Abstract
Sporothrix schenckii is the species responsible for sporotrichosis, a fungal infection caused by the traumatic implantation of this dimorphic fungus. Recent molecular studies have demonstrated that this species constitutes a complex of numerous phylogenetic species. Since the delineation of such species could be of extreme importance from a clinical point of view, we have studied a total of 127 isolates, most of which were received as S. schenckii, including the available type strains of species currently considered synonyms, and also some close morphological species. We have phenotypically characterized all these isolates using different culture media, growth rates at different temperatures, and numerous nutritional tests and compared their calmodulin gene sequences. The molecular analysis revealed that Sporothrix albicans, S. inflata, and S. schenckii var. luriei are species that are clearly different from S. schenckii. The combination of these phenetic and genetic approaches allowed us to propose the new species Sporothrix brasiliensis, S. globosa, and S. mexicana. The key phenotypic features for recognizing these species are the morphology of the sessile pigmented conidia, growth at 30, 35, and 37°C, and the assimilation of sucrose, raffinose, and ribitol.
Sporothrix schenckii is a dimorphic fungus causing sporotrichosis, a severe infection usually acquired by the traumatic inoculation of colonized materials or by inhalation of spores through the respiratory tract (3, 6). Cutaneous lymphatic disease is the most common clinical manifestation, although other types of disease including disseminated infection are also produced. Sporotrichosis has a worldwide distribution, especially in tropical and subtropical areas. The natural habitat of S. schenckii is soil and plants. The teleomorph of this fungus has not yet been discovered, although a close genetic relationship between S. schenckii and the ascomycetous genus Ophiostoma has been demonstrated (2, 3). Contrary to previously reported suggestions, Ophiostoma stenoceras appears (5) not to be the teleomorph (4, 8, 27, 30). In recent years, numerous molecular studies involving S. schenckii have been carried out (13, 15, 22, 23, 25, 32, 34) and have clearly demonstrated the existence of several groups that are genetically different. In a recent multilocus study, we investigated the population structure of S. schenckii and showed the existence of at least six putative phylogenetic species prevalent in different geographical regions (20). In several in vitro antifungal susceptibility studies of clinical isolates of S. schenckii, a wide range of susceptibility to different drugs has been demonstrated (16, 21, 31). This suggests that these isolates could represent different species. If true, knowledge of their various responses to antifungal agents would be critical for appropriate patient management.
The aim of the present study was to phenotypically characterize the different phylogenetic species of the S. schenckii complex in order to find key morphological and/or physiological features that would allow their recognition in the clinical laboratory. Only their reliable identification will allow us to study their epidemiology and to determine if different clinical patterns are associated with each of these species. Numerous additional isolates were included in the study in order to increase the robustness of the isolate sets representing the different species detected within the complex. These isolates were assigned to different lineages on the basis of their calmodulin sequences, the most phylogenetically informative locus found in our previous study (20).
MATERIALS AND METHODS
Fungal isolates.
One hundred twenty-seven isolates were included in the study (Table 1). Isolates consisted of strains that were morphologically identified as being S. schenckii (mainly from clinical origin), the type strain of S. schenckii var. luriei, the available type strains of species currently considered synonyms of S. schenckii (Sporothrix albicans, Sporotrichum tropicale, and Dolichoascus schenckii), and the type strain of Sporothrix inflata, the morphologically closest species to S. schenckii. Isolates were stored on potato dextrose agar (PDA; Difco Laboratories, Detroit, MI) at 4 to 7°C and in slant cultures submerged in mineral oil at room temperature.
TABLE 1.
Fungal species, source code, geographical origin, and GenBank/EMBL/DDBJ accession numbers for the isolates included in the studyc
| Species | Received as species: | Isolate | Source | GenBank/EMBL/DDBJ accession no. (CAL) |
|---|---|---|---|---|
| S. albicans | S. schenckii | CBS 302.73T | Environmental, soil, United Kingdom | AM398396a |
| S. albicans | S. albicans | CBS 111110 | Zootermopsis nevadensis, Germany | AM398382a |
| S. brasiliensis | S. schenckii | CBS 120339T (IPEC 16390) | Clinical, Brazil | AM116899 |
| S. brasiliensis | S. schenckii | IPEC 15572 | Clinical, Brazil | AM116886 |
| S. brasiliensis | S. schenckii | IPEC 16042 | Clinical, Brazil | AM116885 |
| S. brasiliensis | S. schenckii | IPEC 16243 | Clinical, Brazil | AM116877 |
| S. brasiliensis | S. schenckii | IPEC 16456 | Clinical, Brazil | AM116897 |
| S. brasiliensis | S. schenckii | IPEC 16503 | Clinical, Brazil | AM116875 |
| S. brasiliensis | S. schenckii | IPEC 16550 | Clinical, Brazil | AM116892 |
| S. brasiliensis | S. schenckii | IPEC 16864 | Clinical, Brazil | AM116889 |
| S. brasiliensis | S. schenckii | IPEC 16919 | Clinical, Brazil | AM116898 |
| S. brasiliensis | S. schenckii | IPEC 17307 | Clinical, Brazil | AM116896 |
| S. brasiliensis | S. schenckii | IPEC 17331 | Clinical, Brazil | AM116880 |
| S. brasiliensis | S. schenckii | IPEC 17521 | Clinical, Brazil | AM116874 |
| S. brasiliensis | S. schenckii | IPEC 17585 | Clinical, Brazil | AM116887 |
| S. brasiliensis | S. schenckii | IPEC 17608 | Clinical, Brazil | AM116890 |
