Abstract
Analysis of the replication and drug resistance of patient serum hepatitis B virus (HBV) populations can contribute to the therapeutic management of chronic hepatitis B. We developed a procedure for cloning serum HBV quasispecies populations and for phenotypic analysis of the cloned populations for in vitro drug susceptibility. Equivalent sequences were compared to the respective serum HBV DNAs of the cloned quasispecies by population sequencing. Analysis of individual clones revealed that each population contained a diversity of HBV quasispecies. Furthermore, secreted HBV in the supernatant following transfection of the quasispecies populations remained mostly unchanged from the respective input populations. HBV obtained from patients who had developed resistance to adefovir or lamivudine, as demonstrated by development of the rtA181V or rtL180M/M204V mutations in HBV polymerase, respectively, were tested. Phenotypic analysis demonstrated that a population containing the HBV rtA181V mutation showed a 2.9-fold increase in the 50% effective concentration (EC50) for adefovir compared to the wild-type baseline isolate, while the lamivudine-resistant HBV quasispecies population showed a >1,000-fold increase in the lamivudine EC50. In summary, a strategy of cloning full genome HBV quasispecies populations from patient sera was developed, which could provide a useful tool in clinical HBV drug resistance phenotyping and studies of the evolution of clinical viral species.
The availability of oral nucleoside/nucleotide analog anti-hepatitis B virus (HBV) reverse transcriptase (RT) inhibitors has greatly improved the management of patients with chronic hepatitis B, a disease leading to 1 million annual deaths worldwide from resultant illnesses, such as cirrhosis and hepatocellular carcinoma (13). Four nucleoside/nucleotide analog HBV RT inhibitors, lamivudine (LAM), adefovir dipivoxil (ADV), entecavir, and telbivudine, are approved in the United States for the treatment of chronic hepatitis B. Due to the persistent nature of chronic HBV infection, largely attributable to the stability of HBV covalently closed circular DNA (20), these therapies rarely produce HBsAg seroconversion and therefore require prolonged administration to control disease in most patients. Long-term therapy, however, can be associated with the emergence of resistant HBV strains, leading to loss of therapeutic benefit and resumption of liver disease progression. Resistance to LAM results from the selection of HBV RT YMDD mutations (rtM204V and rtM204I) and occurs in about 20% of patients per year of treatment (12). LAM resistance mutations confer cross-resistance to other l-nucleoside analogs, such as telbivudine, clevudine, and emtricitabine, and contribute to resistance to entecavir (25). In contrast, ADV maintains clinical efficacy against LAM resistance mutations (17, 18), but its long-term administration selects for the resistance mutation rtN236T and/or rtA181V, although at much lower incidence than that in LAM therapy (S. Locarnini, X. Qi, S. Arterburn, A. Snow, C. L. Brosgart, G. Currie, M. Wulfsohn, M. Miller, and S. Xiong, presented at the 40th annual meeting of the European Association for the Study of the Liver, Paris, France, 13 to 17 April 2005). Shorter-term clinical studies have indicated that entecavir selects for another set of resistance mutations in RT, I169T, T184S/G, S202I/G, and M250V, which occur in addition to the LAM YMDD mutations (5, 23).
The expanding use and prolonged administration of the approved HBV RT inhibitors, as well as the development of new agents, place an increasing emphasis on the monitoring and identification of new drug resistance mutations in antiviral therapy. Evaluation of the in vitro drug susceptibilities of resistance-associated mutations forms a crucial component of any resistance surveillance program. Phenotypic analysis of HBV clinical isolates would offer more relevant information than that obtained from testing viruses with mutations introduced into laboratory strains, as has been commonly practiced (1, 4, 19). HBV genomes are heterogeneous, consisting of eight distinct genotypes (3, 16, 21), whereas viruses created by site-directed mutagenesis of a laboratory strain would not contain the natural genetic context of a mutation identified in the clinical strains. A novel plasmid expression vector for cloning the entire HBV genome was recently created to facilitate the expression of full-length HBV clinical isolates (26) that were amplified with a pair of primers encompassing a highly conserved region in the HBV genome (9). The cloned clinical isolates could then be transfected into hepatoma cell lines, and in vitro drug susceptibilities could be tested (26). Because of the quasispecies nature of HBV and because the assay was based on testing individual clones of clinical isolates, different isolates showed large variations in replication capacities, even among those from the same serum collections (26). Using this expression vector, we constructed populations of the strains of the predominant serum HBV quasispecies populations. Genotypes of the cloned quasispecies populations were validated by comparison to those generated by direct serum HBV sequencing. The wild-type or mutation-containing quasispecies populations were then tested for in vitro drug susceptibilities. This approach also allows single-genome sequence analysis of the individual clinical isolates.
