Abstract
We developed a multiplex PCR-based reverse line blot (mPCR/RLB) assay to identify 50 uncommon pneumococcal serotypes. In combination with a previously described mPCR/RLB assay (3), all 90 pneumococcal serotypes can be identified individually (32 serotypes) or, because of predictable cross-reactions, to within small groups of two to five related serotypes (58 serotypes), which can be distinguished using serotype-specific antisera.
Recently, we described a multiplex PCR-based reverse line blot (mPCR/RLB) hybridization assay to identify 40 Streptococcus pneumoniae serotypes, including the 23 represented in the polysaccharide vaccine and 17 others that show reproducible cross-reactions (3). In this study, we designed one pair of specific primers and one probe for each of the remaining 50 serotypes to allow provisional identification of all 90 known serotypes by mPCR/RLB (5). The primer and probe sequences were based on recently published full cps gene cluster sequences of all 90 pneumococcal serotypes (1) and on other sequences available in GenBank (4, 7).
Twenty sets of serotype-specific primer pairs and probes were designed; the remaining 30 serotypes shared identical or very similar wzy sequences with one or two others in the same or closely related serogroups, as follows: 7B/7C/40, 10F/10C, 11B/11C, 15F/15A, 19B/19C, 24F/24A/24B, 25F/25A/38, 28F/28A, 32F/32A, 33B/33D, 35F/47F, 35A/35C/42, and 41F/41A. The primers and probes used in this assay are shown in Table 1.
TABLE 1.
Oligonucleotide primers and probes for mPCR/RLB assay used in this study
| Primera | Specificityb | Tm (°C)c | GenBank accession no. | Sequence (5′-3′)d |
|---|---|---|---|---|
| lytASbe | S. pneumoniae | 66.46 | M13812 | 681CAA CCG TAC AGA ATG AAG CGG701 |
| lytAAp | S. pneumoniae | 60.50 | M13812 | 721GTC TTT CCG CCA GTG ATA AT702 |
| lytAAne | S. pneumoniae | 66.68 | M13812 | 999TTA TTC GTG CAA TAC TCG TGC G978 |
| 7B7C40Sb | Serotypes 7B, 7C, and 40 | 60.84 | CR931641 | 11915AAA ACT CAA GTA TCT GTG C(T)CA CCT T11939 |
| 7B7C40Ap | Serotypes 7B, 7C, and 40 | 60.47 | CR931641 | 11967TCC AAA TTT SAG TAA ACC AAC CTA A11943 |
| 7B7C40An | Serotypes 7B, 7C, and 40 | 63.99 | CR931641 | 11990CAT CTC TAT TCG ACC TTG CGT TA11968 |
| 10F10CSb | Serotypes 10F and 10C | 60.73 | CR931652 | 6883TAG TTT TGG TTA CGT AGT TGT TGA CT6908 |
| 10F10CAp | Serotypes 10F and 10C | 61.73 | CR931652 | 6933GAA AAC TTG CCC AAA TCC TT6914 |
| 10F10CAn | Serotypes 10F and 10C | 60.53 | CR931652 | 6963GCA ATA(/G) AAT ACT GTA GCA TAC GAT AGT T6936 |
| 11F11A11DSb | Serotypes 11F, 11A, and 11D | 59.48 | CR931657 | 10665GAA ATA TCG CCA TTC ATC AG10684 |
