Abstract
Performance of antimicrobial susceptibility tests with new agents requires careful consideration of the properties of the antimicrobial to ensure that the tests are standardized, reproducible, and reflect the true potency of the drug. Dalbavancin is a new glycopeptide with potent activity against gram-positive bacterial species. The investigations described here demonstrated that methodologic modifications of procedures are necessary to ensure consistent test results, both for quality control and for routine testing of clinical isolates. Dimethyl sulfoxide is the preferred primary solvent. The addition of 0.002% polysorbate-80 (a surfactant) to dalbavancin-containing wells in the reference broth microdilution assay resulted in consistent and reproducible MIC results for three quality control strains: Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, and Streptococcus pneumoniae ATCC 49619. The same degree of consistency was observed among clinical isolates of gram-positive bacterial species tested in several clinical laboratories. These results indicate that the addition of 0.002% (final concentration) of the surfactant in broth microdilution tests produces optimal dalbavancin MICs required for accurate and reproducible clinical laboratory tests, without untoward influences of substrate binding or media constituents.
Dalbavancin is a new semisynthetic glycopeptide antimicrobial agent with potent activity against gram-positive bacterial species including Staphylococcus aureus (both methicillin-susceptible S. aureus [MSSA] and methicillin-resistant S. aureus [MRSA]), Streptococcus pneumoniae (penicillin susceptible and resistant), beta-hemolytic streptococci, and vancomycin-susceptible enterococci (7-12). Some strains of vancomycin-resistant enterococci (vanA strains) display elevated MICs for dalbavancin, whereas the agent retains activity against strains expressing the vanB or vanC resistance patterns (12). The solubility of dalbavancin is pH dependent in water (approximately 20 μg/ml at neutral pH; B. Goldstein, unpublished data). To attain higher concentrations, solubilization in other solvents such as dimethyl sulfoxide (DMSO) is required. Dalbavancin also readily adsorbs to plastic surfaces, including those used in the manufacture of broth microdilution susceptibility test panels (Goldstein, unpublished). This phenomenon has also recently been observed for another glycopeptide agent (F. F. Arhin, I. Sarmiento, T. J. Parr, and G. Moeck, unpublished data). Investigation and optimization of these parameters has been required in establishing standardized susceptibility test methods for use in quality control and routine clinical testing.
Initial broth microdilution quality control ranges for S. aureus ATCC 29213, Enterococcus faecalis ATCC 29212 and S. pneumoniae ATCC 49619 were developed as part of an eight-laboratory evaluation published in 2003 (1). In January 2004, these proposed quality control MIC ranges for dalbavancin were approved by the Antimicrobial Susceptibility Testing Subcommittee of the Clinical and Laboratory Standards Institute (CLSI; formerly National Committee for Clinical Laboratory Standards) (3, 4). The initial studies performed in 2003 (1) utilized polysobate-80 (P-80), a surfactant commonly utilized in the performance of antimicrobial susceptibility testing to enhance reproducibility. During development of in vitro testing protocols for dalbavancin, concern over the use of P-80 arose as a potential confounding factor for performance of quantitative tests. P-80 is a nonionic surfactant and emulsifier derived from sorbitol which is contained in various types of fruits and is derived primarily from coconut oil. The chemical name is sorbitol methylene mono-oleate. It is used as a dispersion agent for many compounds, including antimicrobials that do not mix well in water and that may stick or adhere to surfaces such as glass or plastics.
This report describes the investigations conducted to ensure that dalbavancin broth microdilution susceptibility tests of both quality control strains and clinical isolates are standardized and reproducible. The aims of the study were to determine (i) optimal P-80 concentrations, (ii) the influence of addition of P-80 into panel wells or inoculum water on the reproducibility of MICs, and (iii) the effect of exposure to plastics used for broth microdilution panels on test results. Validation of MIC ranges as described in the initial quality control evaluation (1) was also sought in the present study.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains utilized in these studies included S. aureus ATCC 29213, E. faecalis ATCC 29212, and S. pneumoniae ATCC 49619 (CLSI quality control strains), as well as 20 S. aureus and 20 beta-hemolytic streptococci acquired from clinical sources. Strains used in a checkerboard interaction experiment of dalbavancin (≤0.002 to 2 μg/ml [log2 scale]) and P-80 (none to 2% [log10 scale]) included S. aureus (eleven strains; five MSSA, five community-associated MRSA, ATCC 29213) and beta-hemolytic streptococci (four strains). Additional clinical isolates were tested using the recommended testing format and have been reported in a companion investigation comparing E-test (AB BIODISK, Solna, Sweden) to the reference broth microdilution method (6) with DMSO and P-80.