MATERIALS AND METHODS
Patients.
Phenotypic testing was performed on serum HBV DNA obtained from two chronic HBV patients enrolled in ADV clinical trials. Patient 1 was treatment naïve and HBV e-antigen negative and had received ADV monotherapy for 160 weeks in study GS-98-438 at the time of the current analysis. Patient 2 had prior LAM treatment, with LAM-resistant mutations. ADV treatment was added to ongoing LAM therapy for 160 weeks in study GS-98-435. Serum HBV DNA was measured using the Roche Amplicor Monitor PCR assay with a lower limit of quantification of 1,000 copies/ml.
Isolation of HBV from patient sera and creation of quasispecies populations.
HBV DNA was extracted either from 200 μl serum using the QIAamp DNA Blood Kit (QIAGEN, Chatsworth, CA) or from 1 ml serum for samples with viral loads of less than 10E4 copies/ml using a QIAamp UltraSens Virus Kit (QIAGEN, Chatsworth, CA). Genome length HBV DNA was amplified by PCR as described by Günther et al. (9), using primers P1 (5′-CCG GAA AGC TTG AGC TCT TCT TTT TCA CCT CTG CCT AAT CA-3′) and P2 (5′-CCG GAA AGC TTG AGC TCT TCA AAA AGT TGC ATG GTG CTG G-3′). Multiple replicates of PCR were carried out and pooled to obtain sufficient amounts of PCR products for subsequent cloning steps. HBV genome length PCR products were purified using a QIAquick PCR purification kit (QIAGEN, Chatsworth, CA). To ensure the purity of the PCR products and to reduce the salt concentration, the column-purified samples were ethanol precipitated and washed twice with 70% ethanol. The purified PCR products were subsequently digested with SapI (NEB, Beverly, MA) and subjected to 1% agarose gel electophoresis, and the 3.2-kbp band was extracted using a QIAEXII Gel Extraction kit (QIAGEN, Chatsworth, CA). The SapI-treated PCR product was then ligated into SapI-digested and shrimp alkaline phosphatase-treated pHY106 plasmid. Plasmid pHY106 contains 180 bp of HBV sequences, which are very well conserved among various HBV genotypes (26), and contains two SapI sites, allowing insertion of the SapI treated PCR-amplified HBV genome, leading to a 1.1-unit length of HBV genome with transcription from the cytomegalovirus immediate-early promoter, resulting in pregenomic mRNA. The ligated samples were then transformed into Escherichia coli using library efficiency DH5α (Invitrogen, Carlsbad, CA). An aliquot of the transformation culture was spread on LB/ampicillin plates to obtain individual clones, and the remaining culture was amplified for large-quantity plasmid preparation. The resultant plasmid preparation contained a mixture of HBV quasispecies genomes (the quasispecies population).
Sequence analysis of serum HBV DNA, cloned quasispecies, and HBV DNA in cell culture supernatant.
Serum HBV DNA was extracted from 100 μl serum diluted in 100 μl phosphate-buffered saline using the QIAamp DNA Blood Kit (QIAGEN, Chatsworth, CA). HBV DNA in the cell culture supernatant was extracted from 200 μl HepG2 culture media 7 days after transfection, using a QIAamp DNA Blood Kit. A pair of primers, HBV302F (5′-GGCCAAAATTCGCAGTCCCAAATCTCCAG-3′) and HBV 1131R (5′-CTGTTTACTTAGAAAGGCCTTGTAAGTTGGCG-3′), were used to amplify a region of the HBV polymerase RT region including the conserved domains A to E. The purified PCR product served as a template for the sequencing of serum HBV DNA, whereas the plasmid minipreps of the recombinant quasispecies clones or the maxipreps of quasispecies populations were used as templates for sequencing clones. The Big Dye terminator sequencing assay using the ABI Prism 3100 Genetic Analyzer (ABI, Foster City, CA) was employed for sequencing reactions. The raw sequencing data were analyzed using the Sequencher program and translated into amino acid sequences covering HBV RT amino acids 75 to 255 of the HBV polymerase.