| 11FAp | Serotype 11F | 60.70 | CR931657 | 10752ATT GAC CCA CTT AAC ATA AAA GTT AAA10726 |
| 11FAn | Serotype 11F | 61.24 | CR931657 | 10770GAT TGT ACC CCA TCA CCG10753 |
| 11B11CSb | Serotypes 11B and 11C | 64.44 | CR931654 | 11092TCT GGT GCT AAG GGG ATC AA11111 |
| 11B11CAp | Serotypes 11B and 11C | 61.93 | CR931654 | 11135TGC ATA AGC TGA TTA TGA GCA TAG11112 |
| 11B11CAn | Serotypes 11B and 11C | 60.34 | CR931654 | 11162CCA ATT ACT CCA TTA TCT ATT GCT AAT11136 |
| 13Sb | Serotype 13 | 61.04 | CR931661 | 12845GAT GGG AAA ATA CGA TAT GCT C12866 |
| 13Ap | Serotype 13 | 61.06 | CR931661 | 12895TGA GCT AAA TGT TGA ATA TTT ATA CCC12869 |
| 13An | Serotype 13 | 60.91 | CR931661 | 12922GAA AAT CGT AAC ATG GAA AAA GTA A12898 |
| 15F15ASb | Serotypes 15F and 15A | 60.57 | CR931666 | 8304TAT TTC CTT CCT ATG GGA CAA C8325 |
| 15F15AAp | Serotypes 15F and 15A | 60.73 | CR931666 | 8369AGT CCT TTC CCA AAT ATA GCA CT8347 |
| 15F15AAn | Serotypes 15F and 15A | 63.72 | CR931666 | 8417GCA AGC ATT TTA CCA AGT TCA TAA A8393 |
| 16FSb | Serotype 16F | 60.91 | CR931668 | 11925TTG TTC TTA CAT TTA GCC GTA GTG11948 |
| 16FAp | Serotype 16F | 60.73 | CR931668 | 11987GTT GAA AGA ATA CGA TTC CTA CAA G11963 |
| 16FAn | Serotype 16F | 62.84 | CR931668 | 20011TCG TCG TTG AAA ACA ATT TCT TAC11988 |
| 16ASb | Serotype 16A | 63.65 | CR931667 | 10908CCG CTC ACG GTA TGG ACT A10926 |
| 16AAp | Serotype 16A | 62.44 | CR931667 | 10952GGA GTA AAT GAT GTG TAG TGA AAA CC10927 |
| 16AAn | Serotype 16A | 61.61 | CR931667 | 10978CCA GCA ATA TAC TCA GGA AAT AAT TC10953 |
| 17ASb | Serotype 17A | 60.22 | CR931669 | 13764GTA GAC TTC TTA GAG CCT ATT GTG G13788 |
| 17AAp | Serotype 17A | 61.79 | CR931669 | 13821TGC TAA ATG TCA TTT TTT TAC CAA G13797 |
| 17AAn | Serotype 17A | 61.77 | CR931669 | 13845 CAT TCG ACC AGA TAT AGG TAC GAT13822 |
| 19B19CSb | Serotypes 19B and 19C | 60.22 | CR931676 | 10179AGA ATT CGG AGA TTT GTG GTA T10200 |
| 19B19CAp | Serotypes 19B and 19C | 60.49 | CR931676 | 10222TTC GTA CTG AAA ATT CAT TTC G10201 |
| 19B19CAn | Serotypes 19B and 19C | 62.26 | CR931676 | 10479CAA TCC ACC TCC ATA AAC GA10460 |
| 21Sn | Serotype 21 | 60.16 | CR931680 | 12674CAA TTC TAC TGA GTC CAT ATT ATG AAA12700 |
| 21Sp | Serotype 21 | 60.51 | CR931680 | 12709GAT AGT TTC TCT GTA TCA AAT AGC GA12734 |
| 21Ab | Serotype 21 | 63.98 | CR931680 | 12755ACC ATC GTA CCT GCA CCA TAA12735 |
| 23ASn | Serotype 23A | 60.54 | CR931683 | 8847TTT ACT TTA ATT TAT AGC TTT TTG GCT AA8875 |
| 23ASp | Serotype 23A | 61.54 | CR931683 | 8876TGC CTT TTT TAA CGA GGT TG8895 |
| 23AAb | Serotype 23A | 63.69 | CR931683 | 8918GGT GCA TGA GTT AGG AGA AAG TG8896 |
| 23BSb | Serotype 23B | 63.02 | CR931684 | 9692GGA TCG TTG TTC ATA GCG G9710 |