| S. brasiliensis | S. schenckii | IPEC 17692 | Clinical, Brazil | AM159127 |
| S. brasiliensis | S. schenckii | IPEC 17786 | Clinical, Brazil | AM116884 |
| S. brasiliensis | S. schenckii | IPEC 17920 | Clinical, Brazil | AM116888 |
| S. brasiliensis | S. schenckii | IPEC 17943 | Clinical, Brazil | AM116878 |
| S. brasiliensis | S. schenckii | IPEC 22468 | Clinical, Brazil | AM116882 |
| S. brasiliensis | S. schenckii | IPEC 22493.1 | Clinical, Brazil | AM116894 |
| S. brasiliensis | S. schenckii | IPEC 22493.2 | Clinical, Brazil | AM116883 |
| S. brasiliensis | S. schenckii | IPEC 22496.4 | Clinical, Brazil | AM116895 |
| S. brasiliensis | S. schenckii | IPEC 22496.5 | Clinical, Brazil | AM116879 |
| S. brasiliensis | S. schenckii | IPEC 22543.2 | Clinical, Brazil | AM116881 |
| S. brasiliensis | S. schenckii | IPEC 22582 | Clinical, Brazil | AM116891 |
| S. brasiliensis | S. schenckii | IPEC 22593 | Clinical, Brazil | AM116893 |
| S. brasiliensis | S. schenckii | FMR 8337 | Environmental, domiciliary dust, Brazil | AM116876 |
| S. brasiliensis | S. schenckii | FMR 9034 | Clinical, Brazil | AM261688 |
| S. brasiliensis | S. schenckii | FMR 9035 | Clinical, Brazil | AM261689 |
| S. globosa | S. schenckii | CBS 120340T (FMR 8600) | Clinical, Spain | AM116908 |
| S. globosa | Sporotrichum tropicaleb | CBS 292.55T | Clinical, United Kingdom | AM490354a |
| S. globosa | S. schenckii | FMR 8594 | Clinical, Spain | AM116906 |
| S. globosa | S. schenckii | FMR 8595 | Clinical, Spain | AM116905 |
| S. globosa | S. schenckii | FMR 8596 | Clinical, Spain | AM116902 |
| S. globosa | S. schenckii | FMR 8597 | Clinical, Spain | AM116907 |
| S. globosa | S. schenckii | FMR 8598 | Clinical, Spain | AM116903 |
| S. globosa | S. schenckii | FMR 8601 | Clinical, Spain | AM116901 |
| S. globosa | S. schenckii | FMR 8602 | Clinical, Spain | AM116909 |
| S. globosa | S. schenckii | FMR 9020 | Clinical, Japan | AM398994a |
| S. globosa | S. schenckii | FMR 9021 | Clinical, Japan | AM398993a |
| S. globosa | S. schenckii | FMR 9022 | Clinical, Japan | AM398995a |
| S. globosa | S. schenckii | FMR 9023 | Clinical, Japan | AM399016a |
| S. globosa | S. schenckii | KMU 4208 | Environmental, wheat, China | AM399002a |
| S. globosa | S. schenckii | KMU 4214 | Environmental, reed, China | AM399003a |
| S. globosa | S. schenckii | KMU 4200 | Environmental, reed, China | AM399004a |
| S. globosa | S. schenckii | KMU 4210 | Environmental, soil, China | AM399005a |
| S. globosa | S. schenckii | KMU 4116 | Environmental, China | AM399019a |
| S. globosa | S. schenckii | IHEM 4178 | Clinical, Italy | AM399018a |
| S. globosa | S. schenckii | UTHSC 04-1485 | Clinical, United States | AM399015a |
| S. globosa | S. schenckii | UTHSC 05-127 | Clinical, United States | AM398992a |
| S. globosa | S. schenckii | UTHSC 99-625 | Clinical, United States | AM398982a |
| S. globosa | S. schenckii | MCCL 220029 | Clinical, India | AM490358a |
| S. globosa | S. schenckii | MCCL 220087 | Clinical, India | AM490359a |
| S. globosa | S. schenckii | MCCL 220038 | Clinical, India | AM490360a |
| S. globosa | S. schenckii | MCCL 220045 | Clinical, India | AM490361a |
| S. globosa | S. schenckii | MCCL 220082 | Clinical, India | AM490362a |
| S. globosa | S. schenckii | MCCL 220084 | Clinical, India | AM490363a |
| S. globosa | S. schenckii | MCCL 220040 | Clinical, India | AM490348a |
| S. globosa | S. schenckii | MCCL 220011 | Clinical, India | AM490349a |
| S. globosa | S. schenckii | MCCL 220030 | Clinical, India | AM490350a |
| S. globosa | S. schenckii | MCCL 220049 | Clinical, India | AM490351a |
| S. globosa | S. schenckii | MCCL 220010 | Clinical, India | AM490352a |
| S. globosa | S. schenckii | MCCL 220085 | Clinical, India | AM490353a |
| S. globosa | S. schenckii | NBRC 5984 | NK | AM116900 |
| S. globosa | S. schenckii | NBRC 6072 | NK | AM116904 |
| S. inflata | S. inflata | CBS 239.68T | Environmental, wheat field, Germany | |
| S. schenckii | S. schenckii | CBS 359.36T | Clinical, United States | AM117437 |
| S. schenckii | Dolichoascus schenckiib | CBS 938.72T | Clinical, France | AM490340a |
| S. schenckii | S. schenckii | NBRC 8158 | NK | AM117438 |
| S. schenckii | S. schenckii | FMR 8604 | Clinical, Peru | AM117429 |
| S. schenckii | S. schenckii | FMR 8605 | Clinical, Peru | AM117442 |
| S. schenckii | S. schenckii | FMR 8606 | Clinical, Peru | AM117431 |
| S. schenckii | S. schenckii | FMR 8607 | Clinical, Peru | AM117428 |
| S. schenckii | S. schenckii | FMR 8608 | Clinical, Peru | AM117441 |
| S. schenckii | S. schenckii | FMR 8609 | Clinical, Peru | AM117439 |
| S. schenckii | S. schenckii | FMR 8677 | Clinical, Argentina | AM117436 |
| S. schenckii | S. schenckii | FMR 8678 | Clinical, Argentina | AM117446 |
| S. schenckii | S. schenckii | FMR 8679 | Clinical, Argentina | AM117445 |
| S. schenckii | S. schenckii | FMR 8716 | Clinical, Peru | AM399006a |
| S. schenckii | S. schenckii | FMR 8717 | Clinical, Peru | AM399017a |
| S. schenckii | S. schenckii | FMR 9051 | Clinical, Venezuela | AM490342a |
| S. schenckii | S. schenckii | FMR 9052 | Clinical, Venezuela | AM490341a |
| S. schenckii | S. schenckii | FMR 9054 | Clinical, Venezuela | AM490343a |
| S. schenckii | S. schenckii | FMR 9055 | Clinical, Venezuela | AM490344a |
| S. schenckii | S. schenckii | FMR 9113 | Clinical, Venezuela | AM490345a |
| S. schenckii | S. schenckii | FMR 9114 | Clinical, Venezuela | AM490346a |
| S. schenckii | S. schenckii | FMR 9115 | Clinical, Venezuela | AM490347a |
| S. schenckii | S. schenckii | FMR 9275 | Clinical, Venezuela | AM490355a |
| S. schenckii | S. schenckii | FMR 9276 | Clinical, Venezuela | AM490356a |
| S. schenckii | S. schenckii | FMR 9277 | Clinical, Venezuela | AM490357a |
| S. schenckii | S. schenckii | FMR 9278 | Clinical, Venezuela | AM490337a |
| S. schenckii | S. schenckii | FMR 9279 | Clinical, Venezuela | AM490338a |
| S. schenckii | S. schenckii | FMR 9280 | Clinical, Venezuela | AM490339a |