Drug susceptibility assays.
The cloned individual HBV genome or the genomes of quasispecies populations were transfected into HepG2 cells as described previously (26). Briefly, six-well culture plates were seeded at 7.5 × 105 HepG2 cells/well a day earlier and transfected with 1 μg DNA using the Fugene 6 reagent following the manufacturer's protocol (Roche Applied Science, Indianapolis, IN). Drug treatment was initiated the next day and lasted for 7 days, with replacement of fresh drug-containing media every 2 or 3 days. The concentrations of adefovir (Gilead Sciences, Foster City, CA) used were 0, 0.01, 0.1, 1, 10, and 25 μM, and those of LAM (Moravek Biochemicals, Brea, CA) were 0, 0.001, 0.01, 0.1, 1, and 10 μM for wild-type HBV and 0, 0.01, 0.1, 1, 10, and 100 μM for HBV containing a LAM resistance mutation. Intracellular core-associated HBV DNA was extracted using a protocol described previously (24) and analyzed by Southern hybridization with 32P-labeled HBV DNA as a probe. The 50% effective concentrations (EC50s) were calculated based on the signal intensities of double-stranded HBV DNA using TableCurve 2D software (SYSTAT Software, Richmond, CA).
RESULTS
Population cloning of full-length genomes of HBV quasispecies from patient sera.
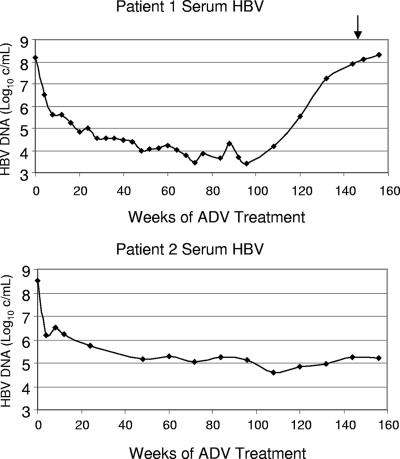
Patient 1 had wild-type HBV RT at baseline and developed the rtA181V ADV-associated mutation as shown by population sequencing of serum HBV DNA at week 144. Patient 2 had the LAM-associated rtL180M/M204I/V mutations at baseline and at week 144 developed an additional mutation, rtS202S/G. The rtS202G mutation is associated with resistance to entecavir (5). The HBV DNA response to therapy with ADV for each patient is shown in Fig. 1.
FIG. 1.
Serum HBV DNA changes of patients 1 and 2 during ADV treatment. The arrow indicates the time point when week 144 serum samples were collected.
Because of the quasispecies nature of the serum HBV strains, we sought to clone the representative patient quasispecies populations following a procedure described in detail in Materials and Methods. The constructed baseline and week 144 populations of patient 1 each had >5,000 and >2,000 individual clones, and those of patient 2 each had >5,000 and >1,000 individual clones, respectively. Sequence analysis of the HBV RT sequences of the plasmids containing the two patient quasispecies populations demonstrated that the cloned populations each matched the corresponding sequence result obtained from direct sequencing of the patient's serum HBV DNA (data not shown). These results demonstrated that amplification of the HBV genome and the subsequent population cloning maintained the composition of the predominant quasispecies of the serum HBV.
Cloned populations contained a diversity of HBV quasispecies.
The diversity of cloned HBV quasispecies was evaluated by sequencing 17 clones from the baseline and week 144 populations obtained from patient 1 and 12 clones from the baseline and week 144 populations obtained from patient 2. Nine of the 17 sequenced clones from the patient 1 baseline or week 144 population and 7 of 12 from the patient 2 baseline or week 144 population each had a unique sequence.
