nonencapsulated and encapsulated H. influenzae strains is poorly understood. In general, NTHi isolates are a genetically heterogeneous group compared with the more clonal encapsulated strains, and therefore most do not appear to be encapsulated organisms that have recently lost the ability to produce capsules (32, 42). Although little evidence has been found to link nonencapsulated and encapsulated H. influenzae, it seems likely that the two share a common ancestor. Even in the absence of capsule-specific genes, the presence of IS1016 in a subset of NTHi may represent a site for the acquisition of capsule genes or be the last remnant reflecting distant loss of capsule genes. The association of IS1016 with additional genetic markers, such as the presence of absence of certain adhesins (hmw negative/hia positive), has been noted and again suggests a closer relationship of IS1016-containing NTHi with encapsulated strains (40). Studies to characterize adhesin gene profiles in these IS1016-positive NTHi strains are ongoing. However, the absence of capsule-associated pgi alleles among the IS1016-positive NTHi strains may argue for horizontal gene transfer rather than strong ancestral relatedness.
In conclusion, we found that H. influenzae isolates were misidentified by conventional H. influenzae serotyping in 17.5% of cases; discrepancies varied by serotype and most often resulted in overreporting of genotypically NTHi isolates as encapsulated strains. Emphasis should be placed on improved laboratory training and adherence to recommended serotyping procedures. PCR capsule typing provided improved accuracy but it requires a higher level of laboratory capacity and commercially available test kits are not available. Molecular capsule typing provides a quality control measure to monitor the ongoing accuracy of traditional SAST. Hib-minus variants were rare in this collection of invasive disease isolates and were only detected by the molecular typing methods. PCR-confirmed NTHi did not contain residual capsule-specific gene sequences or capsule-associated pgi alleles. However, a significant subset of PCR-confirmed, invasive NTHi isolates possessed IS1016, a marker often associated with the H. influenzae capsule gene cluster. A better molecular understanding of clinically relevant NTHi may be important to vaccine development.
Acknowledgments
This work was supported in part by a VA Merit Grant to M.M.F. and the CDC-sponsored Emerging Infections Program.
We thank Christine Marstellar, Kathryn E. Arnold, and Malik Raynor for technical support; Bill Cheek (Georgia Public Health Laboratory) and John Elliott and Richard Facklam (CDC) for biotyping and serotyping; and Nancy Rosenstein Messonnier (CDC) for advice and support.
Footnotes
Published ahead of print on 15 August 2007.
REFERENCES
- 1.Adderson, E. E., C. L. Byington, L. Spencer, A. Kimball, M. Hindiyeh, K. Carroll, S. Mottice, E. K. Korgenski, J. C. Christenson, and A. T. Pavia. 2001. Invasive serotype a Haemophilus influenzae infections with a virulence genotype resembling Haemophilus influenzae type b: emerging pathogen in the vaccine era? Pediatrics 108:E18. [DOI] [PubMed] [Google Scholar]
- 2.Anyanwu, J. N., C. A. Rodriguez, K. E. Fleming, and E. E. Adderson. 2003. pgi genotyping is a surrogate for serotyping of encapsulated Haemophilus influenzae. J. Clin. Microbiol. 41:2080-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bokermann, S., R. C. Zanella, A. P. Lemos, A. L. de Andrade, and M. C. Brandileone. 2003. Evaluation of methodology for serotyping invasive and nasopharyngeal isolates of Haemophilus influenzae in the ongoing surveillance in Brazil. J. Clin. Microbiol. 41:5546-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 5.Bruant, G., S. Watt, R. Quentin, and A. Rosenau. 2003. Typing of nonencapsulated Haemophilus strains by repetitive-element sequence-based PCR using intergenic dyad sequences. J. Clin. Microbiol. 41:3473-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos, J., F. Roman, M. Perez-Vazquez, J. Oteo, B. Aracil, and E. Cercenado. 2003. Infections due to Haemophilus influenzae serotype E: microbiological, clinical, and epidemiological features. Clin. Infect. Dis. 37:841-845. [DOI] [PubMed] [Google Scholar]
