Abstract
Babesia bovis is a deadly disease of cattle resulting in severe economic losses in the vast regions of the world where it is endemic. If reintroduced into the United States, babesiosis would cause significant mortality in the naïve cattle population. In order to address the risk to U.S. cattle, it is essential to quantify the transovarial transmission efficiency in adult female Boophilus microplus ticks following acquisition feeding on persistently infected cattle. This study tested the hypothesis that infection rates are the same for larval progeny derived from females fed to repletion during persistent or acute infection. Increasing parasite levels during acute infection correlated with an increasing number of females harboring kinetes detectable in hemolymph (r = 0.9). The percent infected larvae ranged from 0 to 20% when derived from females fed to repletion on persistently infected calves and from 4 to 6% when derived from females fed to repletion during acute parasitemia. There was no significant difference in infection rates of larval progeny, implying that the risk associated with the introduction of either persistently infected or acutely infected cattle is equal. Parasite levels ranged from 2.4 × 102 to 1.9 × 105 in 3-day-fed larvae derived from females fed to repletion on persistently infected cattle. One group of larvae failed to transmit the parasite, suggesting that a threshold level of parasites must be obtained by larval progeny via transovarial transmission in order for larvae to deliver sufficient parasites to infect a naïve host.
Bovine babesiosis, also known as Texas cattle fever, is endemic to tropical and subtropical regions of the world including Central and South America, Asia, Australia, and Africa and is ranked as the most economically important arthropod-transmitted pathogen of cattle (1). The disease is caused by the tick-borne apicomplexan protozoon Babesia bovis and is characterized by anemia, fever, and, in severe cases, multiorgan failure resulting in death. Young calves are relatively resistant to severe disease and have the potential to recover but remain persistent carriers with no clinical signs of disease (8, 18). While parasite levels in the persistent state are often undetectable (4-6), ticks may still be capable of acquiring infection from these animals. Thus, persistently infected cattle are potentially an important facet in the maintenance of B. bovis in nature as well as in the introduction and spread of the parasite to nonendemic areas where competent vectors are present.
Following outbreaks that resulted in devastating economic losses to the U.S. cattle industry, the major tick vectors of B. bovis, Rhipicephalus (Boophilus) microplus and Rhipicephalus (Boophilus) annulatus, were eradicated from the continental United States by 1943 (2, 7, 10). Today, there remains a quarantine zone along the border between Texas and Mexico that extends from Brownsville to Del Rio. In Mexico, both the parasite and vector remain prevalent, and acaracide-resistant Boophilus ticks are increasingly common (15-17). There is no serological testing of cattle within the quarantine zone, and therefore, movement of cattle is not restricted based on B. bovis infection status. Due to the increase in acaracide-resistant ticks and the lack of clinical signs in persistent cattle, the introduction of infected ticks and/or cattle into the United States is likely. The introduction of babesiosis into the previously unexposed cattle population outside the limits of the quarantine zone would result in significant mortality.
When an adult female Boophilus tick feeds on an infected bovine host, the merozoite stage of B. bovis is acquired. Following gametogenesis and zygote formation within the lumen of the midgut, the kinete stage is released into the hemolymph of the female. The kinete stage can be detected in the hemolymph of the tick during migration from the midgut to ovaries. After invasion of the ovaries, kinetes are transovarially transmitted to developing larvae. Within developing larvae, B. bovis invades salivary glands and develops into infective sporozoites, which are subsequently transmitted when larvae commence feeding on a bovine host.
Determining the efficiency of transmission is crucial to developing strategies to prevent the reintroduction of B. bovis into the United States. If the efficiency of transovarial transmission is equivalent in females acquiring the parasite from either acutely or persistently infected cattle, and should emerging acaracide resistance lead to the reestablishment of B. microplus in the United States, then the absence of serological screening of cattle entering the United States is a definite oversight. In the current study, we began to address this issue by examining the transovarial transmission efficiency of female B. microplus ticks fed to repletion on persistently infected calves. We hypothesized that infection rates of larval progeny from these females would be the same as infection rates of larval progeny from females fed to repletion during acute parasitemia. We examined hemolymph kinete levels in females by light microscopy and nested PCR, determined infection rates of transmission-fed larval progeny by nested PCR, and quantified parasite levels in transmission-fed larvae using real-time PCR.