Consistent with the individual sequencing results, HBV isolates from the constructed quasispecies populations showed great variation in HBV replication efficiency, which was assessed by transfecting each cloned DNA into HepG2 cells and analyzing the intracellular viral core-associated HBV DNA by Southern blotting (Fig. 2). To evaluate if different quasispecies might be preferentially selected in vitro, thus altering the composition of the tested quasispecies population, we performed sequence evaluations of the extracellular HBV DNA 7 days posttransfection of HepG2 cells with the HBV quasispecies populations and compared the results to those obtained for the input populations. Testing was performed on the quasispecies population obtained from patient 1 at baseline and week 144 and for patient 2 at baseline. Sequence results could not be obtained from patient 2 week 144 samples at day 7 posttransfection due to inefficient replication. With the exception of the baseline populations from patient 1, which showed a difference at position 135 (a mixture of Y/C/S/N in the cell culture supernatant compared to a mixture of Y/C in the input population), the rest of the populations had identical sequences when the input plasmid was compared to the cell culture supernatant over the range of analyzed RT sequences (Fig. 3). A close inspection of the sequence chromatographs from the plasmid baseline populations for patient 1 revealed that a mixture of Y/C/S/N at position 135 was present (data not shown).
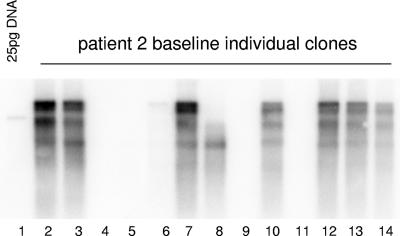
FIG. 2.
HBV replication of individual clones. HBV clones (n = 13) isolated from the constructed clinical quasispecies population (patient 2) were each transfected into HepG2 cells. After 3 days of incubation, HBV core DNA was extracted, fractionated on a 1.5% agarose gel, and detected by Southern hybridization as described in Materials and Methods.
FIG. 3.
Comparison of sequences of input HBV DNA and supernatant HBV following transfection. DNA sequences of patient 1 baseline and week 144 and patient 2 baseline quasispecies populations used for transfection were compared with those secreted in culture supernatants at 8 days posttransfection. Above each section is a consensus HBV sequence, and below are sequences in pairs of transfection input and secreted HBV. Differences from the consensus are shown. Position 135 of the patient 1 baseline supernatant sequence (highlighted in gray) showed a mixture slightly different from that of the input sequence. This position was further inspected in the corresponding sequence chromatograph (data not shown).
Phenotypic analysis of quasispecies populations and individual clones.
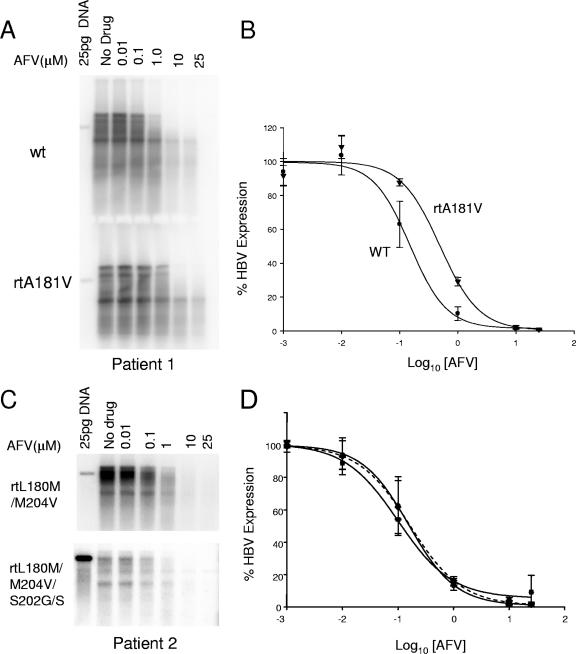
Phenotypic testing was performed on the baseline and week 144 quasispecies populations obtained from each patient to determine the susceptibilities of the isolates to adefovir, and the results are summarized in Table 1. The EC50 value obtained for adefovir on the wild-type baseline population from patient 1 was 0.17 ± 0.076 μM, which is comparable to previously published data for wild-type strains (26). For the week 144 population, which contained the rtA181V mutation, the EC50 increased by 2.94-fold over the baseline population (note the shift in dose response in Fig. 4B). Three individual clones, each isolated from quasispecies populations of patient 1 at baseline and week 144, were also evaluated in vitro for their susceptibilities to adefovir. The average adefovir EC50 value of the baseline clones was 0.14 ± 0.048 μM, with individual values ranging between 0.061 and 0.232 μM. The adefovir EC50 values obtained for the week 144 clones ranged between 0.294 and 0.693 μM, with an average of 0.41 ± 0.18 μM. The average adefovir EC50 value of the week 144 clones was 2.88-fold greater than that of the baseline isolates, similar to the 2.94-fold increase observed in the evaluation of the quasispecies populations for this patient. Therefore, the phenotyping result for the population is consistent with an average of multiple individual clinical isolates.