| 23BAp | Serotype 23B | 60.09 | CR931684 | 9752ATA ATT ACT GGT CTG TGA TTT TTC TTT9726 |
| 23BAn | Serotype 23B | 60.58 | CR931684 | 9782GAT AAT AAA GAA ATT ACT AAC CAT GTC GT9754 |
| 24F24A24BSb | Serotypes 24F, 24A, and 24B | 62.46 | CR931688 | 12110TCA ACA CTT ATG ATG G(A)TG CCT G12131 |
| 24F24A24BAp | Serotypes 24F, 24A, and 24B | 61.44 | CR931688 | 12175CAC AAT CCA AAA CTT AAG TTG TTT C12151 |
| 24F24A24BAn | Serotypes 24F, 24A, and 24B | 60.37 | CR931688 | 12210GCA GAA ACA AAA(/G) GTA AGA ATT ATA GAT ATC12181 |
| 25F25A38Sn | Serotypes 25F, 25A, and 38 | 62.77 | CR931690 | 12994GAC TAC AAA CTG CGG TAG TAG AAA TG13019 |
| 25F25A38Sp | Serotypes 25F, 25A, and 38 | 60.18 | CR931690 | 13020ATA GGA ACT CTA GGG TTT AGT TTT TTC13046 |
| 25F25A38Ab | Serotypes 25F, 25A, and 38 | 61.68 | CR931690 | 13076TGG AAC AAT TCT AAT CGT TAA TAC G13052 |
| 27Sb | Serotype 27 | 63.88 | CR931691 | 8672GCA GCC ACC TCT TCT CAT TC8691 |
| 27Ap | Serotype 27 | 60.18 | CR931691 | 8733CGC CAA ATT CTA TAC CAA CTA GTA T8709 |
| 27An | Serotype 27 | 62.49 | CR931691 | 9047GGA AGG AAC AAC CCA ACA AT9028 |
| 28F28ASb | Serotypes 28F and 28A | 64.23 | CR931693 | 10688CAG AGT TTG GTC GAG GTT CCT A10709 |
| 28F28AAp | Serotypes 28F and 28A | 60.20 | CR931693 | 10739AGA ACT AAA TAC AGT GCA ATA ATT GG10714 |
| 28F28AAn | Serotypes 28F and 28A | 60.11 | CR931693 | 10767GCT CAA CTT TAT TTC TCT AGA ATA AAC A10740 |
| 29Sb | Serotype 29 | 62.01 | CR931694 | 6342CCG AAA ATT GTT CAC AGG ATA C6363 |
| 29Ap | Serotype 29 | 61.37 | CR931694 | 6393TAA CAA GCA GAA TAA GCA AAA TAG C6369 |
| 29An | Serotype 29 | 60.34 | CR931694 | 6418AGC TTT CTT TTG TAC GAC TCT TTT A6394 |
| 31Sb | Serotype 31 | 61.77 | CR931695 | 9419TGA AAA TCC CTT AGT GAC ATC TG9441 |
| 31Ap | Serotype 31 | 60.48 | CR931695 | 9489GAG CCT TCT CAA TAG TCA TAA AAA A9465 |
| 31An | Serotype 31 | 60.36 | CR931695 | 9535GCC ATA ATC AAA AAT AAG TTA GAC ATA A9508 |
| 32F32ASb | Serotypes 32F and 32A | 61.08 | CR931697 | 12671GGT ATG CTT ACA ATG AGA CGC12691 |
| 32F32AAp | Serotypes 32F and 32A | 60.46 | CR931697 | 12711CCA CTT CCC AGA GGA AAA TA12692 |
| 32F32AAn | Serotypes 32F and 32A | 63.63 | CR931697 | 13019AAT TCG TTC CCG GAT AAG ATG12999 |
| 33B33D33CSbf | Serotypes 33B, 33D, and 33C | 61.80 | CR931699 | 13115TCG TTG GAT GAC AAA ACT CTT AC13137 |
| 33B33D33CApf | Serotypes 33B, 33D, and 33C | 62.88 | CR931699 | 13157GCT CAA TGT GAC AGG GAG AA13138 |
| 33B33D33CAnf | Serotypes 33B, 33D, and 33C | 62.28 | CR931699 | 13378CCT CCC TGA GCC AAA ATA AC13359 |
| 33CSb | Serotype 33C | 62.44 | CR931700 | 11186CGA ATC GTC ATA AGG CAA AA11205 |
| 33CAp | Serotype 33C | 60.68 | CR931700 | 11232ACC TAC TGT GAC AGG GAA TAG TAA A11208 |