MATERIALS AND METHODS
Acquisition of B. bovis by B. microplus.
All calves were Holstein calves that were approximately 4 months of age. Calf 1167 was inoculated with 1.4 × 108 infected erythrocytes (strain T2Bo) 13 days following application within a cloth back patch of approximately 20,000 unexposed B. microplus (strain La Minita) larvae hatched from 1 g of B. microplus eggs (14). At the time of inoculation, approximately 1% of B. microplus larvae had molted to the unfed adult stage to ensure that the presence of adult females coincided with acute infection. For acquisition feeding of adult female Boophilus ticks on persistently infected spleen from intact calves, two Holstein calves (designated 1144 and 1158), approximately 4 months of age, were inoculated intravenously with 1.4 × 108 infected erythrocytes (strain T2Bo). At 123 days postinoculation for calf 1144 and 73 days postinoculation for calf 1158, approximately 20,000 larvae were applied to back patches. Both calves were determined to be persistently infected using a RAP-1 competitive enzyme-linked immunosorbent assay (cELISA) at the time of larval application (9). Peripheral blood samples from the jugular vein and capillary samples taken from the distal aspect of the tail were collected daily from each calf beginning at 1% larval molt. To verify B. bovis infection in persistently infected calves following acquisition feeding, nested PCR amplifying msa-1 was performed on jugular blood, brain, skin, and spleen samples following euthanasia. Controls included DNA isolated from preinoculation blood and normal tissues for negative controls and DNA isolated from known B. bovis-infected calves for positive controls. The sensitivity of the nested PCR was determined by making dilutions of a known number of infected erythrocytes and was found to be between 1 and 10 parasites (data not shown).
Transovarial transmission of B. bovis in B. microplus females.
Replete Rhipicephalus females were collected daily from calves 1144, 1158, and 1167 as previously described (11). Briefly, females were rinsed in water and placed into individual wells of tissue culture plates. Females were incubated at 26°C and 92.5% relative humidity during egg production. It was previously reported that a low proportion of eggs laid during the first 5 days of oviposition are infected, and therefore, these eggs were removed and discarded (3, 13). Hemolymphs from individual females were sampled on day 10 postrepletion as previously described (11). Briefly, a distal leg segment was removed, and a drop of exuding hemolymph was placed onto a glass slide and stained using Diff-Quik (Dade Behring, Deerfield, IL). A minimum of 50 high-power fields (hpf) per sample were observed by light microscopy. Samples were read in a blind fashion, and the average number of kinetes identified per field was recorded. Total hemolymph was collected from a subset of females with undetectable kinetes by light microscopy. Nested PCR was performed on these hemolymph samples using primers specific to msa-1 as previously described (11). Eggs produced by females that were hemolymph checked by light microscopy were pooled from each calf, resulting in two pools of eggs from the persistently infected calves and one pool of eggs from the acutely infected calf. Nine weeks after the eggs were pooled, larvae were applied to naïve hosts for transmission feeding.
Larval transmission of B. bovis by B. microplus.
For transmission feeding of larval progeny, three splenectomized Holstein calves approximately 4 months of age and designated calves 1170, 1174, and 5304 were used. Calves received larvae hatched from 1 g of eggs pooled from females that were hemolymph checked by light microscopy after feeding to repletion on a persistently infected or acutely infected calf. Fifty larvae were removed from each calf for DNA isolation at 1 and 3 days postapplication, and individual larvae were tested by nested PCR for the detection of msa-1 as previously described (11). Larval infection rates were calculated by dividing the number of positive larvae by 50. Nonparametric statistical analysis was performed using Kruskal-Wallis one-way analysis of variance followed by Kruskal-Wallis multiple-comparison Z-value test with Bonferroni correction.
Quantification of parasite levels in blood and individual larvae by real-time PCR.