TABLE 1.
In vitro drug susceptibilities of tested clinical isolates
| HBV testeda | RT genotype | Adefovir
|
Lamivudine
|
||
|---|---|---|---|---|---|
| EC50 (μM) | Changeb | EC50 (μM) | Changeb | ||
| Pt1 BL population | Wild type | 0.17 ± 0.076 | 1 | 0.0173 | 1 |
| Pt1 wk 144 population | A181V | 0.5 ± 0.06 | 2.94 | 0.0126 | 0.73 |
| Pt1 BL clones | Wild type | 0.14 ± 0.048 | 1 | ||
| Pt1 wk 144 clones | A181V | 0.41 ± 0.18 | 2.88 | ||
| Pt2 BL population | L180M/M204V | 0.176 ± 0.11 | 1 | 25.32 | >1,000 |
| Pt2 wk 144 population | L180M/S202G/M204V | 0.12 ± 0.014 | 0.7 | ||
| Pt1 wk 144 clone | L180M/S202G/M204V | 0.176 ± 0.1 | 1 | ||
Pt, patient; BL, baseline.
Change (n-fold) from baseline wild type.
FIG. 4.
Adefovir susceptibility tests of patient 1 and 2 quasispecies populations and a week 144 clone from patient 2. (A) A representative Southern blot testing adefovir susceptibilities of patient 1 baseline (wild type [wt]) and week 144 (rtA181V) quasispecies populations. (B) Average dose-response curves of patient 1 populations to adefovir. Genotypes of the wild-type baseline (WT) and the rtA181V week 144 populations are indicated. The error bars indicate standard deviations. (C) A representative Southern blot testing adefovir susceptibilities of patient 2 baseline (rtL180M/M204V) and week 144 (rtL180M/S202G/S/M204V) quasispecies populations. (D) Average dose-response curves of patient 2 populations to adefovir. The dotted line indicates the dose-response curve of a clone (rtL180M/S202G/M204V) isolated from the patient 2 week 144 population.
The adefovir EC50 value obtained for the baseline population of patient 2 harboring the rt180M/204V LAM resistance mutations was 0.176 ± 0.11 μM, similar to that obtained with wild-type isolates. The adefovir EC50 value for the week 144 population, which contained the rtS202S/G mutation, was slightly lower than that obtained for the baseline population, indicating that the additional rtS202G/S change did not decrease the adefovir susceptibility of the baseline rt180M/204V LAM resistance mutations (Table 1). To confirm this result, and to verify that the rt180M plus 202G plus 204V mutations were on the same viral genome, a clone containing rt180M plus 202G plus 204V mutations isolated from the cloned patient 2 week 144 population was tested against adefovir and showed an average EC50 value similar to that of the population (Table 1). Of note, the addition of the rt202G mutation to the rt180M/204V mutations decreased replication levels in vitro (Fig. 4C).
The patient 1 baseline (HBV wild type) and week 144 (rt181V) and patient 2 baseline (mostly rt180M/204V) populations were also evaluated in vitro for their susceptibilities to LAM. The patient 1 baseline and week 144 populations had LAM EC50 values of 0.017 and 0.013 μM, respectively, and the patient 2 baseline population, which consisted predominantly of rt180M/204V but had detectable wild-type sequences, had an EC50 value of 25.32 μM (Table 1).
