Abstract
Indoleamine 2,3-dioxygenase (IDO) is the first and rate-limiting enzyme in the kynurenine pathway of tryptophan catabolism and has been implicated in neurotoxicity and suppression of the antiviral T-cell response in HIV encephalitis (HIVE). Here we show that the Toll-like receptor 3 (TLR3) ligand poly(I:C) (PIC) induces the expression of IDO in human astrocytes. PIC was less potent than gamma interferon (IFN-γ) but more potent than IFN-β in inducing IDO. PIC induction of IDO was mediated in part by IFN-β but not IFN-γ, and both NF-κB and interferon regulatory factor 3 (IRF3) were required. PIC also upregulated TLR3, thereby augmenting the primary (IFN-β) and secondary (IDO and viperin) response genes upon subsequent stimulation with PIC. In HIVE, the transcripts for TLR3, IFN-β, IDO, and viperin were increased and IDO immunoreactivity was detected in reactive astrocytes as well as macrophages and microglia. PIC caused suppression of intracellular replication of human immunodeficiency virus pseudotyped with vesicular stomatitis virus G protein and human cytomegalovirus in a manner dependent on IRF3 and IDO. The involvement of IDO was demonstrated by partial but significant reversal of the PIC-mediated antiviral effect by IDO RNA interference and/or tryptophan supplementation. Importantly, the cytokine interleukin-1 abolished IFN-γ-induced IDO enzyme activity in a nitric oxide-dependent manner without suppressing protein expression. Our results demonstrate that IDO is an innate antiviral protein induced by double-stranded RNA and suggest a therapeutic utility for PIC in human viral infections. They also show that IDO activity can be dissociated from protein expression, indicating that the local central nervous system cytokine and nitric oxide environment determines IDO function.
In the central nervous system (CNS), astrocytes play an important role as regulators of extracellular electrolyte and neurotransmitter balance, as well as in the establishment and maintenance of the blood-brain barrier. Together with microglia, astrocytes are also important modulators of CNS immune and inflammatory reactions (20, 37, 38), participating in both innate and acquired immune responses. Recently, it has been well established that astrocytes express certain members of the Toll-like receptor (TLR) family, in particular, the receptor for double-stranded RNA (dsRNA), TLR3 (9, 22, 34, 59, 65, 72). TLRs play a central role in the generation of innate antimicrobial immune responses through recognition of conserved pathogen-associated molecular patterns (3, 39, 40). The synthetic TLR3 ligand poly(I:C) (PIC) is a potent activator of rat, murine, and human astrocytes (34, 59, 65, 72). Gene profiling studies show that in astrocytes, PIC induces the expression of a wide array of genes that resembles the profile of gene expression activated in these cells by the proinflammatory cytokine interleukin-1 (IL-1) (38, 42, 64). These include cytokine genes, chemokine genes, and antiviral protein genes belonging to the interferon (IFN)-stimulated gene (ISG) family. In preliminary microarray studies, we found that one of the genes highly induced by PIC in human astrocytes was that encoding indoleamine 2,3-dioxygenase (IDO) (∼69-fold, 24 h, n = 3).
IDO is the rate-limiting enzyme in the kynurenine pathway of tryptophan catabolism (51, 52, 54, 77). It is typically induced by IFN-γ in dendritic cells and macrophages and has been shown to have pleiotropic biological functions, chief among them immunosuppressive properties. In addition to a having a role in T-cell suppression and tolerance induction, IDO has also recently been implicated in antimicrobial activity in human cells (14). IDO-sensitive organisms include intracellular facultative parasites such as Toxoplasma gondii, as well as certain bacteria whose replication can be inhibited by depletion of the rare essential amino acid tryptophan by the action of IDO (15, 52). In addition, IDO appears to account for IFN-γ-mediated antiviral activity against human cytomegalovirus (HCMV), herpes simplex virus, and measles virus (2, 6, 58).
In the CNS, IDO has been shown to be induced in brain macrophages in simian immunodeficiency virus encephalitis (SIVE) in macaques, and its expression correlates with that of IFN-γ (8, 19). Consistent with a role for IDO in T-cell suppression, the IDO inhibitor 1-methyl tryptophan has recently been shown to increase viral clearance in a murine model of human immunodeficiency virus encephalitis (HIVE) (62). In addition, increased levels of quinolinic acid, an IDO-dependent tryptophan degradation product and an N-methyl-d-aspartic acid receptor agonist (71), have been detected in the cerebrospinal fluids of HIV-infected individuals (1, 31) and have been implicated in neurodegeneration in HIVE.
Although cells of the monocyte lineage are the major expressers of IDO, studies also demonstrate that nonmacrophage cell types such as astrocytes and neurons can express IDO when stimulated with IFN-γ (30). However, little is known about the regulation of astrocyte IDO expression in these cells, especially via IFN-γ-independent pathways. In this study, we investigated the molecular mechanisms underlying PIC-induced IDO expression and the contribution of IDO to an antiviral innate immune response in astrocytes. We also investigated the relationship between inducible nitric oxide synthase (iNOS) and IDO. The results demonstrate that PIC induces IDO via an interferon regulatory factor 3 (IRF3)- and IFN-β-dependent but IFN-γ-independent mechanism and that IDO limits intracellular viral replication. Furthermore, we show that IDO is inactive when coinduced with iNOS and that this occurs without inhibition of IDO expression.
MATERIALS AND METHODS
Primary human astrocyte culture.
Human fetal CNS cell cultures were prepared from second-trimester abortuses by enzymatic and mechanical dissociation of the cerebral tissues followed by filtration through nylon meshes of 230- and 130μm pore sizes as described previously (44, 48). Cells were plated at 106 to 107 cells per ml in Dulbecco's modified Eagle medium (DMEM) (Cellgro: supplemented with 4.5 g/liter glucose, 4 mM l-glutamine, and 25 mM HEPES; l-tryptophan concentration, 16 μg/ml) supplemented with 5% fetal calf serum (Gemini Bio-products, Woodland, CA), penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (Fungizone; 0.25 μg/ml) for 2 weeks. Highly enriched cultures of human fetal astrocytes (>98% glial fibrillary acidic protein positive) were generated by repeated (two or three times) passage of mixed CNS cell cultures at 2- to 6-week intervals in vitro as described previously (44).
Culture treatment with PIC, IFNs, and antibodies.
PIC and l-tryptophan were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human IFN-γ was purchased from Peprotech (Rocky Hill, NJ; specific activity, 1 ng = 20 U). IFN-β was purchased from PBL Biomedical Laboratories (Piscataway, NJ; 1 ng = 80 U). Confluent astrocyte cultures were treated with standard concentrations of PIC (10 μg/ml) or IFNs (10 ng/ml) (34, 45, 72) in DMEM with 5% fetal calf serum (FCS) for 1 day unless otherwise indicated. For neutralization experiments, astrocytes were incubated with rabbit antisera to IFN-β at 2 × 105 U/ml, a mouse immunoglobulin G1 (IgG1) against IFN-γ at 10 μg/ml, or control serum (normal rabbit serum or normal mouse IgG1), all purchased from PBL. All other reagents were purchased from Sigma unless stated otherwise.
HIV infection of astrocytes.