Daily blood samples and individual larvae that were positive by nested PCR were subsequently quantified by real-time PCR. DNA was isolated using a blood DNA isolation kit (Gentra, Minneapolis, MN). A standard curve was developed using dilutions of known numbers of msa-1 plasmid. Amplification of a 150-bp fragment between bases 604 and 754 of msa-1 (GenBank accession number AF275911) was performed using msa-1-specific primers 5′-GATGCGTTTGCACATGCTAAG-3′ (forward) and 5′-TGAGAGCACCGAAGTACCCG-3′ (reverse). A TaqMan assay was performed utilizing a PE Applied Biosystems fluorogenic probe, 5′-CACGCTCAAGTAGGAAATTTTGTTAAACCTGGA-3′, annealing at bp 628 to 660 under the following conditions: 95.0°C for 10 min, 70 cycles of 95°C for 30 s and 55.8°C for 20 s, and a final extension step at 72.0°C for 1 min. Preinoculation B. bovis blood was used for a background control. Infected blood from a case of known parasitemia was used as a positive control.
RESULTS AND DISCUSSION
A detailed understanding of the efficiency of B. bovis acquisition and transmission by its Rhipicephalus tick vector is critical for evaluating the potential risk of reemergence in U.S cattle. In a recent study, it was determined that blood parasite levels in acutely infected splenectomized calves were directly related to kinete levels in replete females, and parasite levels in individual larvae were associated with kinete levels in hemolymphs of adult females (11). The current study was designed to further evaluate the dynamics of transmission by testing the hypothesis that females fed to repletion on persistently infected calves produce larval progeny with infection rates similar to those of larvae derived from females fed to repletion on acutely infected calves.
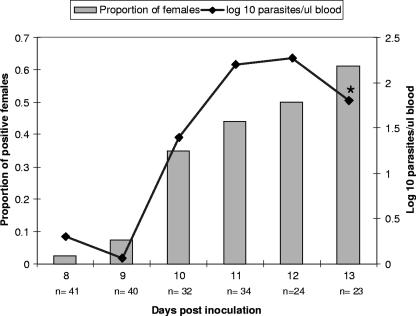
Jugular blood was positive by nested PCR on 6 of 6 days that replete females were collected during acute infection. Infection levels shown in Fig. 1 were lowest on the 9th day postinoculation (1.15 × 103 parasites/ml) and were highest on the 12th day postinoculation (1.9 × 105 parasites/ml). Parasites were not detectable by light microscopy in tail capillary smears until the final day of female collection (0.13% parasitized erythrocytes). Brain and skin samples obtained from acutely infected calf 1167 were positive for msa-1 by nested PCR.
FIG. 1.
B. bovis levels quantified by real-time PCR in daily jugular blood samples from acutely infected calf 1167 and proportion of B. microplus females with detectable kinetes in hemolymph by light microscopy. The * indicates the only day that merozoites were detectable by light microscopy in a capillary tail smear (103.7 parasites/μl blood).
B. bovis was detected in jugular blood by nested PCR on 2 of 5 days and 5 of 6 days that females were collected from persistently infected calves. This is consistent with observations that the detection of B. bovis using conventional PCR is sporadic (4-6), most likely due to sequestration of the parasite in capillaries. Nested PCR-positive jugular blood samples from persistently infected calves were tested by real-time PCR and were below the threshold of quantification using this method. There were no parasites detected by light microscopy in tail capillary smears from either of the persistently infected calves during female tick acquisition. Brain, skin, and spleen samples obtained from calf 1158 posteuthanasia were positive by nested PCR. However, three samples each of brain, skin, and spleen from calf 1144 were negative by nested PCR.
Seventy percent of the females (n = 195) that were replete during acute parasitemia had no detectable kinetes, and 27% had 0 to 1 kinetes/hpf in their hemolymph as determined by light microscopy (Table 1). These results are consistent with a study performed previously by Mahoney et al. in which kinetes were detectable by light microscopy in hemolymphs of 22 to 30% of engorged females fed on spleen-intact, acutely infected calves (12). Two females were observed to have 2 to 4 kinetes/hpf, and two females had 10 or more kinetes/hpf. Similar to the previous study, a positive relationship (r = 0.9) was found between increasing blood parasite levels during acquisition feeding and the number of Rhipicephalus females that had detectable kinetes in their hemolymph (Fig. 1). None of the female ticks that fed to repletion on persistently infected calves 1144 (n = 187) and 1158 (n = 154) had detectable kinetes by light microscopy.