DISCUSSION
The lack of a proofreading function of HBV polymerase, coupled with a high level of virus production in HBV patients, results in an estimated 1010 base-pairing errors daily (reviewed in reference 6). Therefore, HBV in chronically infected patients harbors many virus quasispecies. When a chronic-HBV patient undergoes a nucleoside analog antiviral treatment, the entire virus quasispecies population is subjected to the inhibitory effects. Therefore, in vitro evaluation of HBV replication and drug susceptibility phenotypes of the virus quasispecies populations in patients would be more representative than that of individual clinical isolates or of a laboratory strain. In this report, we describe the development of a procedure, using a previously described vector for cloning HBV genomes, for population cloning to construct the dominant quasispecies population from serum HBV and for in vitro drug susceptibility testing using the constructed quasispecies population. The population-sequencing results for each of the constructed quasispecies populations matched those of their respective patient serum HBVs, indicating that the constructed population represented the dominant serum virus quasispecies. The cloned populations each contained a diversity of different quasispecies, as indicated by the distinct sequences of individual clones and significant variability in in vitro replication levels, consistent with previous findings (9, 10, 26).
While sequence diversities within each cloned population were observed and may represent that of the serum virus quasispecies, the possibility that some of the sequence variation resulted from PCR amplification errors cannot be excluded, even with the use of a high-fidelity PCR polymerase. The cloned quasispecies varied significantly in replication levels, consistent with the previous reports that naturally occurring single-amino-acid changes in HBV are sufficient to drastically change HBV replication levels in vitro (14, 22). Unlike the LAM resistance YMDD mutations, which led to a >1,000-fold increase in the EC50, the adefovir resistance mutations rtN236T and rtA181V showed increases in the EC50 ranging only from 4- to 14-fold and 2.5- to 3-fold, respectively (2). The described testing of cloned quasispecies populations allowed the evaluation of an average change in the EC50 of a population of virus quasispecies pre- and post-antiviral treatment, and the results corresponded to the virological responses observed in these patients (Fig. 1). Patient 1 developed an rtA181V mutation that was associated with rebound in HBV DNA and a corresponding ∼3-fold increase in the EC50 for adefovir compared to the baseline wild-type isolate. By contrast, the adefovir susceptibilities of HBV quasispecies populations from patient 2 at baseline (rtL180M/M204V) and week 144 (rtL180M/M204V plus S202G) were similar, consistent with the clinical observation of a sustained >3-log10-unit drop in HBV DNA and absence of virological rebound. The baseline population containing LAM resistance rtL180M/M204V mutations did confer strong LAM resistance compared to wild-type HBV (>2,000-fold increase in the EC50). The EC50 of LAM (25.32 μM) obtained for the baseline population containing rtL180M/M204V mutations was lower than previously published results (25, 26), presumably attributable to the wild-type HBV that was detected as minor quasispecies populations.
One underlying assumption for phenotypic testing of HBV variants in vitro is that HBV replication in a hepatoma cell line in vitro would represent that in vivo. Our results indicated that at the time of drug susceptibility analysis at 7 days posttransfection, the compositions of the quasispecies populations remained largely unchanged from those of the transfected populations. The drug susceptibility results would presumably then reflect that of the serum HBV itself. Due to the unavailability of cloned quasispecies populations or the low levels of HBV replication following the transfection of serum virus PCR products (9), the composition of the replicating quasispecies population in vitro could not previously be analyzed. To our knowledge, this is the first report to describe a longitudinal sequence analysis of a clinical quasispecies population following transfection into cultured cell lines. It would be interesting in the future to analyze the genotypes following a longer duration of in vitro HBV replication.
Phenotypic testing of HBV quasispecies populations was attempted before with a vector-free approach (9). However, in our experience, the levels of wild-type HBV DNA replication were quite low, either in Huh7 (7, 9) or in HepG2 cells in our experiments (data not shown). With the help of the strong cytomegalovirus immediate-early promoter from the vector used in this study, HBV DNA replication was readily detectable as early as 3 days following transfection. Another cloning vector was recently developed which expresses a 1.1-unit length of HBV from the efficient chicken β-actin promoter (7). However, the 1.1-unit length needed to be amplified as two separate PCR amplicons, followed by a one- or two-step cloning process. Because of the quasispecies nature of serum HBV and the high viral load in many chronic patients, the probability that the two amplicons were from the same genome would be minimal. The vector we used had approximately 180 bp of HBV included, so that once the 3.2-kbp PCR-amplified HBV genome was inserted, the 3.5-kb pregenomic RNA could be generated. The 180-bp HBV fragment included in the vector is highly conserved across different HBV strains (26) and in most cases would not introduce any changes to the otherwise original sequences. In our system, the entire polymerase gene would come from the same HBV genome. Furthermore, insertion of one PCR product allows more efficient population cloning.