Chimeric HIV type 1 was generated by cotransfection of 293 T cells with pVSVg-env and pNL4-3 and added to astrocyte cultures at ∼50 ng/ml of input p24. The chimera expressing the vesicular stomatitis virus G protein and HIV env (VSV-G HIV chimera) results in a high incidence of astrocyte infection, as previously described (13, 65). VSVg-env HIV was also generated by cotransfecting 293 T cells with pVSVg-env and Env-deficient pNL4-3 obtained from Maurizio Federico, Istituto Superiore di Sanità, Rome, Italy (23). Both viruses replicated efficiently in astrocytes. All cultures were washed 24 h after addition of VSV-G HIV to remove input virus, unless stated otherwise. Cultures were fed with fresh DMEM plus 5% FCS with antibiotics without amphotericin B. PIC, l-tryptophan, and other culture additives were added back whenever the culture medium was changed. Cells were harvested for Western blotting or immunocytochemistry 3 days after infection.
IDO knockdown by siRNA.
Astrocytes in 6-cm petri dishes were transfected with 10 nM control nontargeting small interfering RNA (siRNA) or human IDO-specific siRNA (Dharmacon, Chicago, IL) with TransIT-TKO transfection reagents from Mirus, following the manufacturer's instructions. After incubation for 48 to 72 h, cells were washed with fresh medium and then exposed to VSV-G HIV for an additional 3 days as described above. Immunoblotting was performed to confirm suppression of IDO expression by siRNA.
Western blot analysis.
IDO expression was determined by Western blot analysis using a mouse IgG1 against human IDO as described previously (76, 78). Blots were also incubated with a mouse IgG1 against HIV p24 (Dako, Carpinteria, CA) which recognizes a p55 Gag precursor and other processed Gag proteins, including p24 capsid antigen (CA). Blots were also probed with an antibody to IRF3 (C terminal; rabbit IgG from Santa Cruz) which recognizes both endogenous and N-terminally truncated mutant IRF3. All blots were reprobed for vinculin (117 kDa) using a rabbit antibody from Santa Cruz to control for protein loading. Briefly, astrocyte cultures in 6-cm dishes were scraped into lysis buffer (10 mM Tris-HCl [pH 8.8], 50 mM NaCl, 0.5 mM Na3VO4, 30 mM Na4P2O7, 50 mM NaF, 2 mM EDTA, 1% Triton X-100) at various time points. Thirty to seventy micrograms of protein was separated by 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membrane. The blots were blocked in Tris-buffered saline-0.1% Tween-20 containing 5% nonfat milk and then incubated with antibodies at 4°C for 16 h. The secondary antibody was horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG and was used at 1:2,000 to 1:10,000 for 1 h at room temperature. Signals were developed using enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL). Densitometric analysis was performed using Scion NIH Image software (Scion, Frederick, MD).
HIV Gag cell-based ELISA.
Human fetal astrocytes in 96-well tissue culture plates were exposed to indicated amounts of VSV-G HIV as described above. Three days later, cells were fixed with ice-cold methanol and immunostained with a mouse IgG1 against HIV Gag p24 (Dako). Color was developed using nitroblue tetrazolium, and plates were read with a microplate reader at 595 nm, with uninfected cells as blanks. A 100% cell infection resulted in an optical density (OD) of approximately 2.0. Data are expressed as means ± standard errors of the means (SEM) from triplicate samples. The enzyme-linked immunosorbent assay (ELISA) values (ODs) showed a high correlation with the percentage of p24+ cells, as determined by cell counting (data not shown).
Q-PCR.
Quantitative real-time reverse transcription-PCR (Q-PCR) was performed essentially as described previously (65), using porphobilinogen deaminase as an internal control. Primer sequences for viperin, TLR3, and IFN-β were published previously (65). IDO primer sequences were ATGTCCTGGAGGAACTGAGC (forward) and TTCAGTGCTTTGACGTCCTG (reverse). Briefly, total RNA was extracted from astrocytes in culture or from frozen brain tissues (cerebral cortex and white matter) from control and HIVE subjects (Manhattan HIV Brain Bank) with TRIzol (Invitrogen Life Technologies), following the manufacturer's instructions. PCR was performed using a SYBR green PCR mix and conducted with the ABI Prism 7900HT (Applied Biosystems). All values were expressed as the increase relative to the expression of porphobilinogen deaminase. The mean value of the replicates for each sample was calculated and expressed as the cycle threshold (CT; cycle number at which each PCR reaches a predetermined fluorescence threshold, set within the linear range of all reactions). The amount of gene expression was then calculated as the difference between the CT of the sample for the target gene and the mean CT of that sample for the endogenous control.
IDO immunocytochemistry in brain tissues.
Paraffin-embedded sections of HIVE and control brains were obtained from the Manhattan HIV Brain Bank. Sections were boiled in citrate buffer for antigen unmasking and then incubated with anti-IDO antibody (78) for 16 h at 4°C followed by additional incubation at 37°C for 2 h. The staining was completed using the ABC methods described elsewhere (19). Control HIVE sections stained with normal mouse IgG1 revealed no immunoreactivity (not shown).
Measurement of iNOS and IDO activity.
Nitric oxide (NO) production in astrocytes was measured by determining the concentration of the stable metabolite nitrite in the culture supernatants by the Griess reaction as previously described (43, 48). IDO activity was determined by measuring the l-kynurenine (KYN) concentration in the culture supernatants as described previously (16, 50), with modifications. Briefly, 100 μl of supernatant was mixed with 50 μl of 30% trichloroacetic acid, vortexed, and centrifuged at 8,000 × g for 5 min. Then, 75 μl of the supernatant was added to an equal volume of Ehrlich's reagent (100 mg of p-dimethylaminobenzaldehyde in 5 ml of glacial acetic acid) in a 96-well plate, and the A490 was read with a microplate reader. Known concentrations of kynurenine (0 to 200 μM: Sigma-Aldrich) were used to generate a standard curve.
Astrocyte infection with HCMV.
HCMV AD169 was obtained from the NIH AIDS Reagent Repository and propagated in human fetal astrocytes in DMEM with 5% FCS. Culture supernatants were collected at 1, 3, and 7 days and combined with day 7 cell lysates by freeze-thawing. Cell debris was discarded following centrifugation, and the clarified supernatants were used as viral stock. Astrocyte monolayers were infected with culture supernatants containing 2 to 20 50% tissue culture infective doses of HCMV for 1 h. Fresh culture medium was added, and the cells were observed daily for cytopathic effects. Cells were fixed 3 days after infection and immunostained for HCMV using the following antibodies: clone 1-M-12 from Abcam (Cambridge, MA), specific to the late protein envelope glycoprotein B; clone DDG (IgG2a) from Dako, specific to major immediate early protein 1 (IE1; 72 kDa; this Dako antibody cocktail contains another clone, CCH2 [IgG1], specific to the 52-kDa early protein); and clone 1B12 (IgG2a) from Chemicon (Temecula, CA) against IE1 (this clone also reacts with IE2 [84 kDa]). Antibody specificities were confirmed by Western blot analysis of infected astrocytes (data not shown). Pilot studies demonstrated that the frequency of immunoreactive astrocytes (day 3 postinfection) was similar with all three antibodies. All subsequent experiments were performed with the Dako antibody. Cell-based ELISA was performed essentially as described for HIV Gag ELISA, and the ODs were correlated with the percentage of HCMV-immunoreactive astrocytes (data not shown).
Statistical analysis.