- 7.CDC. 2002. Serotyping discrepancies in Haemophilus influenzae type b disease—United States, 1998-1999. Morb. Mortal. Wkly. Rep. 51:706-707. [PubMed] [Google Scholar]
- 8.Corn, P. G., J. Anders, A. K. Takala, H. Kayhty, and S. K. Hoiseth. 1993. Genes involved in Haemophilus influenzae type b capsule expression are frequently amplified. J. Infect. Dis. 167:356-364. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin, M. S., L. Park, and S. M. Borchardt. 2007. The changing epidemiology of invasive Haemophilus influenzae disease, especially in persons >/=65 years old. Clin. Infect. Dis. 44:810-816. [DOI] [PubMed] [Google Scholar]
- 10.Erwin, A. L., S. Allen, D. K. Ho, P. J. Bonthuis, J. Jarisch, K. L. Nelson, D. L. Tsao, W. C. Unrath, M. E. Watson, Jr., B. W. Gibson, M. A. Apicella, and A. L. Smith. 2006. Role of lgtC in resistance of nontypeable Haemophilus influenzae strain R2866 to human serum. Infect. Immun. 74:6226-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erwin, A. L., K. L. Nelson, T. Mhlanga-Mutangadura, P. J. Bonthuis, J. L. Geelhood, G. Morlin, W. C. Unrath, J. Campos, D. W. Crook, M. M. Farley, F. W. Henderson, R. F. Jacobs, K. Muhlemann, S. W. Satola, L. van Alphen, M. Golomb, and A. L. Smith. 2005. Characterization of genetic and phenotypic diversity of invasive nontypeable Haemophilus influenzae. Infect. Immun. 73:5853-5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falla, T. J., D. W. M. Crook, L. N. Brophy, D. Makell, J. S. Kroll, and E. R. Moxon. 1994. PCR for capsular typing of Haemophilus influenzae. J. Clin. Microbiol. 32:2382-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falla, T. J., S. R. Dobson, D. W. Crook, W. A. Kraak, W. W. Nichols, E. C. Anderson, J. Z. Jordens, M. P. Slack, D. Mayon-White, and E. R. Moxon. 1993. Population-based study of non-typable Haemophilus influenzae invasive disease in children and neonates. Lancet 341:851-854. [DOI] [PubMed] [Google Scholar]
- 14.Farley, M. M., D. S. Stephens, P. S. Brachman, Jr., R. C. Harvey, J. D. Smith, J. D. Wenger, et al. 1992. Invasive Haemophilus influenzae disease in adults. A prospective, population-based surveillance. Ann. Intern. Med. 116:806-812. [DOI] [PubMed] [Google Scholar]
- 15.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, J. M. Merrick, K. McKenney, G. Sutton, W. FitzHugh, C. Fields, J. D. Gocayne, J. Scott, R. Shirley, L.-I. Liu, A. Glodek, J. M. Kelley, J. F. Weidman, C. A. Phillips, T. Spriggs, E. Hedblom, M. D. Cotton, T. R. Utterback, M. C. Hanna, D. T. Nguyen, D. M. Saudek, R. C. Brandon, L. D. Fine, J. L. Fritchman, J. L. Fuhrmann, N. S. M. Geoghagen, C. L. Gnehm, L. A. McDonald, K. V. Small, C. M. Fraser, H. O. Smith, and J. C. Venter. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 16.Hammitt, L. L., S. Block, T. W. Hennessy, C. Debyle, H. Peters, A. Parkinson, R. Singleton, and J. C. Butler. 2005. Outbreak of invasive Haemophilus influenzae serotype a disease. Pediatr. Infect. Dis. J. 24:453-456. [DOI] [PubMed] [Google Scholar]
- 17.Hammitt, L. L., T. W. Hennessy, S. Romero-Steiner, and J. C. Butler. 2006. Assessment of carriage of Haemophilus influenzae type a after a case of invasive disease. Clin. Infect. Dis. 43:386-387. [DOI] [PubMed] [Google Scholar]
- 18.Hoiseth, S. K., C. J. Connelly, and E. R. Moxon. 1985. Genetics of spontaneous, high-frequency loss of b capsule expression in Haemophilus influenzae. Infect. Immun. 49:389-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoiseth, S. K., and J. R. Gilsdorf. 1988. The relationship between type b and nontypable Haemophilus influenzae isolated from the same patient. J. Infect. Dis. 158:643-645. [DOI] [PubMed] [Google Scholar]