In clinical practice of human immunodeficiency virus (HIV) therapy, in vitro phenotypic assays were developed to monitor the drug susceptibilities of HIV populations (8, 11). Compared to its use in the HIV field, HBV phenotypic testing is still in its early stage and lacks a standard platform. With the approval of the four nucleoside/nucleotide analog inhibitors LAM, ADV, entecavir, and telbivudine and with more (tenofovir, clevudine, valtorcitabine, and possible drug combinations) in various stages of development, the availability of anti-HBV drugs has certainly expanded greatly. Because many of these anti-HBV agents are potentially cross-resistant (25) and because there is a likely need for long-term treatment using many of these agents (15), HBV clinical management may become similar to that of HIV in that phenotypic testing of patient HBV will contribute to the selection of an effective treatment regimen. Toward that end, we have developed a procedure for cloning patient HBV quasispecies populations that represent the dominant virus strains in serum. With this approach, clinically relevant virus populations can be tested for their in vitro drug susceptibilities. In the future, this procedure will be useful in clinical HBV phenotyping and drug resistance surveillance, as well as in studies of HBV pathogenesis and the evolution of viral species in disease progression.
Acknowledgments
We thank Shelly Xiong and our colleagues at the Department of Clinical Virology of Gilead Sciences, Inc., for their scientific discussions and critique of this work.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Allen, M. I., M. Deslauriers, C. W. Andrews, G. A. Tipples, K.-A. Walters, D. L. Tyrrell, N. Brown, L. D. Condreay, and the Lamivudine Clinical Investigation Group. 1998. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Hepatology 27:1670-1677. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2006. Hepsera® (adefovir dipivoxil) tablets. US prescribing information. Gilead Sciences, Inc. Foster City, CA.
- 3.Arauz-Ruiz, P., H. Norder, B. H. Robertson, and L. O. Magnius. 2002. Genotype H: a new Amerindian genotype of hepatitis B virus revealed in Central America. J. Gen. Virol. 83:2059-2073. [DOI] [PubMed] [Google Scholar]
- 4.Bock, C.-T., H. L. Tillmann, J. Torresi, J. Klempnauer, S. Locarnini, M. P. Manns, and C. Trautwein. 2002. Selection of hepatitis B virus polymerase mutants with enhanced replication by lamivudine treatment after liver transplantation. Gastroenterology 122:264-273. [DOI] [PubMed] [Google Scholar]
- 5.Colonno, R. J., R. Rose, C. J. Baldick, S. Levine, K. Pokornowski, C. F. Yu, A. Walsh, J. Fang, M. Hsu, C. Mazzucco, B. Eggers, S. Zhang, M. Plym, K. Klesczewski, and D. J. Tenney. 2006. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology 44:1656-1665. [DOI] [PubMed] [Google Scholar]
- 6.Doo, E., and T. J. Liang. 2001. Molecular anatomy and pathophysiologic implications of drug resistance in hepatitis B virus infection. Gastroenterology 120:1000-1008. [DOI] [PubMed] [Google Scholar]
- 7.Durantel, D., S. Carrouee-Durantel, B. Werle-Lapostolle, M. N. Brunelle, C. Pichoud, C. Trepo, and F. Zoulim. 2004. A new strategy for studying in vitro the drug susceptibility of clinical isolates of human hepatitis B virus. Hepatology 40:855-864. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Lerma, J. G., and W. Heneine. 2001. Resistance of human immunodeficiency virus type 1 to reverse transcriptase and protease inhibitors: genotypic and phenotypic testing. J. Clin. Virol. 21:197-212. [DOI] [PubMed] [Google Scholar]