Significant differences were evaluated using Student's t tests, except that multiple treatment groups were compared within individual experiments by one-way analysis of variance (ANOVA) with appropriate posthoc tests. Data are expressed as means ± SEM, and probability values of <0.05 were considered significant.
RESULTS
Astrocyte IDO induction by IFN-β, IFN-γ, and PIC.
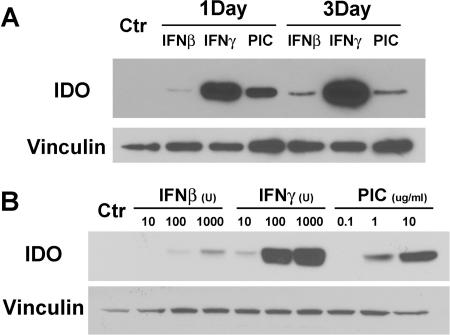
IDO expression was determined by Western blot analysis following treatment with standard concentrations of IFN-β (10 ng/ml), IFN-γ (10 ng/ml), or PIC (10 μg/ml) (34, 45, 72) for 1 or 3 days (Fig. 1A) or with various concentrations of IFN-β (10 to 1,000 U/ml), IFN-γ (10 to 1,000 U/ml), or PIC (0.1 to 10 μg/ml) for 1 day (Fig. 1B). The results show that IFN-β, IFN-γ, and PIC all induced IDO in astrocytes but with different potencies and kinetics. IFN-γ was a potent inducer of IDO, as reported previously (2, 29, 30), while IFN-β was a weak inducer, and PIC showed an intermediate potency. IFN-γ (and IFN-β)-induced IDO was sustained through 3 days, while PIC-induced IDO tended to decrease by 3 days after cell stimulation (Fig. 1A).
FIG. 1.
IDO induction by PIC, IFN-γ, and IFN-β in primary human astrocytes. (A) Astrocyte cultures were stimulated with 10 μg/ml of PIC or 10 ng/ml of recombinant IFN-γ or recombinant IFN-β for 1 or 3 days. The Western blot shows IDO (∼45 kDa) induction by all stimuli, with IFN-γ being the most potent and IFN-β the least potent. Blots were stripped and reprobed for vinculin (117 kDa) as a control for protein loading. (B) Dose-dependent IDO induction in astrocytes. Astrocyte cultures were stimulated with 10 U (0.125 ng/ml), 100 U, or 1,000 U of recombinant IFN-β, 10 U (0.5 ng/ml), 100 U, or 1,000 U of recombinant IFN-γ, or 0.1 μg/ml, 1 μg/ml, or 10 μg/ml of PIC for 1 day. Blots were probed for IDO and then stripped and reprobed for vinculin. Astrocyte IDO is induced in a dose-dependent manner with the following ranking of potency: IFN-γ > PIC > IFN-β. Results are representative of three independent experiments.
IFN-β but not IFN-γ is involved in PIC induction of IDO.
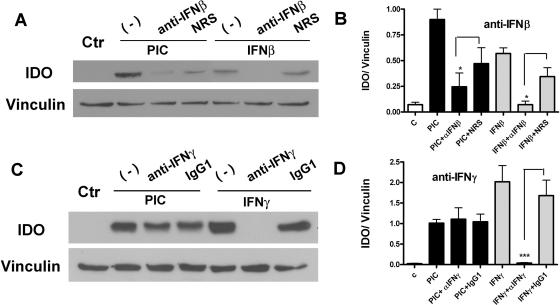
The induction of IDO by PIC in astrocytes has not been reported to date. In previous reports, it was noted that IFN-β failed to induce IDO in astrocytes (2), while IDO induction under most circumstances (regardless of cell type) was attributed to IFN-γ (28). Therefore, we asked whether PIC induction of IDO in astrocytes could be attributed to either IFN-β or IFN-γ, using neutralizing antibodies to IFN-β or IFN-γ. Representative Western blots and pooled densitometric data show that anti-IFN-β significantly reduced the levels of PIC-induced IDO expression in astrocytes (Fig. 2A and B), while anti-IFN-γ had no effect (Fig. 2C and D). Together, these results indicate that PIC induces IDO in astrocytes by a mechanism that is dependent on IFN-β but independent of IFN-γ.
FIG. 2.
PIC-induced expression of IDO is dependent on IFN-β but not IFN-γ. (A) Astrocytes were pretreated for 30 min with neutralizing antibodies to human IFN-β (2 × 105 U/ml; PBL) or control normal rabbit serum (NRS) and then stimulated with PIC (10 μg/ml) or recombinant IFN-β (3 ng/ml) for 24 h. Immunoblotting was performed for IDO and vinculin. Neutralizing IFN-β antibody inhibited PIC-induced IDO expression as well as IFN-β-induced IDO expression in astrocytes. (B) Pooled densitometry data (means plus SEM) from three separate cases show significant inhibition of IFN-β- and PIC-induced astrocyte IDO expression by anti-IFN-β antibody compared to NRS-treated controls (*, P < 0.05, t test). (C) Astrocytes were pretreated for 30 min with a neutralizing antibody to human IFN-γ (10 μg/ml; PBL) or normal mouse IgG1 and then stimulated with PIC or IFN-γ (5 ng/ml) for additional 24 h. Immunoblotting was performed for IDO and vinculin. IFN-γ-induced IDO, but not PIC-induced IDO, was inhibited by anti-IFN-γ antibody in astrocytes. (D) Pooled IDO/vinculin densitometry data (means plus SEM) of Western blots from three different cases show complete inhibition (***, P < 0.001, t test) of IFN-γ-induced IDO by IFN-γ antibody. PIC-induced IDO was not affected by incubation with the anti-IFN-γ antibody.
NF-κB and IRF3 are required for PIC-mediated IDO induction.
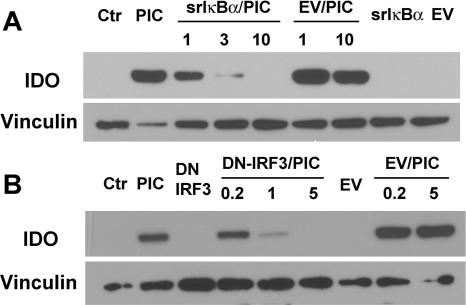
Ligand binding to TLR3 activates the transcription factors IRF3 and NF-κB, which are critical in the induction of IFN-β (primary response gene) (36, 40). The early (3 to 6 h) induction of IFN-β was shown to function in concert with other signal transduction mechanisms to generate effective antiviral responses (46). To determine whether IRF3 and NF-κB are involved in PIC-induced IDO expression, we employed adenovirus-mediated expression of dominant negative (DN) constructs of NF-κB and IRF3. Astrocytes infected with adenoviruses that contain the proteolysis-resistant superrepressor (SR) IκBα, a DN IRF3 lacking the N-terminal domain (41, 64), or empty vectors (EV) as controls were examined for their ability to affect IDO after PIC activation. Representative Western blots (Fig. 3) demonstrate that the SR IκBα and DN IRF3 dose-dependently inhibited astrocyte IDO expression, whereas EV were without effect. These data indicate that both NF-κB and IRF3 are necessary for PIC induction of IDO in astrocytes.
FIG. 3.