TABLE 1.
Detection of B. bovis following acquisition and transovarial transmissiona
| Infection status and calf | Days postinoculation when replete females were obtained | No. of days B. bovis was detected in jugular bloodb/no. of days females were collected | No. of females positive by LM/no. of females checked | No. of positive femalesb/no. of LM-negative females tested | No. of infected larval progeny (%) on day:
|
Transmission of clinical disease by larval progeny | nPCR on transmission host tissues | |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | |||||||
| Persistent | ||||||||
| 1144 | 144-148 | 2/5 | 0/187 | 12/26 | 1/50 (2) | 3/50 (6) | Noc | Negativec |
| 1158 | 94-99 | 5/6 | 0/154 | 0/18 | 0/50 (0) | 10/50 (20) | Yes | Positive |
| Acute | ||||||||
| 1167 | 8-13 | 6/6 | 57/195 | 5/18 | 3/50 (6) | 2/50 (4) | Yes | Positive |
LM, light microscopy; nPCR, nested PCR.
By nested PCR amplifying msa-1.
No transmission with either 1 g or 10 g.
Infection rates of larvae derived from females that were replete during acute infection were not significantly different from those derived from either persistently infected calf (P = 0.368) (Table 1). There was also no significant difference between groups on days 1 and 3 of transmission. Infection rates were lowest (0.02 and 0) on day 1 postapplication for larval groups derived from persistently infected calves (Table 1). The highest infection rate, 0.2, was obtained from larvae derived from females fed to repletion during persistent infection on day 3 postapplication.
Larval infection rates obtained in this study were lower than those reported in the previous study (12 to 48%), which utilized splenectomized, acutely infected calves (11). We attribute these differences to lower parasite levels during acquisition feeding. The current study utilized spleen-intact, persistently infected calves, which harbored dramatically lower parasite levels in peripheral blood, resulting in lower kinete levels in replete females and subsequently lower larval infection rates. The percent infected larvae derived from females fed to repletion on acutely infected calves (4 to 6%) was within the range described previously by Mahoney et al. using light microscopy: up to 14.5%, with lower percentages mostly observed (12). Larvae obtained from field conditions reported previously by Mahoney et al. had much lower infection percentages of 0.04% (12). This level is lower than the average (7%) obtained using the persistently infected calves in this study. We attribute this difference to higher specificity and sensitivity in the method of detection used in this study and variations in experimental design.
Larvae obtained from females fed to repletion during acute infection as well as larvae derived from females fed to repletion on persistently infected calf 1158 were capable of transmitting infection, as indicated by the presence of fever and detection of merozoites by light microscopy in blood of transmission calves. Larval progeny derived from females fed to repletion on persistently infected calf 1144 did not transmit following application to calf 1170. B. bovis DNA was not detected in jugular blood samples or in brain, skin, hemal nodes, or kidney samples from calf 1170. Parasites were also not detected in daily tail capillary smears. A cELISA performed using serum obtained 29 days after larval application was negative. Ten grams of larvae derived from the same group of females but 6 weeks older than the previously applied batch was applied to another splenectomized calf. Again, no transmission occurred, and the calf was cELISA negative 21 days after larval application.
Differences between the persistently infected calves used for acquisition (i.e., a longer period of persistence and lower number of days of infection were detectable in peripheral blood during acquisition feeding) could have resulted in a situation where parasites were detected in larvae but where the number of parasites that were transovarially transmitted was below a threshold required for infection. Of note is a study performed previously by Mahoney et al. in which only three out of five calves infested with 100 larvae and four out of five calves infested with 200 larvae with an estimated 2% infection rate actually became clinically infected (12). Despite the discrepancy in the number of larvae applied by Mahoney et al. and those applied in this study, the results support the hypothesis of a threshold number of parasites required for the development of clinical infection.