Quality control studies utilizing P-80.
P-80 (Tween 80; Sigma, St. Louis, MO) to achieve a final concentration of 0.002% (vol/vol) was included either in the dalbavancin dilutions in cation-adjusted Mueller-Hinton broth (CAMHB) or in the inoculum waters. When we tested S. pneumoniae, 2 to 5% lysed horse blood was incorporated into the medium (3, 4), but the medium formulations in the trays were otherwise the same as for the other quality control strains. Broth microdilution trays were prepared according to the stepwise dilution method appearing in CLSI document M100-S16 (4). In brief, for preparation of the dalbavancin solutions (Vicuron Pharmceuticals, Inc., King of Prussia, PA), the primary stock solution was dissolved in DMSO. Intermediate concentrations were further prepared in DMSO and then diluted 1:100 in CAMHB. Parallel reagent control wells were utilized to determine that neither 0.002% P-80 nor 1% DMSO inhibited or enhanced the growth of the tested bacterial strains.
In the first of two parallel studies, frozen broth microdilution trays containing dalbavancin were prepared at one site with or without P-80 in the broth. P-80 was subsequently added to the inoculum in the trays free of the surfactant. Multiple replicates of the three quality control strains were performed. In the second study each laboratory prepared its own trays, incorporating P-80 into the dalbavancin-containing wells. The panels were used fresh on the first day of preparation and frozen for subsequent testing on three separate occasions. There were a total of 64 replicate values produced for each quality control strain.
Optimization of P-80 concentration.
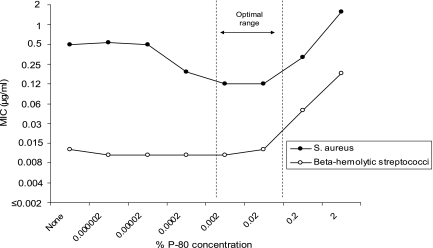
A total of 15 gram-positive clinical isolates and one quality control strain (S. aureus ATCC 29213) were tested by reference MIC methods against dalbavancin with various concentrations of P-80 (none, 0.000002 to 2%) by checkerboard grid analysis to optimize P-80 levels prior to other investigations. The mean MIC results for all 11 S. aureus strains and 4 beta-hemolytic streptococcal strains are plotted in Fig. 1. Generally, dalbavancin was approximately 32-fold more active against beta-hemolytic streptococci (mean MIC of 0.013 μg/ml) than S. aureus (MIC of 0.5 μg/ml) when tested without P-80 and 8-fold more potent when the P-80 concentration recommended by the manufacturer and by CLSI was used (3, 4). Dalbavancin MIC results varied by P-80 concentration for S. aureus, being highest at concentrations of ≤0.0002 and ≥0.2%, but streptococcal MIC results were more uniform at P-80 levels of ≤0.02%. An optimal P-80 range of 0.02 to 0.002% was observed with the 0.002% concentration selected for determining reproducible dalbavancin MICs.
FIG. 1.
Influence of various P-80 concentrations (0.000002 to 2%) on the dalbavancin MIC results for S. aureus (11 strains) and beta-hemolytic streptococci (4 strains).
Studies utilizing clinical isolates.
In two additional studies performed in four laboratories, 60 clinical challenge strains of gram-positive bacteria were tested to investigate possible differences between the incorporation of P-80 directly into panel wells or into the inoculum waters (according to the method used by Anderegg et al. (1). In the first study, 10 MSSA, 10 MRSA, and 20 beta-hemolytic streptococcus (8 S. pyogenes and 12 group B, C and G streptococcus) strains were tested in duplicate in two laboratories. In the second study 10 clinical strains of S. aureus and 10 strains of S. pyogenes were tested in triplicate in one laboratory.
Effect of exposure to plastic in the preparation of dalbavancin test reagents.
S. aureus ATCC 29213 was used to investigate the effect of preparing dalbavancin solutions in glass tubes, followed by exposure to plastic, and the effect of P-80 on MICs. The dalbavancin stock dilution was prepared as described in glass tubes; serial doubling dilutions were made in CAMHB, also in glass tubes (9). These dilutions served as the glass-only controls. A second set of dilutions was prepared by taking 10-ml aliquots from each dalbavancin dilution and transferring each to a 150-mm plastic petri dish. The dishes were gently rocked for 30 min in order to obtain maximum plastic exposure. Two additional sets were prepared by adding P-80 (0.002% [vol/vol]) to glass and plastic dilution series. Microdilution trays were then prepared by using each of the four dilution series, and an inoculum of 2 × 105 to 5 × 105 CFU/ml (without P-80) was added to each well. After overnight incubation at 35°C, the endpoint MIC was read as the complete inhibition of visible growth in the wells. A total of 32 observations were made for each of the glass and plastic tests without P-80, and 8 were made for the tests with P-80.