| S. schenckii | S. schenckii | IHEM 3774 | Clinical, Colombia | AM117447 |
| S. schenckii | S. schenckii | IHEM 3787 | NK, South Africa | AM117435 |
| S. schenckii | S. schenckii | IHEM 15477 | Clinical, Bolivia | AM117444 |
| S. schenckii | S. schenckii | IHEM 15486 | Clinical, Peru | AM117432 |
| S. schenckii | S. schenckii | IHEM 15489 | Clinical, Peru | AM117430 |
| S. schenckii | S. schenckii | IHEM 15499 | Clinical, Peru | AM117434 |
| S. schenckii | S. schenckii | IHEM 15502 | Clinical, Peru | AM117427 |
| S. schenckii | S. schenckii | IHEM 15503 | Clinical, Peru | AM117433 |
| S. schenckii | S. schenckii | IHEM 15508 | Clinical, Peru | AM117443 |
| S. schenckii | S. schenckii | IHEM 15511 | Clinical, Peru | AM117440 |
| S. schenckii | S. schenckii | UTHSC 05-2843 | Clinical, United States | AM399012a |
| S. schenckii | S. schenckii | UTHSC 05-802 | Clinical, United States | AM399008a |
| S. schenckii | S. schenckii | UTHSC 04-2235 | Clinical, United States | AM398984a |
| S. schenckii | S. schenckii | UTHSC 04-1718 | Clinical, United States | AM398990a |
| S. schenckii | S. schenckii | UTHSC 04-1064 | Clinical, United States | AM399014a |
| S. schenckii | S. schenckii | UTHSC 04-1012 | Clinical, United States | AM399020a |
| S. schenckii | S. schenckii | UTHSC 04-771 | Clinical, United States | AM398985a |
| S. schenckii | S. schenckii | UTHSC 04-797 | Clinical, United States | AM399013a |
| S. schenckii | S. schenckii | UTHSC 03-3124 | Clinical, United States | AM398996a |
| S. schenckii | S. schenckii | UTHSC 03-1684 | Clinical, United States | AM398989a |
| S. schenckii | S. schenckii | UTHSC 03-1627 | Clinical, United States | AM398987a |
| S. schenckii | S. schenckii | UTHSC 03-823 | Clinical, United States | AM398991a |
| S. schenckii | S. schenckii | UTHSC 02-2723 | Clinical, United States | AM399007a |
| S. schenckii | S. schenckii | UTHSC 02-510 | Clinical, United States | AM398986a |
| S. schenckii | S. schenckii | UTHSC 01-2137 | Clinical, United States | AM398983a |
| S. schenckii | S. schenckii | UTHSC 00-1734 | Clinical, United States | AM398988a |
| S. schenckii | S. schenckii | UTHSC 00-1488 | Clinical, United States | AM399009a |
| S. schenckii | S. schenckii | UTHSC 00-603 | Clinical, United States | AM399010a |
| S. schenckii | S. schenckii | UTHSC 99-173 | Clinical, United States | AM399011a |
| S. schenckii var. luriei | S. schenckii var. luriei | CBS 937.72T | Clinical, South Africa | |
| S. mexicana | S. schenckii | CBS 120341T | Environmental, soil rose tree, Mexico | AM398393a |
| S. mexicana | S. schenckii | CBS 120342 | Environmental, carnation leaves, Mexico | AM398392a |
Sequences newly generated in this study.
Invalidly published species.
Abbreviations: IPEC, Instituto de Pesquisa Clínica Evandro Chagas, Fiocruz, Brazil; FMR, Facultat de Medicina i Cieǹcies de la Salut, Reus, Spain; IHEM, BCCM/IHEM Biomedical Fungi and Yeasts Collection, Belgium; CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; NBRC, Biological Resource Center, Chiba, Japan; KMU, Kanazawa Medical University, Ishikawa, Japan; UTHSC, Fungus Testing Laboratory, University of Texas Health Science Center; MCCL, Mycology Culture Collection Laboratory, Postgraduate Institute of Medical Education and Research, Chandigarh, India; NK, not known; T, type strain.
DNA extraction, amplification, and sequencing.
The procedures for DNA extraction, amplification, sequencing, and phylogeny analysis of the nuclear calmodulin (CAL) gene were described previously by Marimon et al. (20). The primers used were CL1 and CL2A (24). The phylogenetic analysis was performed by using PAUP*, version 4.0b10 (28). Briefly, the most parsimonious trees were obtained after 100 heuristic searches with random sequence addition and tree bisection-reconnection branch-swapping algorithms, collapsing zero-length branches and saving all minimal-length trees (MulTrees).
Morphological studies.
In order to study macroscopic features and sporulation (5, 8, 22), all the isolates were subcultured on PDA, cornmeal agar (CMA) (30 g corn, 15 g agar, 1 liter tap water), and oatmeal agar (30 g oat flakes, 1 g MgSO4, 1.5 g KH2PO4, 15 g agar, 1 liter tap water) and incubated at 30°C in the dark. The microscopic features were determined primarily from slide cultures made on CMA after 10 to 12 days of incubation at 30°C. Coverslips were mounted in lactic acid and examined under a light microscope (Leitz Dialux 20). At least 25 measurements were recorded as the maximum and minimum values for each type of structure.
Physiologic studies.
The growth rate at various temperatures (20, 25, 30, 35, 37, and 40°C) of all the isolates included in the study was determined on PDA. The petri dishes were centrally inoculated with pieces of the fungus that were approximately 1 mm in diameter, placed upside down. The colony diameters (in millimeters) were measured after 14 and 21 days of incubation. The mean of the diameters was determined to detect differences among isolates.
Assimilation of 35 carbon and seven nitrogen sources was tested in liquid medium according to methods described previously by Yarrow (33). The tests were done in 96-well microplates, with each column containing a standard 150-μl amount of liquid nitrogen base medium (Becton Dickinson Co., Sparks, MD) or carbon base medium (Becton Dickinson Co.), with one test substrate, except those for the negative controls, which had only the base medium, and those for the positive controls, which contained glucose. An inoculum of 50 μl was added to each well of the microdilution trays. The inocula were adjusted to an optical density that ranged from 0.21 to 0.29, which corresponded to a final inoculum in the microplate of 2 × 105 to 2 × 106 CFU/ml. The viability of the conidia was verified by plating 100 μl of serial dilutions of each inoculum onto PDA. Microplates were read after 5 and 10 days of incubation at 25°C.