| 33CAn | Serotype 33C | 60.51 | CR931700 | 11258AAT AGG AGT AAC AAA GAG AAG CCT AA11233 |
| 34Sn | Serotype 34 | 60.67 | CR931703 | 8296TTA AAA GTA TTA TTG GTA GTG ATT CTT TTG8325 |
| 34Sp | Serotype 34 | 61.14 | CR931703 | 8322TTT GTG AAG AGT ACC AAT GGA TT 8344 |
| 34Ab | Serotype 34 | 61.79 | CR931703 | 8366TGT AAA GAC ATT CCC TGT AGG C8345 |
| 35F47FSb | Serotypes 35F and 47F | 60.62 | CR931707 | 6904ATA AAA AGA AAG TCT TTG CCA GAG6927 |
| 35F47FAp | Serotypes 35F and 47F | 60.89 | CR931707 | 6986AAA GTC ACA TC(T)T AAA ATT GAC ACA AC6961 |
| 35F47FAn | Serotypes 35F and 47F | 61.07 | CR931707 | 7012CAA CTT TTG GAA GAT ACT GAA CAT AA6987 |
| 35A35C42Sn | Serotypes 35A, 35C, and 42 | 62.11 | CR931704 | 7662GGA GAC TA(/G)T TAA AAC TTT TTT CGT TC7687 |
| 35A35C42Sp | Serotypes 35A, 35C, and 42 | 60.72 | CR931704 | 7689CCT ACT TTA TTA ATG CCT GTT TGA G7713 |
| 35A35C42Ab | Serotypes 35A, 35C, and 42 | 61.13 | CR931704 | 7739TTA AGT AAG TCT TCG CAA TCC AG7717 |
| 35BSb | Serotype 35B | 63.57 | CR931705 | 7623CTA ATT TGG CTA TGA AGC TAA TCC C7647 |
| 35BAp | Serotype 35B | 60.57 | CR931705 | 7693AAG CGT GAA AAA TTT TAA TAA AAG AC7668 |
| 35BAn | Serotype 35B | 60.29 | CR931705 | 7722TAA CTT AAA TAG GCA TTA ACA AAA TAG GT7694 |
| 36Sb | Serotype 36 | 60.58 | CR931708 | 13687CAA TTT CCC CTT ATT CTG TAG TTC13710 |
| 36Ap | Serotype 36 | 60.38 | CR931708 | 13739AGA TAA ATA CAT CAT TAT TGA CGA ACA13713 |
| 36An | Serotype 36 | 63.39 | CR931708 | 13763TGG AGA TCC CCA AGA GAA AAT A13742 |
| 39Sb | Serotype 39 | 63.67 | CR931711 | 11410ATT GGT TTG GGA ACT TGA TGT C11431 |
| 39Ap | Serotype 39 | 62.70 | CR931711 | 11464TAA TAA CCA TAC TCT TCC GTC GG11442 |
| 39An | Serotype 39 | 62.76 | CR931711 | 11490GCA ATA AGG CAA ATA AGG GAT AAT TA11465 |
| 41F41ASn | Serotypes 41F and 41A | 60.08 | CR931714 | 11239TGA CAC TAT TTA TAA TTG CTT TAT CCT T11266 |
| 41F41ASp | Serotypes 41F and 41A | 60.11 | CR931714 | 11272GGG TGC AAG GTG ATT ATG TAT11292 |
| 41F41AAb | Serotypes 41F and 41A | 63.37 | CR931714 | 11348TAG CGA GAA ACT ATC TGC ATC TTG11325 |
| 43Sb | Serotype 43 | 60.10 | CR931716 | 10493AGA TCA AAT GGT GGT ATT AGG AA10515 |
| 43Ap | Serotype 43 | 61.11 | CR931716 | 10538TCG GGT GTA CAA ATC CTA AAC TA10516 |
| 43An | Serotype 43 | 60.19 | CR931716 | 10564GGA ATA GAT CAT TAA CCC TAA TGA AT10539 |
| 45Sb | Serotype 45 | 64.85 | CR931718 | 13236TAT GCA GGA AAT ATC CGA GAA GG13258 |
| 45Ap | Serotype 45 | 64.64 | CR931718 | 13296CAG CAT ATC TTG CAC GAT AAT GAA13273 |
| 45An | Serotype 45 | 64.31 | CR931718 | 13618CCG TGA AAC AGA AAC GCT ATG13598 |
| 47ASb | Serotype 47A | 63.03 | CR931720 | 10554TAT TTG CCA TAA CGG ACT CTA GAA C10578 |