Additional experiments were performed utilizing clinical strains of S. aureus (n = 35), coagulase-negative staphylococci (n = 20), Enterococcus spp. (n = 10), beta-hemolytic streptococci (n = 15), viridans group streptococci (n = 10), and S. pneumoniae (n = 10). In these investigations, broth microdilution trays were prepared with dalbavancin in CAMHB (with 2 to 5% lysed horse blood for S. pneumoniae), to which 0.002% P-80 was added to one of two sets of dilutions. These trays were inoculated immediately and incubated overnight at 35°C.
RESULTS
The effects of added P-80 (0.002%) in either the broth dilutions or the inoculum waters on the MICs for three quality control strains failed to demonstrate significant differences between the methods of P-80 delivery. In the first study, of a total of 52 measurements, 44 (85%) were identical. There were seven MICs (three E. faecalis and four S. pneumoniae strains) that were one doubling dilution lower and one result (E. faecalis) that was one doubling dilution higher (data not shown). The results of the second study are shown in Table 1. When we used P-80 incorporated into the dalbavancin-containing wells, the MIC results for each quality control strain tested multiple times and using both freshly prepared and frozen trays were all within the approved quality control ranges established by CLSI (1, 4), with prominent modes (70 to 100% of results) occurring at the middle of the published ranges.
TABLE 1.
Dalbavancin MIC distributions in panels containing P-80 compared to CLSI quality control ranges established with P-80 introduced via the inoculum water
| Quality control strain (no. of replicates) | No. of occurrencesa at MIC (μg/ml) of:
|
% of results in CLSI range | ||||
|---|---|---|---|---|---|---|
| 0.008 | 0.015 | 0.03 | 0.06 | 0.012 | ||
| S. aureus ATCC 29213 (64) | - | - | 16* | 45* | 3* | 100.0 |
| E. faecalis ATCC 29212 (64) | - | - | -* | 64* | -* | 100.0 |
| S. pneumoniae ATCC 29619 (64) | 11* | 52* | 1* | - | - | 100.0 |
The results of testing clinical challenge strains of staphylococci and beta-hemolytic streptococci in one study performed in two laboratories are shown in Table 2. In the present study the S. aureus had a tendency toward lower MICs (by approximately one-half of a doubling dilution) when P-80 was added directly to the broth compared to its introduction with the inoculum; however, all results were within ±1 log2 dilution. For beta-hemolytic streptococci, MICs tended to be slightly higher in the presence of P-80 in broth versus in inoculum, with 93.8% of values being within ±1 log2 dilution. Only five readings (6.3%) were ±2 doubling dilutions from the central tendency. For the 20 S. aureus strains, 56% (45 of 80 results) were 1 log2 dilution lower when P-80 was added to the dalbavancin broth dilutions. For the eight S. pyogenes strains, 25% of the results were 1 log2 dilution lower, but there was a broader range of readings. The very low MICs found with the S. pyogenes strains may have contributed to greater variability. The same tendency was observed in the second study performed in one laboratory (data not shown).
TABLE 2.
Comparison of dalbavancin MICs for clinical challenge strains: comparison of P-80 in broth dilutions versus inoculum watersa
| Organisms (no. of strains) | No. of occurrencesb with log2 MIC variations of P-80 in broth vs inoculum of:
|
||||
|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | |
| S. aureus MSSA (10) | - | 21 | 19 | - | - |
| S. aureus MRSA (10) | - | 24 | 16 | - | - |
| Total S. aureus strains (20) | - | 45 | 35 | - | - |
| S. pyogenes (8) | 2 | 8 | 15 | 7 | - |
| Beta-hemolytic streptococcus groups B, C, and G (12) | 2 | 3 | 22 | 20 | 1 |
| Total Streptococcus spp. (20) | 4 | 11 | 37 | 27 | 1 |
| Total clinical strains (40) | 4 | 56 | 72 | 27 | 1 |
Two laboratories tested each strain in duplicate in each condition.
-, No occurrences.