The presence of urease was determined after incubation on Christensen's urea agar slants at 25°C for 8 days. Urease production was noted by the development of a pink color. Acid production was tested on chalk agar (50 g glucose, 5 g CaCO3, 5 g yeast extract, 20 g agar, 1 liter demineralized water) at 25°C, with cultures examined regularly for up to 4 weeks for clearing of the medium around the streaks (33). Gelatin liquefaction was tested on a medium composed of 100 g gelatin, 5 g glucose, 6.7 g nitrogen base medium, and 1 liter of demineralized water and incubated at 25°C for 60 days (33). After incubation, tubes were then refrigerated at 4°C for 1 h to check gelatin hydrolysis. Tolerance to NaCl, MgCl2, and cycloheximide was tested as described above for the assimilation of carbon sources but in liquid nitrogen base medium with 5% glucose (7). Final concentrations were 0.1%, 0.25%, and 1% and 2%, 5%, and 10% for cycloheximide and NaCl and MgCl2, respectively. The results were both read after 7 and 14 days of incubation at 25°C. The formation of extracellular polysaccharide was performed on agar medium in petri dishes, incubated at 25°C for 2 to 3 weeks, and then flooded with diluted Lugol's iodine and examined for the formation of a blue-green color (33).
Testing of the ability of isolates to convert to the yeast phase was performed according to procedures described previously by Ghosh et al. (9). Briefly, mycelial cultures grown on PDA were subcultured on brain heart infusion agar with 5% defibrinated sheep blood at 37°C for 6 to 9 days. Several successive passages were done to achieve the yeast form. The morphology of the yeast cells was examined on wet mounts with 85% lactic acid.
Nucleotide sequence accession numbers.
The newly reported sequences generated in this study were deposited in the GenBank/EMBL/DDBJ database under the accession numbers listed in Table 1.
RESULTS
Phylogeny.
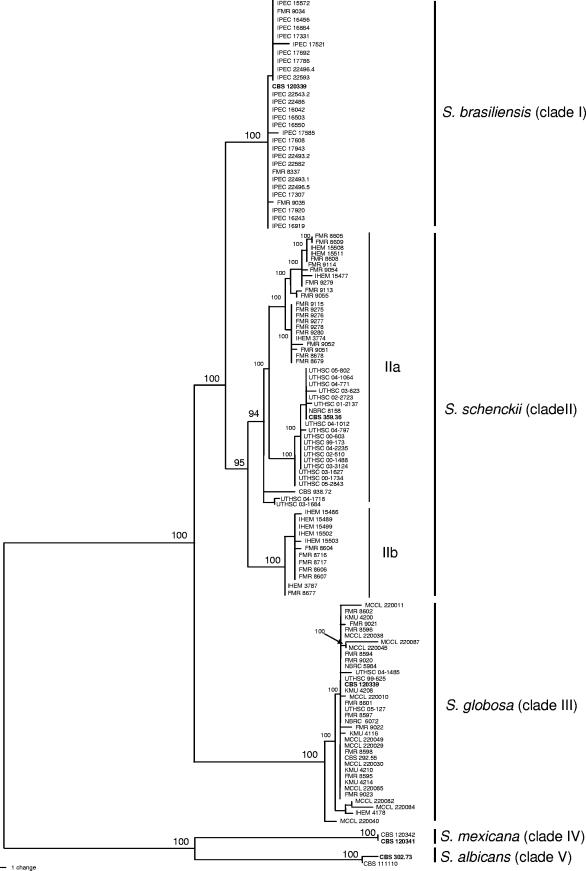
With the primers used, we were able to amplify and sequence 685 bp of CAL loci. Parsimony analysis of the CAL data set yielded 5,000 trees with 278 steps in length in which 20 nodes received 100% bootstrap support. All trees had a consistency index of 0.8885, a retention index of 0.9869, and a homoplasy index of 0.1115. There were 474 constant, 180 parsimony-informative, and 34 variable parsimony-uninformative characters in this fragment, which resulted in a total of 45 haplotypes. One of the most parsimonious trees is shown in Fig. 1. The isolates were distributed in five main clades (clades I to V) that were 100% statistically supported. All isolates of clinical origin were included in the first three clades (clades I to III), which correspond to the same main lineages obtained in our previous study (20). Clade I grouped only Brazilian isolates. Clade II included practically all of the American isolates. As in our previous study (20), clade II was again divided into two highly supported subclades (subclades IIa and IIb). The former subclade grouped most of the isolates from the United States and South America (Argentina, Bolivia, Colombia, Peru, and Venezuela), the type strain of S. schenckii, and the type strain of Dolichoascus schenckii, while subclade IIb included the rest of isolates from South America (Peru and Argentina) and the only existing isolate from South Africa. Clade III was comprised of isolates from China, India, Italy, Japan, Spain, and the United States and the type strain of Sporotrichum tropicale, which was from England. Four environmental isolates formed the two basal clades, clades IV and V. Clade IV grouped two isolates from Mexico, and clade V grouped two isolates identified as being S. albicans, one from England (the type strain) and one from Germany. The only existing isolate of S. schenckii var. luriei and the type strain of S. inflata could not be included in the phylogenetic tree because of sequences that were shorter (ca. 300 bp) than the rest of the isolates due to the low specificity of the degenerate primer CL1. However, the length of these sequences was informative enough to prove that both taxa are considerably genetically distant from the rest of isolates included in this study.
FIG. 1.
One of the 5,000 most parsimonious trees obtained from heuristic searches based on analysis produced from the combined data set. Bootstrap support values above 90% are indicated at the nodes. Type strains are indicated with boldface type.
Physiologic studies.
All isolates demonstrated optimal growth between 20 and 30°C, and none grew at 40°C. The isolates in clades IV and V grew more quickly at 20 and at 30°C, and those in clade I showed the slowest growth at these same temperatures. All the isolates grew well at 35°C; however, those in clades III and IV showed the most restricted growth. Most isolates also grew at 37°C, except for the majority of strains in clade III. The mean colony diameters for isolates in the different clades tested at various temperatures are summarized in Table 2.
TABLE 2.
Mean colony diameter of isolates of the S. schenckii complex on PDA at 21 days
| Clade | Species | Mean colony diam (mm) ± SD at temp:
|
|||
|---|---|---|---|---|---|
| 20°C | 30°C | 35°C | 37°C | ||
| I | S. brasiliensis | 24.7 ± 6.1 | 24.7 ± 5.5 | 16.6 ± 5.9 | 7.6 ± 1.6 |
| IIa | S. schenckii | 28.9 ± 5.4 | 36.0 ± 5.5 | 21.3 ± 7.0 | 6.1 ± 3.0 |
| IIb | S. schenckii | 30.1 ± 3.0 | 33.6 ± 3.0 | 20.8 ± 3.5 | 5.9 ± 1.6 |
| III | S. globosa | 28.5 ± 7.0 | 30.9 ± 5.0 | 11.5 ± 5.3 | 0.4 ± 0.5 |
| IV | S. mexicana | 53.5 ± 0.7 | 67.5 ± 2.1 | 10.8 ± 0.4 | 1.8 ± 0.4 |
| V | S. albicans | 51.0 ± 2.8 | 67.0 ± 4.2 | 28.0 ± 1.4 | 4.2 ± 1.4 |
A relatively large number of carbon and nitrogen sources were assimilated by all the isolates tested, with no significant differences among them (data not shown). The most important variations were observed in the assimilation of sucrose, raffinose, and ribitol (Table 3). All the isolates were able to split urea after 8 days of incubation, none produced extracellular polysaccharides, and variable results were obtained for acid and gelatinase production. All the isolates tolerated cycloheximide at 0.25% and 10% MgCl2. Variable results were obtained for tolerance to different concentrations of NaCl among isolates of the same clade (data not shown).