- 20.Hoiseth, S. K., E. R. Moxon, and R. P. Silver. 1986. Genes involved in Haemophilus influenzae type b capsule expression are part of an 18-kilobase tandem duplication. Proc. Natl. Acad. Sci. USA 83:1106-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes, R. L., L. M. DeFranco, and M. Otto. 1982. Novel method of biotyping Haemophilus influenzae that uses API 20e. J. Clin. Microbiol. 15:1150-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapogiannis, B. G., S. Satola, H. L. Keyserling, and M. M. Farley. 2005. Invasive infections with Haemophilus influenzae serotype a containing an IS1016-bexA partial deletion: possible association with virulence. Clin. Infect. Dis. 41:e97-103. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson, E., and A. Melhus. 2006. Nontypeable Haemophilus influenzae strains with the capsule-associated insertion element IS1016 may mimic encapsulated strains. APMIS 114:633-640. [DOI] [PubMed] [Google Scholar]
- 24.Kilian, M. 1976. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J. Gen. Microbiol. 93:9-62. [DOI] [PubMed] [Google Scholar]
- 25.Kroll, J. S., I. Hopkins, and E. R. Moxon. 1988. Capsule loss in H. influenzae type b occurs by recombination-mediated disruption of a gene essential for polysaccharide export. Cell 53:347-356. [DOI] [PubMed] [Google Scholar]
- 26.Kroll, J. S., B. Loynds, L. N. Brophy, and E. R. Moxon. 1990. The bex locus in encapsulated Haemophilus influenzae: a chromosomal region involved in capsule polysaccharide export. Mol. Microbiol. 4:1853-1862. [DOI] [PubMed] [Google Scholar]
- 27.Kroll, J. S., B. M. Loynds, and E. R. Moxon. 1991. The Haemophilus influenzae capsulation gene cluster: a compound transposon. Mol. Microbiol. 5:1549-1560. [DOI] [PubMed] [Google Scholar]
- 28.Kroll, J. S., S. Zamze, B. Loynds, and E. R. Moxon. 1989. Common organization of chromosomal loci for production of different capsular polysaccharides in Haemophilus influenzae. J. Bacteriol. 171:3343-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaClaire, L. L., M. L. Tondella, D. S. Beall, C. A. Noble, P. L. Raghunathan, N. E. Rosenstein, and T. Popovic. 2003. Identification of Haemophilus influenzae serotypes by standard slide agglutination serotyping and PCR-based capsule typing. J. Clin. Microbiol. 41:393-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason, E. J., S. Kaplan, B. Wiedermann, E. Norrod, and W. Stenback. 1985. Frequency and properties of naturally occurring adherent piliated strains of Haemophilus influenzae type b. Infect. Immun. 49:98-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meats, E., E. J. Feil, S. Stringer, A. J. Cody, R. Goldstein, J. S. Kroll, T. Popovic, and B. G. Spratt. 2003. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J. Clin. Microbiol. 41:1623-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musser, J., S. Barenkamp, D. Granoff, and R. Selander. 1986. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect. Immun. 52:183-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nizet, V., K. F. Colina, J. R. Almquist, C. E. Rubens, and A. L. Smith. 1996. A virulent nonencapsulated Haemophilus influenzae. J. Infect. Dis. 173:180-186. [DOI] [PubMed] [Google Scholar]
- 34.Ohkusu, K., K. A. Nash, and C. B. Inderlied. 2005. Molecular characterisation of Haemophilus influenzae type a and untypeable strains isolated simultaneously from cerebrospinal fluid and blood: novel use of quantitative real-time PCR based on the cap copy number to determine virulence. Clin. Microbiol. Infect. 11:637-643. [DOI] [PubMed] [Google Scholar]