The effects on MICs for S. aureus ATCC 29213 after the exposure of dalbavancin to glass and plastic with or without the addition of P-80 are shown in Table 3. Preparation of dalbavancin in glass alone without P-80 had less effect on the MIC for this quality control strain than plastic alone, where MICs were elevated ≥32-fold after extensive pre- and during-test plastics exposure. Nevertheless, in glass alone, 81% of the results were outside the CLSI quality control range (0.03 to 0.12 μg/ml) for S. aureus ATCC 29213 with dalbavancin. When P-80 was added to the broth dilution wells, all but one result were at 0.06 μg/ml, the midpoint of the quality control range (1, 4).
TABLE 3.
Dalbavancin MICs for S. aureus ATCC 29213 with dilutions prepared in glass or plastic with or without 0.002% P-80 prior to inoculation of microdilution traysa
| Method | No. of observationsb at an MIC (μg/ml) of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | |
| Glass alone | - | 6 | 26 | - | - | - | - | - |
| Glass + P-80 | 7 | 1 | - | - | - | - | - | - |
| Plastic alone | - | - | - | - | - | 8 | 19 | 5 |
| Plastic + P-80 | 8 | - | - | - | - | - | - | - |
Dalbavancin was in contact with glass or plastic for 30 min before inoculation.
-, No observations.
When clinical strains were tested and the dilutions prepared and inoculated directly without permitting dalbavancin to interact with plastic for a prolonged period of time, staphylococci and enterococci showed decreased MICs generally by four- to eightfold (66.2% of strains) when P-80 was present, whereas streptococci showed less effect, with most strains testing within one doubling dilution (97.1%; see Table 4).
TABLE 4.
Variation in dalbavancin MIC ratios for clinical strains tested without or with P-80
| Organism (no. of isolates tested) | No. of observationsa at an MIC ratio (without P-80/with P-80) of:
|
|||||
|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 4 | 8 | |
| S. aureus (35) | - | - | 1 | 3 | 28 | 3 |
| Coagulase-negative staphylococci (20) | - | - | 1 | 11 | 6 | 2 |
| Enterococcus spp. (10) | - | 1 | 2 | 3 | 4 | - |
| Beta-hemolytic streptococci (15) | - | - | 10 | 4 | 1 | - |
| Viridans group streptococci (10) | - | 1 | 5 | 4 | - | - |
| S. pneumoniae (10) | - | - | 8 | 2 | - | - |
| Total (100) | -‡ | 2†‡ | 27*†‡ | 27†‡ | 39‡ | 5 |
-, No observations. *, 27% identical results; †, 56% ± 1 log2 dilution step; ‡, 95% ± 2 log2 dilution steps.
DISCUSSION
The parameters for accurate in vitro antimicrobial susceptibility testing are not often fully appreciated. For some antimicrobial agents, interactions between the agent and assay constituents may result in the diminution of activity. For example, daptomycin in the absence of physiologic concentrations of calcium ions in broth media appears to be relatively inactive (3, 4). Other agents, such as the aminoglycosides, require sufficient concentrations of divalent cations (calcium and magnesium) when testing Pseudomonas aeruginosa (2-4). The carbapenems and trimethoprim-sulfamethoxazole require minimized concentrations of zinc (<3 mg/liter) and thymidine in the medium, respectively (3-5).
Factors such as these must always be considered in the development of susceptibility tests when measuring the activity of new antimicrobial agents. Many commercial manufacturers add surfactants to their inoculum waters to assist dispersion of the antimicrobial agent and organisms in their test systems as an aid in assuring reproducibility of MIC results. For dalbavancin the addition of P-80 (optimal concentration of 0.002%; see Fig. 1) to either the initial broth dilutions of the drug or the inoculum waters has been recognized as important for accurate testing in broth microdilution systems. In an earlier report (6), it did not appear necessary to add P-80 to the agar used in the performance of the Etest assay. The Etest process uses a different dry-form chemistry that appears to disperse dalbavancin without the need to add a surfactant.
Results from four participating laboratories using different manufacturers of trays and incorporating P-80 either into the broth dilutions or inoculum waters demonstrated no differences in dalbavancin results when testing quality control strains. Indeed, more than 85% of all dalbavancin MICs were identical between laboratories, and no results varied by more than one doubling dilution. All results were within the CLSI defined quality control ranges (4) for these strains. When clinical challenge strains of S. aureus and beta-hemolytic streptococci were tested in more than one laboratory and on multiple occasions, there appeared to be slight, species-specific shifts in MICs, but all S. aureus and 94% of the streptococcal MICs were within one doubling dilution, regardless of whether P-80 was added to the reference broth dilutions or inoculum waters.