TABLE 3.
Physiological key characteristics for differentiating the clades of the S. schenckii complex
| Clade | Species | % of isolates that assimilate carbon source:
|
||
|---|---|---|---|---|
| Sucrose | Raffinose | Ribitol | ||
| I | S. brasiliensis | 0 | 0 | 18.5 |
| IIa | S. schenckii | 100 | 100 | 100 |
| IIb | S. schenckii | 100 | 100 | 33.3 |
| III | S. globosa | 100 | 0 | 90.9 |
| IV | S. mexicana | 100 | 100 | 100 |
| V | S. albicans | 100 | 0 | 50 |
Most isolates were able to convert, at least partially, to a yeast phase, and no significant differences were observed among clades.
Morphological studies.
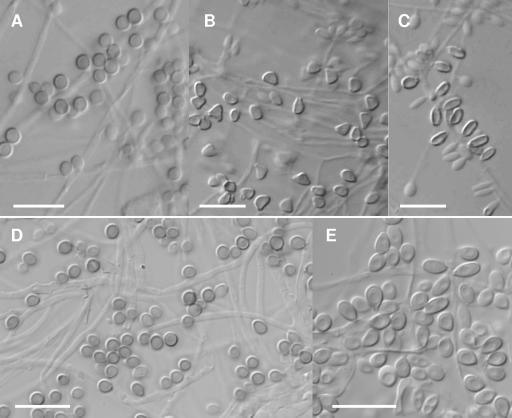
The macroscopic morphologies of all isolates were similar regardless of the medium on which they were grown. After 21 days of incubation, colonies on PDA were pale orange to gray-orange, and on CMA and oatmeal agar, they were brown to dark brown. An exception was noted in clade V and in two isolates of the clade IIa (CBS 359.36, the type strain of S. schenckii, and NBRC 8158), where colonies remained colorless. Since all the isolates sporulated considerably better on CMA than on other media tested and no growth differences were observed at 25 and 30°C, this culture medium, incubated at 30°C, was used to compare microscopic features among clades. Practically all the isolates developed terminal or intercalary conidial clusters on more or less differentiated conidiophores. The conidia were produced sympodially on denticulate conidiogenous cells. These conidia were hyaline or slightly pigmented, usually obovoid or pear-shaped, and measured 2 to 8 μm long by 1.5 to 2.5 μm wide. In addition, practically all the isolates produced another type of conidia, which were thick walled, dark brown, and usually borne individually on short denticles along the sides of the vegetative hyphae. These conidia, which are regarded as sessile conidia, measured 2 to 6 μm long by 2 to 3.5 μm wide and showed different shapes among clades. The presence or absence of these sessile conidia and their morphologies were key features to distinguish the different clades obtained in the phylogenetic analysis. Clades I and III showed globose to subglobose sessile conidia (Fig. 2A and D). Most of the isolates of clade II produced, in greater or lesser degree, triangular to cuneiform sessile conidia (Fig. 2 B). Only two isolates in this clade (FMR 8677 and IHEM 3787), which were phylogenetically distinct from the rest of the isolates, showed a different type of sessile conidia, which were obovoid, elongated, or irregularly shaped (Fig. 2C). Isolates of clade IV produced subglobose, obovoidal, or ellipsoidal sessile conidia (Fig. 2E). Neither the isolates in clade V nor the two isolates in clade IIa produced pigmented sessile conidia. These conidial differences correlated with those observed for growth rate and carbohydrate assimilations mentioned above (Tables 2 and 3) and confirmed that clades I, III, IV, and V represent species that are different from S. schenckii. The first three clades are here proposed as new species, and clade V corresponded with S. albicans.
FIG. 2.
Morphology of the sessile conidia of the S. schenckii species complex. (A) S. brasiliensis CBS 120339 (clade I). (B and C) S. schenckii (clade II) and FMR 8608 (clade IIa) (B) and FMR 8677 (clade IIb) (C). (D) S. globosa CBS 120340 (clade III). (E) S. mexicana CBS 120341 (clade IV). Bars, 10 μm.
Sporothrix brasiliensis Marimon, Gené, Cano, et Guarro, sp. nov. = Sporothrix schenckii, clade I sensu Marimon et al. (20). Coloniae in PDA ad 30°C post 21 dies 15 vel 38 mm diametri. Augmentum fit in temperatura 37°C. Conidia sympodialia hyalina vel subhyalina, obovoidea, 2 vel 6 per 1 vel 4 μm. Conidia sessilis brunnea vel atrobrunnea, crassitunicata, plerumque globosa vel subglobosa, 2.5 vel 5 per 2 vel 3 μm. Teleomorphosis ignota. Assimilantur ribitolum variabile. Non assimilantur sucrosum et raffinosum.
The colonies on PDA attained a diameter of 15 to 38 mm after 21 days of incubation at 30°C. Conidiogenous cells were usually terminal or intercalary on more or less differentiated conidiophores, were slightly swollen, and produced conidia sympodially on a few denticles. Sympodial conidia were usually hyaline to subhyaline, obovoidal, and 2 to 6 μm long by 1 to 4 μm wide. Sessile conidia were brown to dark brown, thick walled, globose to subglobose, and 2.5 to 5 μm long by 2 to 3 μm wide. A teleomorph was not developed by any isolate. The maximum growth temperature was 37°C (5 to 10 mm in diameter after 21 days). The fungus did not grow at 40°C and was unable to assimilate sucrose and raffinose. Variable results were seen for the assimilation of ribitol (81.5% of isolates were negative). The holotype is IMI 394469, from Rio de Janeiro, Brazil. Ex-type living cultures include CBS 120339, FMR 8309, and IPEC 16490. Etymology refers to the country of origin of the isolates.
Sporothrix globosa Marimon, Gené, Cano, et Guarro, sp. nov. = Sporothrix schenckii, clade III sensu Marimon et al. (20). Coloniae in PDA ad 30°C post 21 dies 18 vel 40 mm diametri. Augmentum fit in temperatura 35°C. Conidia sympodialia hyalina vel subhyalina, obovoidea, 2.5 vel 5 per 1 vel 3 μm. Conidia sessilis brunnea vel atrobrunnea, crassitunicata, plerumque globosa vel subglobosa, 3 vel 4 per 2 vel 3.5 μm. Teleomorphosis ignota. Assimilantur sucrosum et ribitolum. Non assimilantur raffinosum.