| 47AAp | Serotype 47A | 59.71 | CR931720 | 10659TTT TTA ACA ACC TTG TAT AGA ATA CCT C10632 |
| 47AAn | Serotype 47A | 60.23 | CR931720 | 10710GCT AAA ATA ATA AAT AGC GAA CTT ACT ACA10681 |
| 48Sb | Serotype 48 | 63.24 | CR931722 | 12838GCA TTT GGA GTT ATT GCC CTA C12859 |
| 48Ap | Serotype 48 | 60.13 | CR931722 | 12884CCT ATA AAC ACA CTC AAA ACT AGC A12860 |
| 48An | Serotype 48 | 61.76 | CR931722 | 12927CGA CGG AAT CAA TAT AAA TAA GTG ATA12901 |
S, sense; A, antisense; b, biotin-labeled primer (primers were biotin labeled at the 5′ end); p, probe (probes were 5′ end labeled with a C-6 amine); n, non-biotin-labeled primer.
Based on published sequence data for the whole cps gene cluster, it was not possible to design primers/probes that could distinguish some individual serotypes from one to three closely related ones, usually (but not always) belonging to the same serogroup.
Tm, melting temperature. Values were provided by the primer synthesizer (Sigma-Aldrich).
Numbers represent the base positions at which primer/probe sequences start and finish (starting at position 1 of the corresponding GenBank sequence).
One pair of previously published primers (11) were used as species-specific primers.
Some 33C strains may have positive results with the primers/probe (see the text for further explanation).
Probes and primers were designed with similar physical characteristics to allow simultaneous amplification and hybridization in a multiplex reaction (5) and were synthesized by Sigma-Aldrich (Sydney, Australia). Primers were biotinylated at the 5′ end, and probes had a 5′ amine group (5). DNA extraction (6) and RLB hybridization were performed as previously described (5).
mPCR was performed according to the QIAGEN Hotstar Taq polymerase kit instructions (5) under the following conditions: 95°C for 15 min; 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min; 72°C for 10 min; and a hold at 22°C. The 34-primer-pair mPCR mixture was prepared to contain the following: 2 μl template DNA, 0.075 μl of each forward (100 pmol μl−1) and reverse (100 pmol μl−1) primer, 2.4 μl deoxynucleoside triphosphates (2.5 mM of each deoxynucleoside triphosphate), 3 μl 10× PCR buffer, 4.2 μl 25 mM MgCl2 (final concentration, 5 mM), 0.2 μl QIAGEN Hotstar Taq polymerase (5 units μl−1), and water to 30 μl.
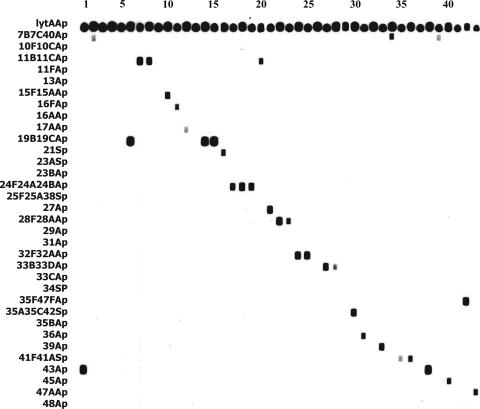
Preliminary testing of reference strains of all 90 serotypes (Statens Serum Institut, Copenhagen, Denmark) showed that all 50 target serotypes (and none of the other 40) were amplified and hybridized by the mPCR/RLB system; 20 serotypes reacted only with the corresponding primers and probe, and 30 exhibited the predicted cross-reactions (Table 1; Fig. 1).