PIC-induced expression of IDO is dependent on NF-κB and IRF-3. (A) Astrocytes were infected for 48 h with increasing concentrations (multiplicities of infection) of adenoviral vectors expressing a DN NF-κB (srIκBα) or EV. Cells were then stimulated with 10 μg/ml of PIC for 24 h, and total cell homogenates were prepared and immunoblotted for IDO and vinculin. PIC-induced IDO was dose dependently inhibited by the SR IκBα but not EV. Adenovirus expressing the SR IκBα or EV alone did not induce IDO. (B) The role of DN IRF3 was examined by adenoviral vectors as for panel A. The results showed that IDO expression was dose dependently inhibited by DN IRF3, whereas EV were without effect. The data shown are from one of two separate experiments with identical results.
Activated astrocytes upregulate TLR3, a mechanism that amplifies IDO expression.
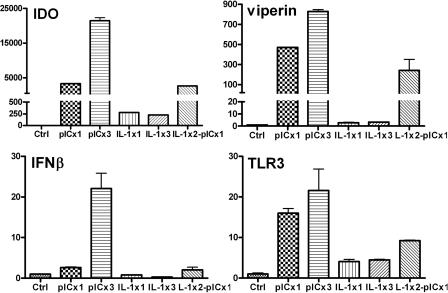
Because TLR3 has been shown to be upregulated by inflammatory stimuli, we asked whether repeated stimulation of astrocytes with PIC increases the expression of antiviral genes such as those for IDO and viperin (65). We compared single treatment with repeated treatment for the expression of TLR3, IFN-β, IDO, and viperin mRNA on day 4 by Q-PCR. We compared the PIC response to that of IL-1, a proinflammatory cytokine that fails to induce antiviral responses in astrocytes (65). Figure 4 demonstrates that repeated stimulation of astrocytes with PIC increases the expression of IDO and viperin (secondary response genes), as well as IFN-β (primary response gene) and TLR3. These results, together with the demonstration that PIC-induced gene transcription was suppressed by the inhibitor of endosomal acidification NH4Cl (data not shown), implicate receptor (TLR3) upregulation as the underlying mechanism for the increased gene expression (also see Discussion). IL-1, on the other hand, induced smaller amounts of genes, with repeated stimulation providing no benefit. Astrocytes prestimulated with IL-1 still retained their ability to respond to PIC, indicating that TLR3 in these cells was functional. Together, these results show that PIC provides a much stronger stimulus than IL-1 in inducing antiviral genes and that increased expression of TLR3 under inflammatory conditions could lead to enhanced antiviral responses in vivo.
FIG. 4.
Astrocytes pretreated with PIC show upregulation of TLR3, IFN-β, and IDO mRNA. The effect of pretreatment of astrocytes with PIC or IL-1 on IDO expression was examined. Astrocytes were treated with medium (Ctrl), PIC once on day 1 (pICx1), PIC daily for 3 days (pICx3), IL-1 once on day 1 (IL-1x1), IL-1 daily for 3 days (IL-1x3), or IL-1 on day 1 and 2 and PIC on day 3 (IL-1x2-pICx1). Total RNA was harvested on day 4 and subjected to Q-PCR for IDO, viperin/cig5, IFN-β, and TLR3 mRNA expression as described in Materials and Methods. Results are means plus SEM from triplicate samples. Note that some error bars are too small to be seen. Also note that failure to induce IFN-β in some samples reflects the discordant time points for RNA harvest (24 h) and the peak IFN-β mRNA expression (3 to 6 h).
IDO expression in astrocytes in HIVE.
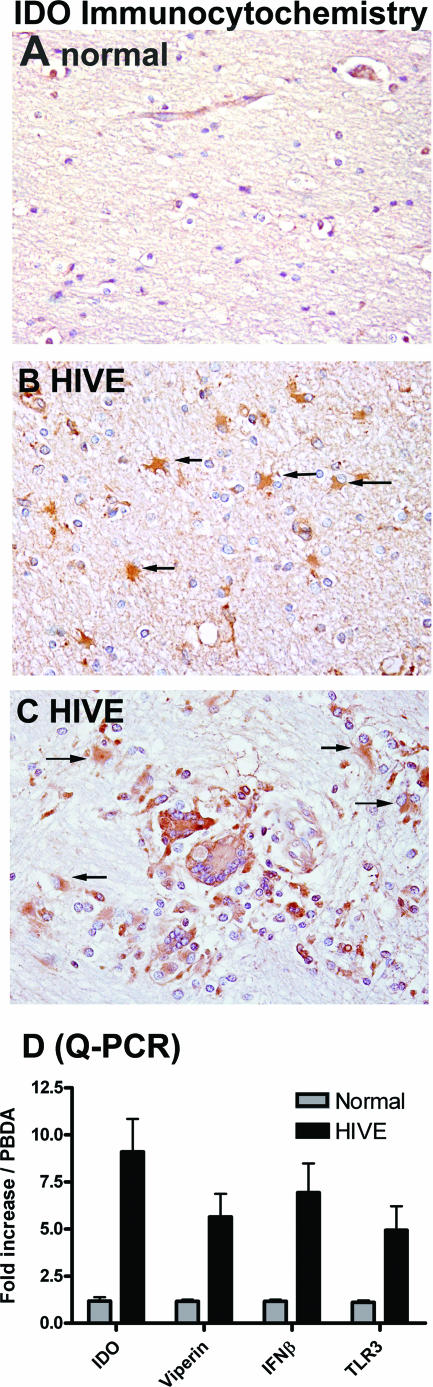
IDO was previously shown to be induced in macrophages in SIVE. To determine whether IDO can be induced in astrocytes in vivo, we examined IDO expression in HIVE by immunocytochemistry. IDO gene expression in HIVE and control brain tissues was also analyzed by Q-PCR. While HIV-seronegative brains showed no IDO immunoreactivity (Fig. 5A), robust IDO expression was detected in HIVE brains in astrocytes (Fig. 5B and C), as well as macrophages and microglia (Fig. 5C). The IDO-positive astrocytes were found in the vicinity of HIVE lesions composed of multinucleated giant cells and microglial nodules (Fig. 5C), as well as in areas remote from such lesions (Fig. 5B). Therefore, astrocytes, in addition to macrophages, express IDO in HIVE. Q-PCR analysis also demonstrated that IDO mRNA was increased severalfold in HIVE compared to healthy controls. Additionally, transcripts for viperin, IFN-β, and TLR3, which we found to be increased in astrocytes treated with PIC (Fig. 4), were also increased in HIVE brain tissues (Fig. 5D). These results demonstrate that astrocytes in vivo can express IDO and that antiviral innate immune responses are activated in vivo in HIVE.
FIG. 5.
Astrocyte IDO expression in vivo in HIVE. IDO immunohistochemistry was performed on paraffin-embedded brain sections of healthy control and HIVE subjects. While no immunoreactivity was detected in controls (A), abundant IDO expression was detected in reactive astrocytes (arrows) in the white matter remote from the sites of HIV infection (B), as well as those (arrows) in the vicinity of infected macrophages and microglia forming microglial nodules (C) in HIVE. Note the IDO immunoreactivity in multinucleated giant cells and microglia in HIVE lesions (C, center). Astrocytes, macrophages, and microglia were identified by their characteristic morphology and their immunoreactivity to glial fibrillary acidic protein and CD68 in serial sections (not shown). (D) Q-PCR analysis of IDO, viperin, IFN-β, and TLR3 mRNA in brain homogenates of HIVE (n = 3) normalized to that of healthy brain (n = 3). Data are means plus SEM.