The colonies on PDA attained a diameter of 18 to 40 mm after 21 days of incubation at 30°C. Conidiogenous cells were often terminal or intercalary on more or less differentiated conidiophores, were often swollen, and produced conidia sympodially on numerous denticles. Sympodial conidia were usually hyaline to subhyaline, obovoidal, and 2 to 5 μm long by 1 to 3 μm wide. Sessile conidia were brown to dark brown, thick walled, predominantly globose to subglobose, and 2.5 to 4 μm long by 2 to 3.5 μm wide. A teleomorph was not developed by any isolate. The maximum growth temperature was 35°C (2.5 to 20 mm in diameter after 21 days). All isolates were unable to grow at 37°C, with the exception of four strains, which showed a very restricted growth (up to 2 mm in diameter after 21 days). The fungus did not grow at 40°C and was unable to assimilate raffinose. Ribitol was assimilated by 90.9% of the isolates. The holotype is IMI 394470 from Zaragoza, Spain. Ex-type living cultures include CBS 120340 and FMR 8600. Etymology refers to the spherical shape of the lateral conidia.
Sporothrix mexicana Marimon, Gené, Cano, et Guarro, sp. nov. = Sporothrix schenckii, clade IV, from the present study. Coloniae in PDA ad 30°C post 21 dies 66 vel 69 mm diametri. Augmentum fit in temperatura 37°C. Conidia sympodialia hyalina vel subhyalina, obovoidea, 3 vel 5.5 per 2 vel 2.5 μm. Conidia sessilis brunnea vel atrobrunnea, crassitunicata, subglobosa, obovoides vel ellipsoidea, 3 vel 4 per 2 vel 3.5 μm. Teleomorphosis ignota. Assimilantur ribitolum, sucrosum, et raffinosum.
The colonies on PDA attained a diameter of 66 to 69 mm after 21 days of incubation at 30°C. Conidiogenous cells were usually terminal or intercalary on more or less differentiated conidiophores, were often swollen, and were densely denticulate. Sympodial conidia were usually hyaline to subhyaline, obovoidal, and 3 to 5.5 μm long by 2 to 2.5 μm wide. Sessile conidia were brown to dark brown, thick walled, predominantly subglobose, obovoidal, or ellipsoidal, and 3 to 4 μm long by 2 to 3.5 μm wide. A teleomorph was not developed by any isolate. The maximum growth temperature was 37°C (1.5 to 2.5 mm in diameter after 21 days). The fungus did not grow at 40°C and was able to assimilate sucrose, raffinose, and ribitol. The holotype is IMI 394471 from Puebla, Mexico. Ex-type living cultures include CBS 120341 and FMR 9108. Etymology refers to the country of origin of the isolates.
DISCUSSION
Up to now, sporothrichosis, the most common subcutaneous fungal infection in South America, had been attributed to a unique species, S. schenckii. However, a recent molecular study demonstrated that S. schenckii is a complex of at least six putative phylogenetic species (20). Indeed, something similar has occurred in many other pathogenic fungi where the use of different molecular markers has demonstrated that they are genetically more complex than was initially thought. This is the case for Pneumocystis carinii (1), Coccidioides immitis (17), Aspergillus fumigatus (26), Candida parapsilosis (29), and Pseudallescheria boydii (10), among others. All of them constitute species complexes that are often difficult to differentiate phenotypically but sometimes with different clinical manifestations and infecting different body sites or even different hosts.
The most useful and significant finding of the present study has been the demonstration of a clear correlation between molecular data and phenotypic features, which allowed us to differentiate three new Sporothrix species, two of which have been associated with human infections (S. brasiliensis and S. globosa). Another interesting aspect of this study has been to confirm that the CAL gene is a good marker for the recognition of these species. Thus, by sequencing only this one locus and including many additional strains in the analysis, we were able to obtain the same main groups as in the previous study, where we sequenced three different loci (20). In addition, the analysis of the CAL sequences was useful to demonstrate that S. albicans, S. inflata, and S. schenckii var. luriei are clearly different species from S. schenckii.
In order to confirm the uniqueness of the new species described here, and knowing that several ascomycetes belonging mainly to Ophiostoma species can form Sporothrix anamorphs and that β-tubulin sequences of several of these ascomycetes are deposited in the GenBank database, we sequenced a representative isolate of each of the new species (S. brasiliensis AM116946, S. globosa AF116966, and S. mexicana AM498344) for comparison. In all cases, the degree of homology with the deposited sequences was very low.
Our study, to some extent, has confirmed previous molecular studies carried out by other authors, who already demonstrated high genetic variability in S. schenckii (13, 14, 15, 22, 32). Watanabe et al. (32), using restriction fragment length polymorphism (RFLP) analysis of the internal transcribed spacer region, grouped 204 isolates into four types, which correlated with their geographical origins. Type I was found predominantly in Africa and America, type II was found predominantly in South America, type III was found predominantly in North America, and type IV was found predominantly in Australia and Asia. This geographical distribution is similar to that obtained here. However, in our case, the Asian isolates grouped with the European ones, forming a highly supported group, which was the basis of the newly proposed species S. globosa. This agrees with data from a study reported previously by Ishizaki et al. (14), who used RFLP analysis of mitochondrial DNA, where the European isolates (all from Spain) were nested in the same phylogenetic group as isolates from Korea, China, and Japan. In the S. globosa clade, five Chinese environmental isolates were included which had previously been identified as being S. schenckii isolates by mitochondrial DNA RFLP by Ishizaky et al. (15). These isolates showed the same key phenotypic features as the rest of the isolates in the clade. With the exception of the Brazilian isolates and three isolates from the United States, the rest of the American isolates were included in clade II, as in the previous study (20). This clade was the most genetically heterogeneous group and probably encompasses a few more putative phylogenetic species. However, we did not find enough phenotypic characteristics to distinguish them. This clade also included the type strain of S. schenckii, which was also of American origin.