FIG. 1.
mPCR/RLB results for a representative sample of 43 clinical isolates (see Table 1 for descriptions and specificities of the probes listed on the left). Conventional serotype-mPCR/RLB results for the isolates shown, from left to right, are 43, 7B, 9A,* 9L,* 9V,* 19B, 11B, 11C, 12A,* 15F, 16F, 17A, 18F,* 19B, 19C, 21, 24F, 24A, 24B, 11B, 27, 28F, 28A, 32F, 32A, 33A,* 33B, 33B, 33F,* 35C, 36, 37,* 39, 40, 41F, 41A, 44,* 43, 7B, 45, 46,* 47F, and 47A. Isolates marked with asterisks belong to one of 40 serotypes identified by our original mPCR/RLB assay (3) and were not amplified in this assay.
The method was further evaluated using 173 previously studied (4, 7) clinical isolates from China, Australia, and Canada, which included one to four isolates of all pneumococcal serotypes except for 10C, 11F, 23B, 25A, and 33D (for which clinical isolates were not available). They were tested by mPCR/RLB without knowledge of the serotype. Sixty-eight isolates belonging to 1 of 40 serotypes identified by our original mPCR/RLB assay were not amplified. One nonserotypable isolate was also not amplified by either the previous (3) or new mPCR, although it was confirmed to be S. pneumoniae by species-specific PCR and phenotypic characteristics. This result suggests that this isolate either contains a significant cps deletion or mutation (8) or belongs to a novel serotype (10).
Forty-four isolates were identified as being of individual serotypes corresponding with those identified by conventional serotyping, and 59 were identified as being one of a group of two or three related serotypes which, with one exception, included the serotype identified by conventional serotyping (Table 2).
TABLE 2.
mPCR/RLB and serotype identification results for 173 previously studied isolates from China, Australia, and Canada
| Serotype(s) | No. of isolates | mPCR/RLB result |
|---|---|---|
| Forty serotypes represented in previously described mPCR/RLB assay (3) | 68 | Negative |
| 7B | 2 | 7B/7C/40 |
| 7C | 2 | 7B/7C/40 |
| 40 | 1 | 7B/7C/40 |
| 10F | 2 | 10F/10C |
| 10C | 0 | |
| 11B | 3 | 11B/11C |
| 11C | 2 | 11B/11C |
| 11F | 0 | |
| 13 | 4 | 13 |
| 15F | 3 | 15F/15A |
| 15A | 3 | 15F/15A |
| 16F | 3 | 16F |
| 16A | 1 | 16A |
| 17A | 2 | 17A |
| 19B | 2 | 19B/19C |
| 19C | 3 | 19B/19C |
| 21 | 3 | 21 |
| 23A | 3 | 23A |
| 23B | 0 | |
| 24F | 3 | 24F/24A/24B |
| 24A | 1 | 24F/24A/24B |
| 24B | 1 | 24F/24A/24B |
| 25F | 2 | 25F/25A/38 |
| 25A | 0 | |
| 38 | 4 | 25F/25A/38 |
| 27 | 4 | 27 |
| 28F | 4 | 28F/28A |
| 28A | 3 | 28F/28A |
| 29 | 1 | 29 |
| 31 | 4 | 31 |
| 32F | 1 | 32F/32A |
| 32A | 2 | 32F/32A |
| 33B | 3 | 33B/33D |
| 33D | 0 | |
| 33Ca | 1 | 33B/33D |
| 34 | 4 | 34 |
| 35F | 3 | 35F/47F |
| 47F | 1 | 35F/47F |
| 35A | 1 | 35A/35C/42 |
| 35C | 3 | 35A/35C/42 |
| 42 | 1 | 35A/35C/42 |
| 35B | 3 | 35B |
| 36 | 3 | 36 |
| 39 | 2 | 39 |
| 41F | 2 | 41F/41A |
| 41A | 1 | 41F/41A |
| 43 | 2 | 43 |
| 45 | 1 | 45 |
| 47A | 1 | 47A |
| 48 | 3 | 48 |
| Nonserotypableb | 1 | Negative |
One isolate from China, which was identified as 33B/33D by single PCR and mPCR/RLB, was shown to be 33C by using serotype-specific antiserum.