The level of parasites detected within individual larvae supports this hypothesis, although low infection rates resulted in only 19 larvae for testing by real-time PCR, and 12 of those tested were below quantifiable levels (Table 2). Following 3 days of transmission feeding, parasite levels in larvae that did transmit the parasite ranged from 2.4 × 102 to 1.9 × 105, values comparable to those found in the previous study in groups of larvae derived from females harboring elevated levels of kinetes in their hemolymph (11). Only one of four larvae that did not transmit the parasite was quantifiable by real-time PCR, and its level (4.3 × 102) was comparable to those found in our previous study, which were derived from females with no detectable kinetes by light microscopy and with PCR-positive hemolymph. Overall, the lower number of parasites quantified by real-time PCR in transmission-fed larvae may be related to the lower parasitemia during acquisition feeding. The highest msa-1 copy numbers were detected in larval progeny from females fed to repletion on a persistently infected calf (1.9 × 105 parasites) as well as females fed to repletion an acutely infected calf (2.3 × 104 parasites).
TABLE 2.
Quantification of B. bovis in individual larvae by real-time PCR
| Transmission calf (larval progeny) | No. of quantifiable larvae/no. of larvae tested | No. of parasitesa | Day of transmission feed |
|---|---|---|---|
| 5304 (acute larvae) | 1/5 | 2.30E+04 | 1 |
| 1174 (persistent larvae) | 5/10 | 2.40E+02 | 3 |
| 6.40E+02 | 3 | ||
| 1.40E+03 | 3 | ||
| 1.50E+03 | 3 | ||
| 1.90E+05 | 3 | ||
| 1170 (persistent larvae) | 1/4 | 4.30E+02 | 3 |
Per individual larva tested.
This study tested the hypothesis that females fed to repletion on persistently infected spleen-intact calves produce larval progeny with infection rates similar to those of females fed to repletion on acutely infected spleen-intact calves. There was no significant difference between infection rates of these groups, and therefore, the hypothesis is accepted. These data suggest that females fed on persistent carriers, despite low blood parasite levels, are capable of acquiring the parasite and passing it transovarially to larval offspring. The fact that larval infection rates were not significantly different implies that the risk of transmission from the introduction of a persistently infected animal into the United States is comparable to the risk associated with the introduction of an acutely infected animal. We have shown that B. microplus is capable of transmitting B. bovis following acquisition on a clinically normal host, suggesting the need for more rigorous serological screening in the quarantine zone. Our data also suggest that there is a threshold limit below which larvae are not capable of transmission. Additional experiments are necessary to further characterize this threshold level.
Acknowledgments
We appreciate the expert technical assistance of Ralph Horn, Will Harwood, Carl Johnson, and Nancy Kumpula. We also thank James Allison and Melissa Flatt for the outstanding animal care they provided.
