Abstract
We report a case of Aspergillus tamarii keratitis. Ocular injury was known to be a predisposing factor. Topical natamycin and econazole treatment and subsequent systemic ketoconazole treatment proved effective. The isolate was identified by morphological characteristics and sequence analysis as A. tamarii, a member of Aspergillus section Flavi not hitherto reported from keratomycosis.
CASE REPORT
A 32-year-old female from Coimbatore was presented to the Aravind Eye Hospital, Coimbatore, South India, on December 27, 2005, with complaints of pain, redness, and defective vision of a 4-day duration in the left eye. She indicated that she had suffered an ocular injury caused by an iron piece while hammering 4 days earlier. On examination, her uncorrected visual acuities in the right and left eyes were 6/9 (partial) and 1/2/60, respectively. An anterior segment examination of the left eye showed lid edema and conjunctival congestion. The cornea showed a central 3-by-3-mm ulcer with an anterior midstromal infiltrate with feathery edges and surrounding edema. The anterior chamber showed a moderate number of cells (2+ grade). The lens was clear. The anterior segment of the right eye and the posterior segments of both eyes were within normal limits.
With due aseptic precautions, the ulcer was scraped and two smears were made on glass slides for a 10% KOH wet mount and Gram staining. The microscopic examination of the KOH wet mount and Gram staining showed fungal filaments. Material from scraping was also directly inoculated onto potato dextrose agar and incubated at 25°C. Based on the colony appearance, the fungus was identified as an Aspergillus sp. Topical antifungal therapy was started with 5% natamycin suspension and 2% econazole drops every half hour, along with 1% homatropine three times a day.
When reviewed after 3 days, the patient's uncorrected visual acuity in the left eye had improved to 6/36, but the corneal midstromal infiltrate was still active. The anterior chamber showed a hypopyon of 0.5 mm. The patient was admitted as an inpatient and advised to continue the same medications along with 200 mg oral ketoconazole and 0.2% subconjunctival fluconazole based on the results of our previous study (6). The patient showed improvement during the next 3 weeks; the infiltrate reduced gradually, and the anterior segment inflammations subsided. On the last review, the anterior segment of the left eye showed a central nebular scar, with the best corrected visual acuity having improved to 6/12. The patient was advised to use glasses and to report for review after 6 months.
The clinical isolate was further examined at the CBS Fungal Biodiversity Centre and at the University of Szeged for species assignment and antifungal susceptibility tests.
Mycological study and diagnosis.
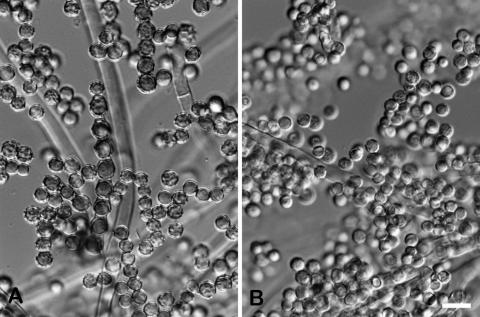
The fungus was subcultured on malt extract agar plates and identified as Aspergillus tamarii Kita based on the colony morphology and microscopic features of the isolate (Fig. 1 and 2). Colonies on malt extract agar at room temperature attained diameters of 6.0 to 7.0 cm in 10 days, producing abundant conidial heads in dull yellowish green shades becoming metallic bronze at maturity (24). The conidiophore stipe was hyaline and rough walled; the conidial heads were radiate; the vesicles were globose to subglobose, 25 to 50 μm in diameter. The phialides were borne directly on the vesicle or on metulae (mostly on large heads). The conidia were globose to subglobose, 5 to 6.5 μm in diameter, and brownish yellow. However, in contrast with those of typical wild A. tamarii isolates (Fig. 2A), some conidia of this isolate were not ornamented with tubercules and warts but were smooth walled and hyaline (Fig. 2B). The isolate grew well at 37°C but was unable to grow at 42°C on malt extract agar medium.