PIC induces astrocyte antiviral activity via IRF3.
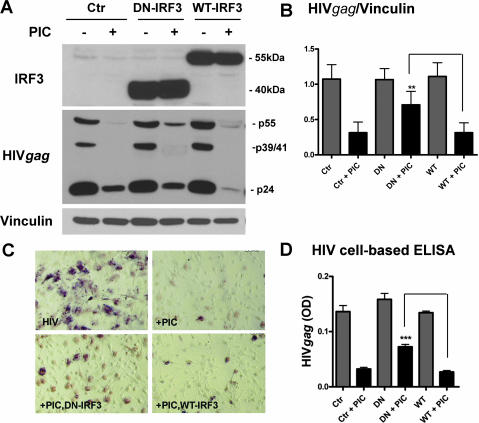
We next determined the extent to which PIC-induced cell activation pathways participate in the induction of antiviral immunity. We first examined the requirement for IRF3 in PIC-mediated suppression of HIV replication in astrocytes. For astrocyte HIV infection, we employed VSV-G pseudotyped HIV, which enters astrocytes highly efficiently. Astrocytes were first infected with adenovirus containing DN IRF3 or wild-type (WT) IRF3 for 2 days and then infected with VSV-G HIV for an additional 3 days with or without PIC. Expression of WT and DN IRF3 was confirmed by Western blot (Fig. 6A, top). HIV Gag expression was also determined by Western blotting and immunocytochemistry. As shown in Fig. 6, astrocytes were productively infected with VSV-G HIV, and PIC suppressed HIV production in control, DN IRF3-expressing, and WT IRF3-expressing astrocyte cultures. However, pooled densitometric data from several cases demonstrated a significant reversal of the inhibitory effect of PIC in cultures infected with the DN IRF3 constructs compared to those infected with the WT IRF3 construct (Fig. 6B). By HIV Gag immunocytochemistry and cell-based ELISA, a significant reversal of PIC-mediated HIV inhibition was also observed in DN IRF3-expressing astrocytes (Fig. 6C and D). Together, these results demonstrate an essential role for IRF3 in PIC-induced HIV suppression. Although overexpression of WT IRF3 has been shown to increase cell activation in certain systems, including our own (64, 69), we did not see an increase of PIC-induced anti-HIV activity in IRF3 overexpressing astrocytes. Therefore, in primary human astrocytes activated by PIC, IRF3 phosphorylation may be the major regulatory step in the induction of antiviral immunity. Interestingly, we see only partial reversal of PIC's antiviral effect by adenoviral DN IRF3, while adenoviral DN IRF3 potently inhibited PIC-induced IDO expression (Fig. 6 and 3, respectively). We believe the cell populations infected by HIV and by adenovirus may be incompletely overlapping, while PIC can reach all cell populations. For example, adenoviral vectors have been reported to interfere with HIV replication in macrophages (63), which may result in only partial reversal of the PIC's antiviral effect by adenoviral DN IRF3.
FIG. 6.
Astrocyte HIV replication is inhibited by PIC in an IRF3-dependent manner. (A) Astrocytes were first infected with adenovirus (DN IRF3 or WT IRF3). Two days later, cells were infected with VSV-G HIV with or without 10 μg/ml of PIC for an additional 3 days, as described in Materials and Methods. Cells were harvested for HIV Gag expression by Western blot analysis. Overexpression of DN (N-terminally truncated) IRF3 and WT IRF3 was confirmed by immunoblotting using an antibody against the C terminus of IRF3. A faint 55-kDa endogenous IRF3 is visible in control (Ctr) cultures. Results show that PIC suppresses HIV Gag expression, and this is partially reversed by DN IRF3 overexpression. (B) Pooled densitometric ratios (means plus SEM) of HIV Gag (total band densities including p55 Gag, p39/41 MA-CA when present, and p24 CA) to vinculin from three cases. PIC-induced inhibition of HIV expression was significant in control (Ctr) or WT IRF3-overexpressing (WT) cultures but not in DN IRF3-overexpressing (DN) cultures. Overexpression of DN IRF3 significantly reversed the antiviral effect of PIC (**, P < 0.01 by t test versus WT + PIC). (C, D) Astrocytes in 96-well plates were exposed to PIC, adenovirus, and HIV as described for panel A, and HIV Gag expression was determined by immunocytochemistry and cell-based ELISA. The number of HIV+ cells (C) and the ODs (D) (means plus SEM) are reduced by PIC, and this was significantly reversed by DN IRF3 (***, P < 0.001 by t test versus WT + PIC).
IDO as an antiviral protein.
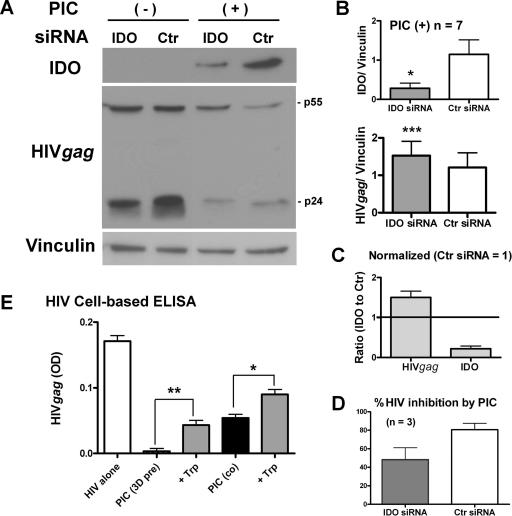
In several cell types, including human astrocytoma cell lines, IFN-γ has been shown to limit microbial proliferation via induction of IDO (2, 6, 14). Therefore, we tested whether IDO played a role in PIC-induced HIV replication using siRNA-mediated knockdown of IDO. Astrocytes were transfected with an IDO-targeting siRNA or a control nontargeting siRNA and then tested for their ability to limit HIV expression in the presence of PIC (Fig. 7). Successful knock-down of IDO in PIC-treated cultures was verified by Western blotting (representative data are shown in Fig. 7A and cumulative data in 7B). Western blot analysis also demonstrated that the PIC-induced suppression of HIV Gag was indeed reversed by IDO knockdown, albeit slightly (Fig. 7A). Pooled densitometric data from seven cases were compared by the paired t test, and the P values were 0.0019 (two-tailed) and <0.0001 (one-tailed), indicating that this reversal was significant (Fig. 7B). This reciprocal relationship between low IDO and high HIV Gag in these siRNA experiments is evident when data are normalized to the control siRNA values (Fig. 7C). The percent HIV Gag inhibition by PIC was calculated for the three cases in which matching IDO siRNA and control siRNA samples were available, and the results showed a smaller amount of HIV inhibition by IDO knockdown (48 ± 12%) than controls (80 ± 6%) (Fig. 7D). Since IDO is thought to mediate its antimicrobial activity by depleting the microorganisms of the essential amino acid tryptophan, we tested the effect of tryptophan supplementation (100 μg/ml). As shown in Fig. 7E, tryptophan caused significant reversal of the PIC-induced anti-HIV effect, whether the culture was pretreated or cotreated with PIC. Supplementation of tryptophan alone (without PIC) did not change HIV expression in these cultures (n = 3: data not shown). Taken together, these results demonstrate that IDO is one of the downstream antiviral effector proteins induced by PIC in astrocytes.