This work was supported by U.S. Department of Agriculture-ARS-ADRU project 5348-32000-028-00D and NIH training program T32-AI07025.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Bock, R., L. Jackson, A. de Vos, and W. Jorgensen. 2004. Babesiosis of cattle. Parasitology 129:S247-S269. [DOI] [PubMed] [Google Scholar]
- 2.Bram, R. A., J. E. George, R. E. Reichard, and W. J. Tabachnick. 2002. Threat of foreign arthropod-borne pathogens to livestock in the United States. J. Med. Entomol. 39:405-416. [DOI] [PubMed] [Google Scholar]
- 3.Cafrune, M. M., D. H. Aguirre, A. J. Mangold, and A. A. Guglielmone. 1995. Experimental studies of the rate of infection of Boophilus microplus eggs with Babesia bovis. Res. Vet. Sci. 58:284-285. [DOI] [PubMed] [Google Scholar]
- 4.Calder, J. A., G. R. Reddy, L. Chieves, C. H. Courtney, R. Littell, J. R. Livengood, R. A. Norval, C. Smith, and J. B. Dame. 1996. Monitoring Babesia bovis infections in cattle by using PCR-based tests. J. Clin. Microbiol. 34:2748-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahrimal, Y., W. L. Goff, and D. P. Jasmer. 1992. Detection of Babesia bovis carrier cattle by using polymerase chain reaction amplification of parasite DNA. J. Clin. Microbiol. 30:1374-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueroa, J. V., L. P. Chieves, G. S. Johnson, W. L. Goff, and G. M. Buening. 1994. Polymerase chain reaction-based diagnostic assay to detect cattle chronically infected with Babesia bovis. Rev. Latinoam. Microbiol. 36:47-55. [PubMed] [Google Scholar]
- 7.George, J. E., R. B. Davey, and J. M. Pound. 2002. Introduced ticks and tick-borne diseases: the threat and approaches to eradication. Vet. Clin. N. Am. Food Anim. Pract. 18:401-416. [DOI] [PubMed] [Google Scholar]
- 8.Goff, W. L., W. C. Johnson, S. M. Parish, G. M. Barrington, W. Tuo, and R. A. Valdez. 2001. The age-related immunity in cattle to Babesia bovis infection involves the rapid induction of interleukin-12, interferon-gamma and inducible nitric oxide synthase mRNA expression in the spleen. Parasite Immunol. 23:463-471. [DOI] [PubMed] [Google Scholar]
- 9.Goff, W. L., J. B. Molloy, W. C. Johnson, C. E. Suarez, I. Pino, A. Rhalem, H. Sahibi, L. Ceci, G. Carelli, D. S. Adams, T. C. McGuire, D. P. Knowles, and T. F. McElwain. 2006. Validation of a competitive enzyme-linked immunosorbent assay for detection of antibodies against Babesia bovis. Clin. Vaccine Immunol. 13:1212-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham, O. H., and J. L. Hourrigan. 1977. Eradication programs for the arthropod parasites of livestock. J. Med. Entomol. 13:629-658. [DOI] [PubMed] [Google Scholar]
- 11.Howell, J. M., M. W. Ueti, G. H. Palmer, G. A. Scoles, and D. P. Knowles. 2007. Transovarial transmission efficiency of Babesia bovis tick stages acquired by Rhipicephalus (Boophilus) microplus during acute infection. J. Clin. Microbiol. 45:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahoney, D. F., and G. B. Mirre. 1971. Bovine babesiosis: estimation of infection rates in the tick vector Boophilus microplus (Canestrini). Ann. Trop. Med. Parasitol. 65:309-317. [DOI] [PubMed] [Google Scholar]
- 13.Mahoney, D. F., and G. B. Mirre. 1977. The selection of larvae of Boophilus microplus infected with Babesia bovis (syn B argentina). Res. Vet. Sci. 23:126-127. [PubMed] [Google Scholar]
- 14.Pereira, M. C. 1998. Daily mean number of eggs laid by the southern cattle tick (Acari: Ixodidae) compared with mean egg mass weight. J. Econ. Entomol. 91:153-158. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Vivas, R. I., M. A. Alonso-Diaz, F. Rodriguez-Arevalo, H. Fragoso-Sanchez, V. M. Santamaria, and R. Rosario-Cruz. 2006. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet. Parasitol. 136:335-342. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Vivas, R. I., A. L. Rivas, G. Chowell, S. H. Fragoso, C. R. Rosario, Z. Garcia, S. D. Smith, J. J. Williams, and S. J. Schwager. 2007. Spatial distribution of acaricide profiles (Boophilus microplus strains susceptible or resistant to acaricides) in southeastern Mexico. Vet. Parasitol. 146:158-169. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Vivas, R. I., F. Rodriguez-Arevalo, M. A. Alonso-Diaz, H. Fragoso-Sanchez, V. M. Santamaria, and R. Rosario-Cruz. 2006. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev. Vet. Med. 75:280-286. [DOI] [PubMed] [Google Scholar]
- 18.Trueman, K. F., and G. W. Blight. 1978. The effect of age on resistance of cattle to Babesia bovis. Aust. Vet. J. 54:301-305. [DOI] [PubMed] [Google Scholar]