- 35.O'Neill, J. M., J. W. St. Geme III, D. Cutter, E. E. Adderson, J. Anyanwu, R. F. Jacobs, and G. E. Schutze. 2003. Invasive disease due to nontypeable Haemophilus influenzae among children in Arkansas. J. Clin. Microbiol. 41:3064-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pittman, M. 1931. Variation and type specificity in the bacterial species Haemophilus influenzae. J. Exp. Med. 53:471-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satola, S. W., P. L. Schirmer, and M. M. Farley. 2003. Complete sequence of the cap locus of Haemophilus influenzae serotype b and nonencapsulated b capsule-negative variants. Infect. Immun. 71:3639-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapiro, E. D., and J. I. Ward. 1991. The epidemiology and prevention of disease caused by Haemophilus influenzae type b. Epidemiol. Rev. 13:113-142. [DOI] [PubMed] [Google Scholar]
- 39.Singleton, R., L. Hammitt, T. Hennessy, L. Bulkow, C. DeByle, A. Parkinson, T. E. Cottle, H. Peters, and J. C. Butler. 2006. The Alaska Haemophilus influenzae type b experience: lessons in controlling a vaccine-preventable disease. Pediatrics 118:e421-e429. [DOI] [PubMed] [Google Scholar]
- 40.St. Geme, J. W., III, V. V. Kumar, D. Cutter, and S. J. Barenkamp. 1998. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect. Immun. 66:364-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.St. Geme, J. W., III, A. Takala, E. Esko, and S. Falkow. 1994. Evidence for capsule gene sequences among pharyngeal isolates of nontypeable Haemophilus influenzae. J. Infect. Dis. 169:337-342. [DOI] [PubMed] [Google Scholar]
- 42.Stuy, J. H. 1978. On the nature of nontypable Haemophilus influenzae. Antonie Leeuwenhoek 44:367-376. [DOI] [PubMed] [Google Scholar]
- 43.Sukupolvi-Petty, S., S. Grass, and J. W. St. Geme III. 2006. The Haemophilus influenzae type b hcsA and hcsB gene products facilitate transport of capsular polysaccharide across the outer membrane and are essential for virulence. J. Bacteriol. 188:3870-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swaminathan, B., T. J. Barrett, S. B. Hunter, and R. V. Tauxe. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg. Infect. Dis. 7:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turk, D. C. 1982. Clinical importance of Haemophilus influenzae, p. 3-9. In S. H. Sell and P. F. Wright (ed.), Haemophilus influenzae: epidemiology, immunology, and prevention of disease. Elsevier/North-Holland Publishing Co., New York, NY.
- 46.Turk, D. C. 1984. The pathogenicity of Haemophilus influenzae. J. Med. Microbiol. 18:1-16. [DOI] [PubMed] [Google Scholar]
- 47.Urwin, G., J. A. Krohn, K. Deaver-Robinson, J. D. Wenger, M. M. Farley, et al. 1996. Invasive disease due to Haemophilus influenzae serotype f: clinical and epidemiologic characteristics in the H. influenzae serotype b vaccine era. Clin. Infect. Dis. 22:1069-1076. [DOI] [PubMed] [Google Scholar]
- 48.van Eldere, J., L. Brophy, B. Loynds, P. Celis, I. Hancock, S. Carman, J. S. Kroll, and E. R. Moxon. 1995. Region II of the Haemophilus influenzae type b capsulation locus is involved in serotype-specific polysaccharide synthesis. Mol. Microbiol. 15:107-118. [DOI] [PubMed] [Google Scholar]
- 49.Waggoner-Fountain, L. A., J. O. Hendley, E. J. Cody, V. A. Perriello, and L. G. Donowitz. 1995. The emergence of Haemophilus influenzae types e and f as significant pathogens. Clin. Infect. Dis. 21:1322-1324. [DOI] [PubMed] [Google Scholar]
- 50.Ward, J., J. Lieberman, and S. Cochi. 1994. Haemophilus influenzae vaccines, 2nd ed. WB Sanders, Philadelphia, PA.
- 51.Wenger, J. D., R. Pierce, K. Deaver, R. Franklin, G. Bosley, N. Pigott, C. V. Broome, et al. 1992. Invasive Haemophilus influenzae disease: a population-based evaluation of the role of capsular polysaccharide serotype. J. Infect. Dis. 165(Suppl. 1):S34-S35. [DOI] [PubMed] [Google Scholar]
- 52.Wilcox, K. W., and H. O. Smith. 1975. Isolation and characterization of mutants of Haemophilus influenzae deficient in an adenosine 5′-triphosphate-dependent deoxyribonuclease activity. J. Bacteriol. 122:443-453. [DOI] [PMC free article] [PubMed] [Google Scholar]