FIG. 7.
IDO siRNA partially reverses the anti-HIV effect of PIC. Astrocytes were transfected with IDO siRNA or control siRNA for 2 to 3 days as described in Materials and Methods. Cells were infected with VSV-G HIV with or without PIC for an additional 3 days. (A) Immunoblot showing the effect of IDO siRNA on IDO and HIV expression. (B and C) Pooled densitometric ratios of IDO and HIV Gag to vinculin from seven cases. (B) IDO siRNA suppressed PIC-induced IDO expression compared to control siRNA (*, P < 0.05, n = 7). In the same cases, IDO siRNA increased the expression of HIV Gag compared to control siRNA (***, P < 0.001, n = 7), indicating a significant reversal of PIC-induced anti-HIV activity by IDO knockdown. (C) IDO and HIV Gag expression in PIC-treated, IDO siRNA-treated samples (n = 7) are normalized to the levels in control (Ctr) siRNA samples, showing a reciprocal relationship. (D) Percent HIV (HIV Gag/vinculin) inhibition by PIC in three cases with matching IDO siRNA and control (Ctr) siRNA samples show a decrease in inhibition by IDO knockdown. (E) Tryptophan supplementation also reverses the anti-HIV effect of PIC. Astrocytes were pretreated with PIC for 3 days or cotreated with PIC simultaneously with HIV with or without l-tryptophan (Trp; 100 μg/ml). HIV Gag expression was determined by cell-based ELISA. Pretreatment with PIC shows a more potent antiviral effect than cotreatment. l-Tryptophan significantly reversed PIC effects in both treatment regimens (**, P < 0.01; *, P < 0.05). Data are means plus SEM and are representative of two experiments with similar results.
PIC inhibits CMV replication in astrocytes: role of IDO.
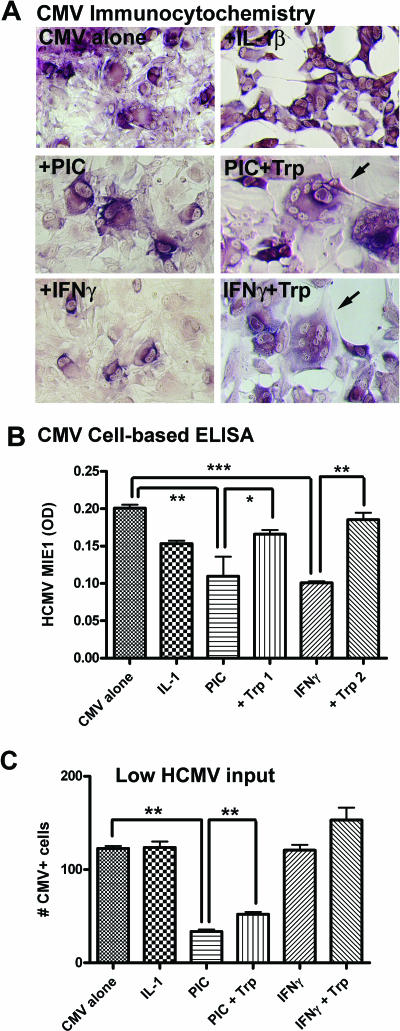
Because the innate immune response is not pathogen specific, we tested another human virus that productively infects astrocytes in vivo as a target of TLR3-induced antiviral immunity. Astrocytes were infected with HCMV (strain AD169), and the effect of PIC activation was determined by examining HCMV IE1 (72 kDa) expression by immunocytochemistry and cell-based ELISA (Fig. 8). HCMV-infected astrocytes display cytopathic effects identical to those noted in vivo, i.e., cytomegaly, intranuclear inclusions, multinucleated giant cell formation, and cytolysis (Fig. 8A). Cell-based ELISA showed that astrocyte HCMV expression was suppressed by PIC or IFN-γ, but not by IL-1, and that the anti-HCMV activity induced by PIC or IFN-γ was significantly reversed by supplementing the cultures with 100 μg/ml l-tryptophan (Fig. 8B). Tryptophan alone (without PIC or IFN-γ) did not change the level of HCMV expression in these cultures (data not shown). The results together indicate that PIC induces anti-HCMV activity in astrocytes in part dependent on IDO, similar to that shown for IFN-γ in this and other (6) studies. The effect of cytokines was also determined at very low HCMV concentrations, which resulted in approximately 1 to 3% cell infection on day 3. Using this regimen, the CMV+-cell counts demonstrated that neither IL-1 nor IFN-γ showed significant anti-HCMV activity, while the effect of PIC was highly significant (Fig. 8C). Addition of l-tryptophan resulted in a small but significant reversal of the PIC effect at a low viral input as well (Fig. 8C). These results together demonstrate that the mechanism by which PIC induces anti-HCMV activity is different from those induced by cytokines (60, 75).
FIG. 8.
PIC limits CMV replication in astrocytes: the effect of tryptophan supplementation and the role of IRF3. (A) Astrocyte cultures in 96-well plates were infected with the AD169 strain of HCMV with IL-1β (10 ng/ml), PIC (50 μg/ml), and IFN-γ (10 ng/ml) with or without 100 μg/ml of l-tryptophan (Trp). Three days later, cultures were fixed and immunostained for HCMV IE1, as described in Materials and Methods. Astrocytes infected with CMV demonstrate IE1 immunoreactivity (purple) as well as typical CMV cytopathic effects such as cytomegaly, multinucleated giant cell formation (arrows), and cytolysis (empty areas devoid of cells, as evident in IL-1β-treated cultures). (B) Cell-based ELISA demonstrates that both PIC and IFN-γ reduced CMV expression (**, P < 0.01; ***, P < 0.001), while IL-1β had no significant effect. Tryptophan significantly reversed the effect of PIC and IFN-γ (*, P < 0.05; **, P < 0.01). These results support the conclusion that IDO mediates its anti-CMV effect through tryptophan starvation. (C) Effect of cytokines at low HCMV concentrations. Astrocyte cultures in four replicate wells were infected with low-dose HCMV (∼2 μl), which resulted in 1 to 3% cell infection on day 3. CMV+-cell counts were determined by enumerating all IE1+ cells in a random 100× microscopic field per well. Data are means plus SEM from four wells. IFN-γ is no longer effective at low viral doses, while PIC is effective (ANOVA with Dunnett's test). Tryptophan partially reverses the PIC effect (P < 0.01, t test). Data are representative of two experiments with similar results.
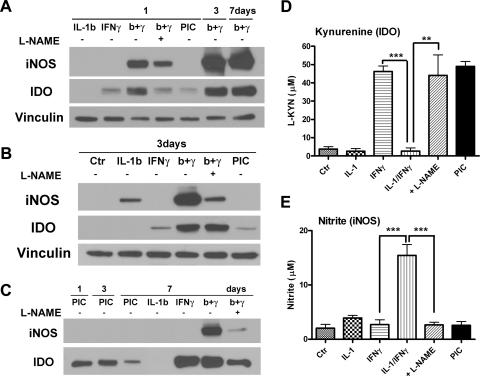
Nitric oxide (NO) inactivates IDO in astrocytes.