This isolate was amplified and hybridized by S. pneumoniae-specific primers and probes (targeting lytA) and had optochin susceptibility and bile solubility.
The exception was an isolate from China, which was identified as serotype 33B/33D by mPCR/RLB and as serotype 33C by conventional serotyping. Both methods were repeated and the initial (discrepant) results confirmed. Single PCR was positive with 33B/33D-specific and negative with 33C-specific primers. Sequencing of a portion of wzy of this isolate showed that it was different from that of any known serotype (1, 7) but more closely related to that of 33C than to that of 33B/33D. Although the discrepancy between molecular and immunological serotype identifications remained unresolved, our results suggest that wzy is not the only determinant of antigenic specificity (3). Further investigation of this isolate is in progress.
Finally, the mPCR/RLB assay was used to test 152 clinical isolates collected during 2000-2007 at the Centre for Infectious Diseases and Microbiology and selected as belonging to one of the 50 uncommon serotypes identified by the new mPCR/RLB system.
Thirteen isolates were not initially amplified by mPCR. Of these, 11 were successfully identified after modification of the DNA extraction method (5) by adding 2 μl of proteinase K (20 mg/ml; Sigma, Australia) to tubes before heating them at 100°C for 10 min. Two isolates that still were not amplified had been identified, using antisera, as serotypes 11F and 16A. Conventional serotyping was repeated and showed that they belonged to serotypes 11A and 18C, respectively (not represented in the current mPCR/RLB system). Two isolates, previously identified as serotypes 35A and 29, were identified by mPCR/RLB as 35F and 35B, respectively. Retesting with antisera confirmed the mPCR/RLB results.
Seventy-three isolates were identified by mPCR/RLB as single serotypes corresponding with those of conventional serotyping; 75 isolates gave positive results with probes cross-reacting with two or three related serotypes, which included the serotypes identified by conventional serotyping. The mPCR/RLB-predicted serotypes for these 152 isolates are shown in Table 3.
TABLE 3.
mPCR/RLB and conventional serotyping results for 152 clinical isolates from Centre for Infectious Diseases and Microbiology
| Serotype | No. of isolates | mPCR/RLB result |
|---|---|---|
| 7C | 9 | 7B/7C/40 |
| 10F | 1 | 10F/10C |
| 11F | 1 | Negative (11A)a |
| 13 | 4 | 13 |
| 15A | 5 | 15F/15A |
| 16F | 41 | 16F |
| 16A | 1 | Negative (18C)a |
| 21 | 2 | 21 |
| 24F | 1 | 24F/24A/24B |
| 25A | 2 | 25F/25A/38 |
| 38 | 24 | 25F/25A/38 |
| 28F | 1 | 28F/28A |
| 28A | 1 | 28F/28A |
| 31 | 3 | 31 |
| 34 | 4 | 34 |
| 35Fb | 30 | 35F/47F |
| 35C | 1 | 35A/35C/42 |
| 35Bc | 20 | 35B |
| 36 | 1 | 36 |
Serotypes in parentheses are corrected serotypes after retesting by conventional serotyping; these serotypes are not included in the new mPCR/RLB assay.
One isolate initially identified as serotype 35A was found to be 35F upon being retested.
One isolate initially identified as serotype 29 was found to be 35B upon being retested.
Because serotype-specific targets within cps gene clusters are not always available, this mPCR/RLB assay is less discriminatory—although more objective and reproducible—than conventional serotyping (2, 4). In combination with our previous mPCR/RLB assay (3), it can identify 32 serotypes precisely and 58 as belonging to one of two to five related serotypes. This is the first report of a practical molecular assay that can predict all 90 serotypes without sequencing. Potentially, it can be used to identify multiple serotypes directly in clinical (including culture-negative) specimens. It could be adapted to other platforms, such as “liquid” molecular beacons (9) and solid microarrays (12).