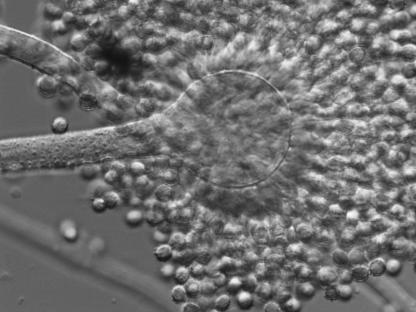
FIG. 1.
Conidial head of A. tamarii 2342/05.
FIG. 2.
Conidia of a typical A. tamarii strain (CBS 484.65) (A) and that of A. tamarii 2342/05 (B). The scale bar represents 10 μm.
For purposes of molecular identification, mycelia grown in liquid YPG medium (0.5% Bacto yeast extract, 0.5% Bacto peptone, 1% glucose) for 1 day were subjected to DNA isolation by a Masterpure yeast DNA purification kit (Epicenter Biotechnologies, Madison, WI) according to the manufacturer's instructions. The internal transcribed spacer (ITS) region of the rRNA gene complex, incorporating ITS 1, the 5.8S rRNA gene, and ITS 2, was amplified using primers ITS1 and ITS4 (26). A segment of the calmodulin gene was amplified using primers cmd5 and cmd6 as described by Hong et al. (12), while a segment of the β-tubulin gene was amplified using primers bT2a and bT2b (9). DNA sequences were determined using a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems Inc., Foster City, CA) and an ABI 3100 DNA sequencer. Both strands of each fragment were sequenced. The resulting sequences were deposited in the GenBank database. Sequence analysis was carried out by a BLASTN similarity search (2) at the website of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/blast/).
Table 1 lists A. tamarii sequences with complete homology to those of the case isolate. The ITS, tubulin, and calmodulin sequences of the case isolate proved to be completely identical to the corresponding sequences of A. tamarii strain NRRL 25565 (13).
TABLE 1.
GenBank sequences with 100% similarity to the ITS (EF525554), β-tubulin (EF525555), and calmodulin (EF525556) sequences of strain 2342/05
| Locus | GenBank accession no. | Species | Strain designation | Reference |
|---|---|---|---|---|
| ITS | AB008420 | A. tamarii | JCM2259 | 19 |
| ITS | AF004932 | A. tamarii | NRRL 26066 | 21 |
| ITS | AF272579 | A. tamarii | NRRL 26594 | 13 |
| ITS | AF272576 | A. tamarii | NRRL 25565 | 13 |
| ITS | AF004929 | A. tamarii | NRRL 20818 | 13 |
| ITS | D84358 | A. tamarii | A0754 | 16 |
| ITS | AY373870 | A. tamarii | SRRC 1088 | 11 |
| ITS | AY213635 | A. tamarii | UWFP534 | 22 |
| β-Tubulin | AF255074 | A. tamarii | NRRL 26594 | 13 |
| β-Tubulin | AF255071 | A. tamarii | NRRL 25565 | 13 |
| β-Tubulin | AF255069 | A. tamarii | NRRL 20818 | 13 |
| Calmodulin | AF255033 | A. tamarii | NRRL 25565 | 13 |
| Calmodulin | AF255032 | A. tamarii | NRRL 26594 | 13 |
Living cultures were deposited at the Department of Microbiology, Aravind Eye Hospital and Postgraduate Institute of Ophthalmology, Coimbatore, India (strain number 2342/05), and at the Centraalbureau voor Schimmelcultures (CBS 121598).
Antifungal susceptibility testing.
The Etest method (AB BIODISK, Solna, Sweden) for molds was applied for the determination of MICs of amphotericin B, fluconazole, itraconazole, ketoconazole, and voriconazole according to the manufacturer's instructions on RPMI 1640 agar (15 g in 1,000 ml) supplemented with 20 g glucose per 1,000 ml medium (1). The Etest drug concentrations ranged from 0.016 to 256 μg/ml for fluconazole and from 0.002 to 32 μg/ml for itraconazole, ketoconazole, voriconazole, and amphotericin B. The MIC was read as the drug concentration at which the elliptical inhibition zone intersected the scale of the Etest strip. The MICs of natamycin and econazole were determined by the broth microdilution method according to NCCLS M38-A in RPMI broth (18). The MICs for natamycin, amphotericin B, fluconazole, itraconazole, ketoconazole, voriconazole, and econazole proved to be >1,024, 0.125, >256, 0.064, 0.25, 0.125, and 0.064 μg/ml, respectively.