IDO biosynthesis is regulated at several steps. In particular, IDO expression and/or activity have been shown to be downregulated by NO in several cell culture and cell-free systems (25, 33, 52). We therefore asked whether NO generated by astrocyte iNOS can affect IDO expression or activity. Astrocyte IDO expression was measured by Western blotting and IDO activity by determining the KYN concentration in the culture supernatants as described in Materials and Methods. In addition, iNOS expression was measured by Western blot and iNOS activity by determining the nitrite concentration in the culture supernatants by the Griess reaction (45, 48). Nω-Nitro-l-arginine methyl ester (l-NAME) was used to block iNOS activity. As shown in Fig. 9A, astrocyte iNOS was induced by IL-1β/IFN-γ to a greater extent than by IL-1β and not by IFN-γ or PIC. IFN-γ-induced IDO expression was increased by IL-1β, and l-NAME decreased (or did not alter) IL-1β/IFN-γ-induced IDO expression. The IDO expression profile was similar on days 1, 3, and 7 after cell stimulation, excluding the possibility that iNOS alters IDO half-life. Thus, the results demonstrate that iNOS (NO) increases or has no effect on IDO expression in human astrocytes. This is different from the results obtained in other systems, where NO decreased IDO expression (33, 52). In contrast to protein expression, astrocyte IDO activity as determined by KYN production was markedly reduced by NO (Fig. 9D and E). IFN-γ-induced IDO activity was inhibited by IL-1, and this inhibition was reversed by l-NAME. PIC induced only KYN and not nitrite, while IL-1 induced only nitrite and not KYN. This indicates that in astrocytes, PIC and IL-1 create an IDO- and iNOS-predominant environment, respectively. Taken together, these results demonstrate that iNOS/NO inactivates IDO in human astrocytes without reducing IDO expression.
FIG. 9.
Endogenous NO inactivates IDO in cytokine-treated astrocytes. (A to C) To determine the relationship between iNOS expression and IDO expression in human astrocytes, cells were treated with cytokines (IL-1β, IFN-γ, or together) for 1 to 7 days as indicated and immunoblotted for iNOS and IDO. The results are compared with those obtained with PIC-treated astrocytes. l-NAME (NOS inhibitor) at 100 μM was added to cultures to determine the effect of NO. (D) IDO activity was also determined by measurement of KYN concentrations in day 3 culture supernatants as described in Materials and Methods. Data are means plus SEM from three cases. (E) iNOS activity was determined by measurement of nitrite in day 3 culture supernatants. Data are means plus SEM from four cases. Statistics in panels D and E are repeated-measure ANOVA with Tukey's post hoc test (**, P < 0.01; ***, P < 0.001). KYN induction by both IFN-γ and PIC was significant (P < 0.01 and P < 0.001 versus control). The results show that coinduction of iNOS by the cytokine IL-1 inactivates IDO enzyme in astrocytes without decreasing the amounts of IDO expression.
Summary and hypothesis.
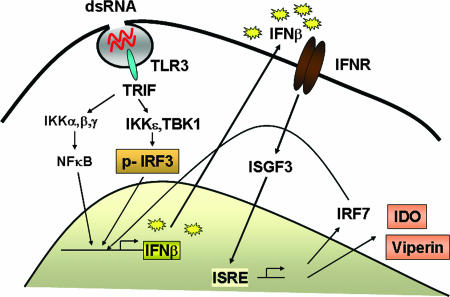
Figure 10 illustrates a schematic representation of the TLR3 signaling which results in innate antiviral responses. Ligand (PIC/dsRNA) binding to the intracellular (endosomal) TLR3 activates two critical transcription factors, NF-κB and IRF3, via the TLR IL-1 receptor domain containing the adaptor protein TRIF, thereby activating the primary IFN-β gene response (36,40). IFN-β acts in an autocrine manner to stimulate the Jak/Stat signal transduction pathway to activate the transcription of ISGs. IRF7 synergizes with IRF3 in the induction of type I (α and β) IFNs, as well as chemokines such as RANTES (46, 47, 69). In addition, IFN-β also upregulates TLR3 expression (79), demonstrating multiple mechanisms for amplification of innate antiviral immunity by dsRNA. We have identified two molecules, viperin (65) and IDO (this paper), as innate antiviral proteins that are induced by TLR3 activation in astrocytes.
FIG. 10.
Schematic representation of the TLR3 signaling leading to the antiviral immunity in astrocytes. TLR3 located in the intracellular endosomal membrane is triggered by binding with dsRNA such as PIC. TLR3, unlike other TLRs, uses the adaptor protein TLR IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) to transduce signals to activate IRF3 kinases, such as TBK1 or IKKɛ. Both NF-κB and IRF3 are necessary to transactivate the human IFN-β gene (primary response gene). Secreted IFN-β then binds to the type I IFN receptor (IFNR) in an autocrine manner and transactivates the ISGs by formation of the ISG factor 3 (ISGF3:Stat1, Stat2, and IRF9) that simulates the IFN-stimulated response element (ISRE) of the gene promoter. One of the genes induced in this manner is the transcription factor IRF7, which synergizes with IRF3 in the activation of IFN-β gene and is necessary for the activation of all human IFN-α genes, thereby establishing a positive feedback mechanism for ISG expression (46). Another positive feedback mechanism appears to include upregulation of the receptor TLR3 itself by PIC. We have identified IDO and viperin (65) as antiviral effector molecules induced by TLR3, in addition to classical IFN-induced antiviral proteins, such as dsRNA-dependent protein kinase and 2′,5′-oligoadenylate synthetase.
DISCUSSION
In this study we have shown that IDO can be induced in human fetal astrocytes by the TLR3 ligand PIC and have further demonstrated that in these cells, IDO has antiviral activity, as determined by inhibition of infection with a pseudotyped HIV and HCMV. Analysis of the signaling pathways involved demonstrated a requirement for the transcription factors NF-κB and IRF3 and the production of the primary response gene encoding IFN-β. This represents a novel pathway of IDO production, in addition to the well-established induction of this gene by IFN-γ, particularly in cells of the monocyte/macrophage series. A role for IFN-β in IDO induction was further supported by the observation that this cytokine could directly induce IDO in astrocytes, albeit only weakly. This difference between the two ligands likely reflects the potent positive feedback response of TLR3 signaling, where IRF3- and NF-κB-dependent transactivation of the human IFN-β gene (primary response) sets off a second wave of Stat1-dependent gene expression that includes IRF7 (46) (Fig. 10). IRF7 then synergizes with IRF3 in the induction of type I IFNs and chemokines (46, 47, 69). This aspect of synergism between IRF3 (TLR3-induced) and other Stat1-dependent (IFN-β-induced) genes constitutes one mechanism for the potent innate immunity elicited by TLR3. Furthermore, we show that the dsRNA receptor TLR3 itself is amplified by PIC (and other inflammatory stimuli, including IL-1), adding to the positive-feedback aspect of dsRNA signaling (22, 34, 65). Importantly, TLR3 upregulation has been observed in experimental SIV infection in vivo (68), as well as in human brains with HIVE (this study). We also show that in HIVE, astrocytes, in addition to macrophages and microglia, express IDO, a finding also reported by others (62). These results suggest that enhanced TLR3 expression during viral infection could predispose the cells and/or tissues to a greater response and that triggering TLR3 by PIC might be a viable therapeutic option for human viral infections.