- 9.Günther, S., B.-C. Li, S. Miska, D. H. Krüger, H. Meisel, and H. Will. 1995. A novel method for efficient amplification of whole hepatitis B virus genomes permits rapid functional analysis and reveals deletion mutants in immunosuppressed patients. J. Virol. 69:5437-5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunther, S., G. Sommer, F. Von Breunig, A. Iwanska, T. Kalinina, M. Sterneck, and H. Will. 1998. Amplification of full-length hepatitis B virus genomes from samples from patients with low levels of viremia: frequency and functional consequences of PCR-introduced mutations. J. Clin. Microbiol. 36:531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna, G. J., and R. T. D'Aquila. 2001. Clinical use of genotypic and phenotypic drug resistance testing to monitor antiretroviral chemotherapy. Clin. Infect. Dis. 32:774-782. [DOI] [PubMed] [Google Scholar]
- 12.Lai, C.-L., J. Dienstag, E. Schiff, N. W. Y. Leung, M. Atkins, C. Hunt, N. Brown, M. Woessner, R. Boehme, and L. Condreay. 2003. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin. Infect. Dis. 36:687-696. [DOI] [PubMed] [Google Scholar]
- 13.Lee, W. M. 1997. Hepatitis B virus infection. N. Engl. J. Med. 337:1733-1745. [DOI] [PubMed] [Google Scholar]
- 14.Lin, X., Z. H. Yuan, L. Wu, J. P. Ding, and Y. M. Wen. 2001. A single amino acid in the reverse transcriptase domain of hepatitis B virus affects virus replication efficiency. J. Virol. 75:11827-11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lok, A. S. 2005. The maze of treatments for hepatitis B. N. Engl. J. Med. 352:2743-2746. [DOI] [PubMed] [Google Scholar]
- 16.Magnius, L. O., and H. Norder. 1995. Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene. Intervirology 38:24-34. [DOI] [PubMed] [Google Scholar]
- 17.Perrillo, R., H.-W. Hann, D. Mutimer, B. Willems, N. Leung, W. M. Lee, A. Moorat, S. Gardner, M. Woessner, E. Bourne, C. L. Brosgart, and E. Schiff. 2004. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology 126:81-90. [DOI] [PubMed] [Google Scholar]
- 18.Peters, M. G., H. W. Hann, P. Martin, J. E. Heathcote, P. Buggisch, R. Rubin, M. Bourliere, K. Kowdley, C. Trepo, D. F. Gray, M. Sullivan, K. Kleber, R. Ebrahimi, S. Xiong, and C. L. Brosgart. 2004. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 126:91-101. [DOI] [PubMed] [Google Scholar]
- 19.Pichoud, C., B. Seignères, Z. Wang, C. Trépo, and F. Zoulim. 1999. Transient selection of a hepatitis B virus polymerase gene mutant associated with a decreased replication capacity and famciclovir resistance. Hepatology 29:230-237. [DOI] [PubMed] [Google Scholar]
- 20.Seeger, C., and W. S. Mason. 2000. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64:51-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuyver, L., S. De Gendt, C. Van Geyt, F. Zoulim, M. Fried, R. F. Schinazi, and R. Rossau. 2000. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J. Gen. Virol. 81:67-74. [DOI] [PubMed] [Google Scholar]
- 22.Suk, F. M., M. H. Lin, M. Newman, S. Pan, S. H. Chen, J. D. Liu, and C. Shih. 2002. Replication advantage and host factor-independent phenotypes attributable to a common naturally occurring capsid mutation (I97L) in human hepatitis B virus. J. Virol. 76:12069-12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenney, D. J., S. M. Levine, R. E. Rose, A. W. Walsh, S. P. Weinheimer, L. Discotto, M. Plym, K. Pokornowski, C. F. Yu, P. Angus, A. Ayres, A. Bartholomeusz, W. Sievert, G. Thompson, N. Warner, S. Locarnini, and R. J. Colonno. 2004. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrob. Agents Chemother. 48:3498-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto, T., S. Litwin, T. Zhou, Y. Zhu, L. Condreay, P. Furman, and W. S. Mason. 2002. Mutations of the woodchuck hepatitis virus polymerase gene that confer resistance to lamivudine and 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil. J. Virol. 76:1213-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang, H., X. Qi, A. Sabogal, M. Miller, S. Xiong, and W. E. Delaney IV. 2005. Cross-resistance testing of next-generation nucleoside and nucleotide analogues against lamivudine-resistant HBV. Antivir. Ther. 10:625-633. [PubMed] [Google Scholar]
- 26.Yang, H., C. Westland, S. Xiong, and W. E. Delaney IV. 2004. In vitro antiviral susceptibility of full-length clinical hepatitis B virus isolates cloned with a novel expression vector. Antivir. Res. 61:27-36. [DOI] [PubMed] [Google Scholar]