Discussion.
Corneal infections of fungal etiology are very common and represent 30% to 40% of all cases of culture-positive infectious keratitis. Combating fungal keratitis is of special importance in India, which harbors the largest agrarian population at risk for developing blindness due to the limited availability of antifungal drugs and the lack of response during therapy. Certain Aspergillus species, mainly Aspergillus flavus (23, 25), Aspergillus terreus (25), Aspergillus fumigatus (23, 25), and Aspergillus niger (4), have long been regarded as important pathogens in eye infections, especially keratitis. Other members of the genus less frequently occurring in keratitis include Aspergillus glaucus and Aspergillus ochraceus. Most of the Aspergillus strains isolated from keratomycosis patients are being identified and reported at the genus level only (10). Their molecular identification at the species level would be of great importance, as the pathogenic potential may vary between different species of the genus.
A. tamarii is a member of Aspergillus section Flavi (8). This species is widely used in the food industry for the production of soy sauce (known as red Awamori koji) (14) and in the fermentation industry for the production of various enzymes, including amylases, proteases, and xylanolytic enzymes (3, 7, 17). Although A. tamarii is able to produce several toxic secondary metabolites, including cyclopiazonic acid and fumigaclavines (24), it has rarely been encountered as a human pathogen. The only known cases are an eyelid infection (5), invasive nasosinusal aspergillosis in an immunocompetent patient (20), and onychomycosis in a 3-year-old boy (15). To our knowledge, the present case of fungal keratitis is the first report on an ocular infection caused by A. tamarii and the fourth known case worldwide involving this unusual opportunistic human pathogen.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences of the case isolate are EF525554 (ITS), EF525555 (β-tubulin), and EF525556 (calmodulin).
Acknowledgments
This work was supported by the Indian National Science Academy and the Hungarian Academy of Sciences within the frames of the Indo-Hungarian bilateral exchange program (no. IA/INSA-HAS Project/2007). L.K. is a grantee of the János Bolyai Research Scholarship.
Footnotes
Published ahead of print on 15 August 2007.
REFERENCES
- 1.AB BIODISK. 1999. Etest technical guide 10. Antifungal susceptibility testing of moulds. AB BIODISK, Solna, Sweden.
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Anandan, D., W. N. Marmer, and R. L. Dudley. Isolation, characterization and optimization of culture parameters for production of an alkaline protease isolated from Aspergillus tamarii. J. Ind. Microbiol. Biotechnol. 34:339-347. [DOI] [PubMed]
- 4.Chowdhary, A., and K. Singh. 2005. Spectrum of fungal keratitis in North India. Cornea 24:8-15. [DOI] [PubMed] [Google Scholar]
- 5.Degos, R., G. Segretain, G. Badillet, and A. Maisler. 1970. Aspergillose de la paupiére. Bull. Soc. Fr. Dermatol. Syphiligr. 77:732-734. [PubMed] [Google Scholar]
- 6.Dev, S., R. Rajaraman, and A. Raghavan. 2006. Severe fungal keratitis treated with subconjunctival fluconazole. Am. J. Ophthalmol. 141:783-784. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira, G., C. G. Boer, and R. M. Peralta. 1999. Production of xylanolytic enzymes by Aspergillus tamarii in solid state fermentation. FEMS Microbiol. Lett. 173:335-339. [Google Scholar]
- 8.Gams, W., M. Christensen, A. H. S. Onions, J. I. Pitt, and R. A. Samson. 1985. Infrageneric taxa of Aspergillus, p. 55-62. In R. A. Samson and J. I. Pitt (ed.), Advances in Penicillium and Aspergillus systematics. Plenum Press, New York, NY.