For routine serotyping of pure pneumococcal cultures, our first mPCR/RLB assay (identification of 40 serotypes) would be used initially to identify the most prevalent serotypes (>95% of invasive isolates, based on unpublished data from the NSW Pneumococcal Reference Laboratory) (3). The current assay would then be needed, infrequently, to identify the small proportion of isolates belonging to less-common serotypes.
Acknowledgments
Fanrong Kong and Fei Zhou had similar contributions to the work and should be seen as co-first authors.
We thank Mitchell Brown and Shahin Oftadeh for conventional serotyping and for selecting appropriate clinical isolates.
This study was funded, in part, by National Health and Medical Research Council project grant 358351 (to G. L. Gilbert).
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Bentley, S. D., D. M. Aanensen, A. Mavroidi, D. Saunders, E. Rabbinowitsch, M. Collins, K. Donohoe, D. Harris, L. Murphy, M. A. Quail, G. Samuel, I. C. Skovsted, M. S. Kaltoft, B. Barrell, P. R. Reeves, J. Parkhill, and B. G. Spratt. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong, F., M. Brown, A. Sabananthan, X. Zeng, and G. L. Gilbert. 2006. Multiplex PCR-based reverse line blot hybridization assay to identify 23 Streptococcus pneumoniae polysaccharide vaccine serotypes. J. Clin. Microbiol. 44:1887-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong, F., and G. L. Gilbert. 2003. Using cpsA-cpsB sequence polymorphisms and serotype-/group-specific PCR to predict 51 Streptococcus pneumoniae capsular serotypes. J. Med. Microbiol. 52:1047-1058. [DOI] [PubMed] [Google Scholar]
- 5.Kong, F., and G. L. Gilbert. 2006. Multiplex PCR-based reverse line blot hybridization assay (mPCR/RLB)—a practical epidemiological and diagnostic tool. Nat. Protoc. 1:2668-2680. [DOI] [PubMed] [Google Scholar]
- 6.Kong, F., S. Gowan, D. Martin, G. James, and G. L. Gilbert. 2002. Serotype identification of group B streptococci by PCR and sequencing. J. Clin. Microbiol. 40:216-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong, F., W. Wang, J. Tao, L. Wang, Q. Wang, A. Sabananthan, and G. L. Gilbert. 2005. A molecular-capsular-type prediction system for 90 Streptococcus pneumoniae serotypes using partial cpsA-cpsB sequencing and wzy- or wzx-specific PCR. J. Med. Microbiol. 54:351-356. [DOI] [PubMed] [Google Scholar]
- 8.Llull, D., P. Veiga, J. Tremblay, and S. Kulakauskas. 2005. Immobilization-based isolation of capsule-negative mutants of Streptococcus pneumoniae. Microbiology 151:1911-1917. [DOI] [PubMed] [Google Scholar]
- 9.Marras, S. A., S. Tyagi, and F. R. Kramer. 2006. Real-time assays with molecular beacons and other fluorescent nucleic acid hybridization probes. Clin. Chim. Acta 363:48-60. [DOI] [PubMed] [Google Scholar]
- 10.Park, I., D. G. Pritchard, R. Cartee, A. Brandao, M. C. Brandileone, and M. H. Nahm. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki, N., M. Yuyama, S. Maeda, H. Ogawa, K. Mashiko, and Y. Kiyoura. 2006. Genotypic identification of presumptive Streptococcus pneumoniae by PCR using four genes highly specific for S. pneumoniae. J. Med. Microbiol. 55:709-714. [DOI] [PubMed] [Google Scholar]
- 12.Wen, L., Q. Wang, Y. Li, F. Kong, G. L. Gilbert, B. Cao, L. Wang, and L. Feng. 2006. Use of a serotype-specific DNA microarray for identification of group B streptococcus (Streptococcus agalactiae). J. Clin. Microbiol. 44:1447-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]