The potential for dalbavancin binding to test constituents was clearly exhibited when the antimicrobial agent was exposed to glass or plastic without added P-80 and subsequently tested against a well-characterized S. aureus control strain. Only 9% of observations (and none with exposure to plastic) were within the CLSI established quality control range of 0.03 to 0.12 μg/ml. When P-80 was added, all results were within the established range, and 94% were at the midpoint of the range. When clinical strains of gram-positive bacteria were tested in Mueller-Hinton broth with or without P-80 and the susceptibility tests were promptly performed, there was still an effect with MICs being elevated in the absence of P-80 with staphylococci and enterococci, but less so with streptococci, probably due to effects of the lysed horse blood supplement.
The results of these studies indicate that 0.002% P-80 should be added to reference broth microdilution antimicrobial susceptibility tests for dalbavancin (1, 3, 4). As recommended by the CLSI (4) and Jones and coworkers (1, 6), addition of P-80 can most easily be incorporated during the preparation of the broth dilution series for dalbavancin without introducing P-80 to the testing wells of other agents via the inoculum water. Performance characteristics were shown to be more stable when dilutions were prepared by adding P-80 at the outset. In this format, accurate tests for dalbavancin susceptibility can be performed in commonly utilized plastic trays and become the standard to which other dalbavancin susceptibility testing devices (Etest and others) should be compared during product validation trials (6).
Acknowledgments
We acknowledge the skilled technical assistance of C. Brosnikoff, L. Turnbull, J. DiFranco, P. Rhomberg, G. Moet, J. Streit, and N. Holliday.
This study was supported by grants-in-aid from Vicuron Pharmaceuticals, Inc., and Pfizer, Inc.
Footnotes
Published ahead of print on 1 August 2007.
REFERENCES
- 1.Anderegg, T. R., D. J. Biedenbach, and R. N. Jones. 2003. Initial quality control evaluations for susceptibility testing of dalbavancin (BI397), an investigational glycopeptide with potent gram-positive activity. J. Clin. Microbiol. 41:2795-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. L., L. B. Reller, G. H. Miller, J. A. Washington, F. D. Schoenknect, L. R. Peterson, R. S. Hare, and C. Knapp. 1992. Revision of standards for adjusting the cation content of Mueller-Hinton broth for testing susceptibility of Pseudomonas aeruginosa to aminoglycosides. J. Clin. Microbiol. 30:585-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; 7th ed. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. Approved standard M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Daly, J. S., R. A. Dodge, R. H. Glew, D. T. Soja, B. A. DeLuca, and S. Hebert. 1997. Effect of zinc concentration in Mueller-Hinton agar on susceptibility of Pseudomonas aeruginosa to imipenem. J. Clin. Microbiol. 35:1027-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritsche, T. R., R. P. Rennie, B. P. Goldstein, and R. N. Jones. 2006. Comparison of dalbavancin MIC values determined by Etest (AB BIODISK) and reference dilution methods using gram-positive organisms. J. Clin. Microbiol. 44:2988-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein, B. P., R. N. Jones, T. R. Fritsche, and D. J. Biedenbach. 2006. Microbiologic characterization of isolates from a dalbavancin clinical trial for catheter-related bloodstream infections. Diagn. Microbiol. Infect. Dis. 54:83-87. [DOI] [PubMed] [Google Scholar]
- 8.Jones, R. N., T. R. Fritsche, H. S. Sader, and B. P. Goldstein. 2005. Antimicrobial spectrum and potency of dalbavancin tested against clinical isolates from Europe and North America (2003): initial results from an international surveillance protocol. J. Chemother. 17:593-600. [DOI] [PubMed] [Google Scholar]
- 9.Jones, R. N., M. G. Stilwell, H. S. Sader, T. R. Fritsche, and B. P. Goldstein. 2006. Spectrum and potency of dalbavancin tested against 3,322 gram-positive cocci isolated in the United States Surveillance Program (2004). Diagn. Microbiol. Infect. Dis. 54:149-153. [DOI] [PubMed] [Google Scholar]
- 10.Lin, G., K. Credito, L. M. Ednie, and P. C. Appelbaum. 2005. Antistaphylococcal activity of dalbavancin, an experimental glycopeptide. Antimicrob. Agents Chemother. 49:770-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Streit, J. M., T. R. Fritsche, H. S. Sader, and R. N. Jones. 2004. Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. Diagn. Microbiol. Infect. Dis. 48:137-143. [DOI] [PubMed] [Google Scholar]
- 12.Streit, J. M., H. S. Sader, T. R. Fritsche, and R. N. Jones. 2005. Dalbavancin activity against selected populations of antimicrobial-resistant gram-positive pathogens. Diagn. Microbiol. Infect. Dis. 53:307-310. [DOI] [PubMed] [Google Scholar]