- 9.Glass, N. L., and G. C. Donaldson. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61:1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopinathan, U., P. Garg, M. Fernandes, S. Sharma, S. Athmanathan, and G. N. Rao. 2002. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea 21:555-559. [DOI] [PubMed] [Google Scholar]
- 11.Haugland, R. A., M. Varma, L. J. Wymer, and S. J. Vesper. 2004. Quantitative PCR analysis of selected Aspergillus, Penicillium and Paecilomyces species. Syst. Appl. Microbiol. 27:198-210. [DOI] [PubMed] [Google Scholar]
- 12.Hong, S. B., H. S. Cho, H. D. Shin, J. C. Frisvad, and R. A. Samson. 2006. Novel Neosartorya species isolated from soil in Korea. Int. J. Syst. Evol. Microbiol. 56:477-486. [DOI] [PubMed] [Google Scholar]
- 13.Ito, Y., S. W. Peterson, D. T. Wicklow, and T. Goto. 2001. Aspergillus pseudotamarii, a new aflatoxin producing species in Aspergillus section Flavi. Mycol. Res. 105:233-239. [Google Scholar]
- 14.Jong, S. C., and J. M. Birmingham. 1992. Current status of fungal culture collections and their role in biotechnology, p. 993-1024. In D. K. Arora, R. P. Elander, and K. G. Murekji (ed.), Handbook of applied mycology, vol. 4. Fungal biotechnology. Marcel Dekker Inc., New York, NY. [Google Scholar]
- 15.Kristensen, L., J. Stenderup, and A. Otkjaer. 2005. Onychomycosis due to Aspergillus tamarii in a 3-year-old boy. Acta Derm. Venereol. 85:261-262. [DOI] [PubMed] [Google Scholar]
- 16.Kumeda, Y., and T. Asao. 2001. Heteroduplex panel analysis, a novel method for genetic identification of Aspergillus Section Flavi strains. Appl. Environ. Microbiol. 67:4084-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreira, F. G., V. Lenartovicz, and R. M. Peralta. 2004. A thermostable maltose-tolerant alpha-amylase from Aspergillus tamarii. J. Basic Microbiol. 44:29-35. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. NCCLS document M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 19.Nikkuni, S., H. Nakajima, S. I. Hoshina, M. Ohno, C. Suzuki, Y. Kashiwagi, and K. Mori. 1998. Evolutionary relationships among Aspergillus oryzae and related species based on the sequences of 18S rRNA genes and internal transcribed spacers. J. Gen. Appl. Microbiol. 44:225-230. [DOI] [PubMed] [Google Scholar]
- 20.Paludetti, G., M. Rosignoli, E. Ferri, M. R. Cesari, G. Morace, M. Fantoni, and J. Galli. 1992. Invasive nasosinusal aspergillosis in an immunocompetent patient. Acta Otorhinolaryngol. Ital. 12:581-591. (In Italian.) [PubMed] [Google Scholar]
- 21.Peterson, S. W., B. W. Horn, Y. Ito, and T. Goto. 2000. Genetic variation and aflatoxin production in Aspergillus tamarii and A. caelatus, p. 447-458. In R. A. Samson and J. I. Pitt (ed.), Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood Academic Publishers. Amsterdam, The Netherlands.
- 22.Rakeman, J. L., U. Bui, K. Lafe, Y. C. Chen, R. J. Honeycutt, and B. T. Cookson. 2005. Multilocus DNA sequence comparisons rapidly identify pathogenic molds. J. Clin. Microbiol. 43:3324-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha, R., and S. Das. 2006. Mycological profile of infectious keratitis from Delhi. Indian J. Med. Res. 123:159-164. [PubMed] [Google Scholar]
- 24.Samson, R. A., E. S. Hoekstra, and J. C. Frisvad. 2004. Introduction to food- and airborne fungi, 7th edition. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 25.Thomas, P. A. 2003. Current perspectives on ophthalmic mycoses. Clin. Microbiol. Rev. 16:730-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. H. Innes, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols. Academic Press, San Diego, CA.