The saprophytic form of S. schenckii is usually characterized by the two types of conidia (5, 19) described above, i.e., sessile and sympodial. While the shape of the former type of conidia is very variable (globose, subglobose, ellipsoidal, triangular, etc.) among isolates, the morphology of the latter is more constant, being more or less obovoidal in general. By examining the morphology of the sessile conidia, we found robust differences among clades, which, combined with physiological data, allowed the differentiation of some cryptic species within the set of isolates that we received as S. schenckii. Other authors also correlated the morphology of the sessile conidia with some physiological features when environmental isolates of S. schenckii were studied. For instance, strains reported previously by Mackinnon (18), which were isolated from different environmental sources, showed oval dark-pigmented conidia and multiple spicules on the hyphae after conidial detachment. These isolates grew at 37°C and were pathogenic to mice. By contrast, other environmental strains isolated previously by Howard and Orr (12) formed dark-pigmented conidia firmly attached to the hyphae. These isolates did not grow at 37°C and were nonpathogenic to mice. Mesa-Arango et al. (22) found significant differences in conidial size among several groups generated by random amplified polymorphic DNA analysis, which coincided with different geographical locations. However, they did not indicate to what type of conidia they referred, and it is important to take into account that, in general, the sporulation of Sporothrix spp., especially the production of the sessile pigmented conidia, can vary considerably when routine culture media such as Sabouraud dextrose agar or PDA are used. We recommend the use of CMA for determining the microscopic features of these fungi, as the morphology remains stable when this medium is used. We were surprised by the fact that the type strain of S. schenckii (CBS 359.36) produced only hyaline sympodial conidia. This strain has traditionally been described as having pigmented conidia (11); however, we were unable to observe any dark, sessile conidia, and we presume that the isolate has degenerated, thereby losing its ability to produce these conidia. Isolate NBRC 8158, which is morphologically and genetically identical to the above-described isolate and of unknown origin, is probably a subculture of the type strain.
Only a few studies on the physiology of S. schenckii have been published. Ghosh et al. (9) previously tested 49 isolates from India, and none of them tolerated a 10% salt concentration. In contrast, approximately one-half of the isolates in our study tolerated this concentration, although isolates of S. brasiliensis showed the most restricted growth under these conditions. We also noticed an important discrepancy with the results reported by Ghosh et al. (9) concerning the carbohydrate assimilation tests, specifically inositol and mannitol. Both tests were negative for all the isolates tested previously by Ghosh et al. (9), while in our study, they were consistently positive. The results of assimilation studies for cellobiose, ribitol, d-galactose, d-glucose, glycerol, maltose, sorbitol, trehalose, and d-xylose were similar in both studies. Ghosh et al. (9) found variable results for the assimilation of raffinose, rhamnose, and dextrin within a given geographical area. We also found variable results for raffinose; however, all our isolates were able to assimilate rhamnose and dextrin. One explanation for these important discrepancies could lie in the fact that Ghosh et al. (9) used an auxanographic method with discs impregnated with saturated solutions of the carbohydrates on solid medium incubated at 37°C, while we used liquid media incorporated into microplates and incubated at 25°C. Although we evaluated the responses to more than 40 physiological tests, we found that only the assimilation of sucrose, raffinose, and ribitol was useful in discriminating members of the S. schenckii complex. Our studies are in agreement with those described previously by Dixon et al. (8) with regard to the ability of environmental isolates to grow at 35°C. The growth of all clinical isolates at 37°C was not, however, supported by our findings, as S. globosa failed to grow at this temperature.
In conclusion, S. schenckii must no be longer considered a single species. Using the procedures described here and the data reported in Table 4, the different species within the complex can be easily and reliably identified without the need for molecular techniques. As more epidemiological data become available for these species, we should gain a clear understanding of their geographic distribution, their role in disease, and the potentially different responses to antifungal agents.
TABLE 4.
Summary of the key features for species differentiation
| Species | Presence of sessile pigmented conidia | Colonies on PDA at 30°C exceeding 50 mm in 21 days | Growth at 37°C | Assimilation test result
|
|
|---|---|---|---|---|---|
| Sucrose | Raffinose | ||||
| S. albicans | No | Yes | Yes | + | − |
| S. brasiliensis | Yes | No | Yes | − | − |
| S. globosa | Yes | No | No | + | − |
| S. mexicana | Yes | Yes | Yes | + | + |
| S. schenckii | Yes | No | Yes | + | + |
Acknowledgments
We are indebted to the curators of the Centraalbureau voor Schimmelcultures (Utrecht, The Netherlands), BCCM/IHEM Biomedical Fungi and Yeasts Collection (Brussels, Belgium), A. Espinosa (Centro de Investigaciones en Ciencias Microbiológicas, Universidad Autónoma de Puebla, Mexico), J. M. Torres (IMIM, Hospital del Mar, Barcelona, Spain), C. Rubio (Hospital Universitario Lozano Blesa, Zaragoza, Spain), R. Negroni (Hospital de Infecciosas Francisco Javier Muñiz, Buenos Aires, Argentina), L. Trilles (Serviçio de Micología Médica, Instituto Evandro Chagas, Fiocruz, Rio de Janeiro, Brazil), P. Godoy (Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil), M. Paniso (Instituto Nacional de Higiene Rafael Rangel, Caracas, Venezuela), C. Hartung (Instituto de Medicina Tropical, Caracas, Venezuela), and A. Chakrabarti (Center for Advance Research in Medical Mycology, Chandigarh, India) for supplying many of the strains used in the study.
This study was supported by the Spanish Ministerio de Ciencia y Tecnología, grant CGL 2005-07394.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Beard, C. B., J. L. Carter, S. P. Keely, L. Huang, N. J. Pieniazek, I. N. Moura, J. M. Roberts, A. W. Hightower, M. S. Bens, A. R. Freeman, S. Lee, J. R. Stringer, J. S. Duchin, C. del Rio, D. Rimland, R. P. Baughman, D. A. Levy, V. J. Dietz, P. Simon, and T. R. Navin. 2000. Genetic variation in Pneumocystis carinii isolates from different geographic regions: implications for transmission. Emerg. Infect. Dis. 6:265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berbee, M. L., and J. W. Taylor. 1992. 18S ribosomal RNA gene sequence characters place the human pathogen Sporothrix schenckii in the genus Ophiostoma. Exp. Mycol. 16:87-91. [Google Scholar]
- 3.da Rosa, A. C., M. L. Scroferneker, R. Vettorato, R. L. Gervini, G. Vettorato, and A. Weber. 2005. Epidemiology of sporotrichosis: a study of 304 cases in Brazil. J. Am. Acad. Dermatol. 52:451-459. [DOI] [PubMed] [Google Scholar]
- 4.de Beer, Z. W., T. C. Harrington, H. F. Vismer, B. D. Wingfield, and M. J. Wingfield. 2003. Phylogeny of the Ophiostoma stenoceras-Sporothrix schenckii complex. Mycologia 95:434-441. [PubMed] [Google Scholar]
- 5.de Hoog, G. S. 1974. The genera Blastobotrys, Sporothrix, Calcarisporium and Calcarisporiella gen. nov. Stud. Mycol. 7:1-84. [Google Scholar]
- 6.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 7.de Hoog, G. S., F. D. Marvin-Sikkema, G. A. Lahpoor, J. C. Gottschall, R. A. Prins, and E. Guého. 1994. Ecology and physiology of the emerging opportunistic fungi Pseudallescheria boydii and Scedosporium prolificans. Mycoses 37:71-78. [DOI] [PubMed] [Google Scholar]
- 8.Dixon, D. M., I. F. Salkin, R. A. Duncan, N. J. Hurd, J. H. Haines, M. E. Kemna, and F. B. Coles. 1991. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J. Clin. Microbiol. 29:1106-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh, A., P. K. Maity, B. M. Hemashettar, V. K. Sharma, and A. Chakrabarti. 2002. Physiological characters of Sporothrix schenckii isolates. Mycoses 45:449-454. [PubMed] [Google Scholar]
- 10.Gilgado, F., J. Cano, J. Gené, and J. Guarro. 2005. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J. Clin. Microbiol. 43:4930-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hektoen, L., and C. F. Perkins. 1900. Refractory subcutaneous abscesses caused by Sporothrix schenckii. A new pathogenic fungus. J. Exp. Med. 5:77-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard, D. H., and G. F. Orr. 1963. Comparison of strains of Sporotrichum schenckii isolated from nature. J. Bacteriol. 85:816-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishizaki, H., M. Kawasaki, M. Aoki, T. Matsumoto, A. A. Padhye, M. Mendoza, and R. Negroni. 1998. Mitochondrial DNA analysis of Sporothrix schenckii in North and South America. Mycopathologia 142:115-118. [DOI] [PubMed] [Google Scholar]
- 14.Ishizaki, H., M. Kawasaki, M. Aoki, S. Wu, J. Lin, J. A. Kim, Y. H. Won, and C. R. Calvo. 2004. Mitochondrial DNA analysis of Sporothrix schenckii from China, Korea and Spain. Nippon Ishinkin Gakkai Zasshi 45:23-25. [DOI] [PubMed] [Google Scholar]
- 15.Ishizaki, H., M. Kawasaki, M. T. Mochizuki, X. Z. Jin, and S. Kagawa. 2002. Environmental isolates of Sporothrix schenckii in China. Nippon Ishinkin Gakkai Zasshi 43:257-260. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, E. M., A. Szekely, and D. W. Warnock. 1998. In-vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J. Antimicrob. Chemother. 42:741-745. [DOI] [PubMed] [Google Scholar]
- 17.Koufopanou, V., A. Burt, T. Szaro, and J. W. Taylor. 2001. Gene genealogies, cryptic species and molecular evolution in the human pathogen Coccidioides immitis and relatives (Ascomycota, Onygenales). Mol. Biol. Evol. 18:1246-1258. [DOI] [PubMed] [Google Scholar]
- 18.Mackinnon, J. E. 1970. Ecology and epidemiology of sporotrichosis, p. 169-181. In Proceedings of the International Symposium on Mycoses. Pan American Health Organization, Washington, DC.
- 19.Mariat, F., P. Lavalle, and P. Destombes. 1962. Recherches sur la Sporotrichose. Etude mycologique et pouvior pathogene de souches Mexicaines de Sporothrichum schenkii. Sabouraudia 2:60-79. [Google Scholar]
- 20.Marimon, R., J. Gené, J. Cano, L. Trilles, M. Dos Santos Lazera, and J. Guarro. 2006. Molecular phylogeny of Sporothrix schenckii. J. Clin. Microbiol. 44:3251-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGinnis, M. R., N. Nordoff, R. K. Li, L. Pasarell, and D. W. Warnock. 2001. Sporothrix schenckii sensitivity to voriconazole, itraconazole and amphotericin B. Med. Mycol. 39:369-371. [DOI] [PubMed] [Google Scholar]
- 22.Mesa-Arango, A. C., M. R. Reyes-Montes, A. Pérez-Mejía, H. Navarro-Barranco, V. Souza, G. Zúñiga, and C. Toriello. 2002. Phenotyping and genotyping of Sporothrix schenckii isolates according to geographic origin and clinical form of sporotrichosis. J. Clin. Microbiol. 40:3004-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neyra, E., P. Fonteyne, D. Swinne, F. Fauche, B. Bustamante, and N. Nolard. 2005. Epidemiology of human sporotrichosis investigated by amplified fragment length polymorphism. J. Clin. Microbiol. 43:1348-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Donnell, K. 2000. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 92:919-938. [Google Scholar]
- 25.O'Reilly, L. C., and S. A. Altman. 2006. Macrorestriction analysis of clinical and environmental isolates of Sporothrix schenckii. J. Clin. Microbiol. 44:2547-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pringle, A., D. M. Baker, J. L. Platt, J. P. Wares, J. P. Latge, and J. W. Taylor. 2005. Cryptic speciation in the cosmopolitan and clonal human pathogenic fungus Aspergillus fumigatus. Evolution 59:1886-1899. [PubMed] [Google Scholar]
- 27.Suzuki, K., M. Kawasaki, and H. Ishizaki. 1988. Analysis of restriction profiles of mitochondrial DNA from Sporothrix schenckii and related fungi. Mycopathologia 103:147-151. [DOI] [PubMed] [Google Scholar]
- 28.Swofford, D. L. 2001. PAUP*. Phylogenetic analysis using parsimony (*and other methods) (version 4.0). Sinauer Associates, Sunderland, MA.
- 29.Tavanti, A., A. D. Davidson, N. A. R. Gow, and M. C. J. Maiden. 2005. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43:284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travassos, L. R., and K. O. Lloyd. 1980. Sporothrix schenckii and related species of Ceratocystis. Microbiol. Rev. 44:683-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trilles, L., B. Fernández-Torres, M. Dos Santos Lazera, B. Wanke, A. de Oliveira Schubach, R. de Almeida Paes, I. Inza, and J. Guarro. 2005. In vitro antifungal susceptibilities of Sporothrix schenckii in two growth phases. Antimicrob. Agents Chemother. 49:3952-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe, S., M. Kawasaki, T. Mochizuki, and H. Ishizaki. 2004. RFLP analysis of the internal transcriber spacer regions of Sporothrix schenckii. Jpn. J. Med. Mycol. 45:165-175. [DOI] [PubMed] [Google Scholar]
- 33.Yarrow, D. 1998. Methods for the isolation, maintenance and identification of yeasts, p. 95-96. In C. P. Kurtzman, and J. W. Fell (ed.), The yeasts, a taxonomic study, 4th ed. Elsevier, Amsterdam, The Netherlands.
- 34.Zhang, Z., X. Liu, G. Yang, X. Gao, L. Jin, and L. An. 2006. Genotyping of Sporothrix schenckii by analysis of ribosomal DNA regions. Mycoses 49:305-310. [DOI] [PubMed] [Google Scholar]