Although we assumed TLR3 to be the PIC (dsRNA) receptor in astrocytes, recent studies have identified additional intracellular (cytosolic) helicases that function as dsRNA sensors, namely, retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA-5) (3, 32, 73). While we have not ruled out the role of RIG-I and MDA-5, suppression of PIC activity by inhibitors of cell acidification such as acridine orange (65) and ammonium chloride (this report) in astrocytes supports the role for TLR3. In addition, nontransfected PIC is likely to bind to the membrane receptor (TLR3) rather than the cytosolic receptor (24). PIC has also been shown to signal primarily through TLR3 in murine astrocytes and microglia in vivo (80). Further experiments with more specific molecular tools should clarify the role of each of these receptors in the astrocyte innate immune response.
Our results demonstrate that PIC-induced antiviral gene expression in astrocytes translates into antiviral activity. This functional confirmation is important because IL-1 also induces IFN-β and IFN response genes in astrocytes (64) but does not display antiviral activity, as shown for HIV (65) and HCMV (this study). The antiviral activity of PIC was also dependent on IRF3, confirming the pivotal role this transcription factor plays in the innate antiviral immune response. Furthermore, our data support the conclusion that IDO forms part of the innate host defense mechanism. To our knowledge, this is the first demonstration of the antimicrobial role of IDO against HIV using RNA interference. We find that while the effect of IDO knockdown was highly significant, the extent to which IDO contributed to the overall antiviral response was small. This is perhaps not surprising, since PIC induces other well-known antiviral proteins, such as dsRNA-dependent protein kinase and 2′,3′-oligoadenylate synthase (35, 65). Furthermore, it has been shown that PIC induces viperin/Cig5, another protein with activity against HIV and HCMV (11, 65). Together, these studies add IDO and viperin to the long list of newly discovered anti-HIV proteins. Novel anti-HIV proteins inducible by IFNs include the recently discovered apolipoprotein B mRNA-editing enzyme catalytic polypeptide 3G (APOBEC3G) (12, 55, 61).
The best known physiological ligands for TLR3 are viral dsRNA intermediates that are produced during viral infection. Positive-strand RNA viruses, as well as some negative-strand RNA and DNA viruses, have been shown to form dsRNA intermediates during the viral life cycle (21, 70, 74, 83). However, even viruses that are known to form dsRNA intermediates may not be recognized by the cellular dsRNA receptors, and others are known to inhibit IRF3 activation induced by PIC (70, 83). Furthermore, studies with mice have failed to detect a contribution of TLR3 signaling to the antiviral immune response during viral infection in vivo (21). These data suggest that viruses have developed various strategies to evade TLR-mediated innate immune responses. Indeed, we observe that while PIC induces robust amounts of IFN-β and IDO, HIV fails to induce detectable amounts of IFN-β or IDO in infected astrocytes (Fig. 7 and data not shown). Together, these findings suggest that while TLR3 is upregulated during viral infection, efficient TLR3 signaling and antiviral immunity may not take place in HIVE. Moreover, although the increase in TLR3, IFN-β, IDO, and viperin in HIVE (this study) suggests that some degree of antiviral immune response is activated in vivo (also demonstrated in studies of SIVE [5, 66]), the receptor(s)/signal transduction pathways triggered during SIVE/HIVE might be different from those triggered by PIC. For example, IL-1 can induce TLR3, IFN-β, IDO, and viperin, although it is much less potent than PIC (for example, see Fig. 4 and reference 64).
IDO expression is regulated at multiple steps by transcriptional, translational, and posttranslational mechanisms (52, 67). In several cell systems, IDO expression and activity have been shown to be inhibited by NO. For example, NO enhances proteosomal degradation of IDO, and NO donors inactivate IDO by inducing nitration of critical tyrosine residues (25, 33). Therefore, we investigated the relationship between IDO and iNOS expression in astrocytes. We first observed that in human astrocytes, IL-1 induces iNOS but very little IDO, while PIC induces IDO with virtually no iNOS. This was surprising because PIC induces robust amounts of iNOS and NO in rodent astrocytes (9, 72). Secondly, we found that IDO expression and IDO activity could be dissociated. While IL-1 increased the amount of IDO induced by IFN-γ, IL-1 inactivated IDO in an NO-dependent manner. IL-1 is an essential cytokine for human iNOS induction in several cell types (10, 26, 48); thus, these results suggest that IDO function in vivo could be severely restricted in a cytokine environment where IL-1 predominates (48, 49). Furthermore, species-dependent differences in the regulation of iNOS (for example, iNOS is not induced in human macrophages under most circumstances [18, 48]) might also influence IDO biology such that IDO may be more effective in human cells than in rodent cells (14, 52, 57).
Because the innate immune response is not pathogen specific and because astrocyte HIV infection in vivo is a minor occurrence (4, 7, 27), we tested another human virus (HCMV) that productively infects astrocytes in vitro and in vivo to test the efficacy of PIC. Human astrocytes infected with CMV in vitro essentially recapitulate the cytopathic effects of their in vivo counterparts (53). Both CMV infection and cytolysis were prevented in astrocytes treated with PIC. These results are similar to those recently reported for adult astrocytes (81). The role of IDO in PIC (and IFN-γ)-induced anti-CMV activity was demonstrated by tryptophan supplementation, confirming previous results obtained with cytokine-stimulated human retinal epithelial cells (6).
Unlike HIV, CMV has been reported to activate IRF3 as well as ISGs in infected cells (17, 82). The responses of HCMV to IFN treatment are complex. Interestingly, IFN-γ-activated sequence-like consensus sequences are present in HCMV immediate early genes that lead to the induction of viral genes in response to IFNs. These sequences are also required for HCMV growth at low viral doses (56), such as might occur during reactivation infection in vivo. Thus, IFN-γ may (paradoxically) increase HCMV replication when the viral concentration is low. Indeed, we observed that IFN-γ was not effective against HCMV at low viral concentrations (similar to IL-1), whereas PIC continued to suppress HCMV replication under these conditions (Fig. 8C). These results point to some of the important differences in the antiviral immunity generated by dsRNA and IFNs and suggest that PIC offers a unique advantage in triggering effective innate antiviral immunity for human infections.
Acknowledgments
This study was supported by NIH RO1 MH55477, Molecular Neuropathology Training grant T32 NS007098, and Einstein CFAR P30 AI051519.
We thank the Einstein Human Fetal Tissue Repository and the Manhattan HIV Brain Bank (Susan Morgello, director) for human tissues and the NIH AIDS Research and Reference Reagent Program for HIV plasmids and HCMV. We are also grateful to Mark J. Czaja for the adenoviral SR IκBα, John Hiscott for IRF3 plasmids, and Louis Weiss for providing help with the kynurenine assay.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Achim, C. L., M. P. Heyes, and C. A. Wiley. 1993. Quantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brains. J. Clin. Investig. 91:2769-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, O., K. Besken, C. Oberdorfer, C. R. MacKenzie, O. Takikawa, and W. Daubener. 2004. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J. Virol. 78:2632-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, C. E., G. S. Tomlinson, B. Pauly, F. W. Brannan, A. Chiswick, R. Brack-Werner, P. Simmonds, and J. E. Bell. 2003. Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection. Neuropathol. Appl. Neurobiol. 29:378-388. [DOI] [PubMed] [Google Scholar]
- 5.Barber, S. A., D. S. Herbst, B. T. Bullock, L. Gama, and J. E. Clements. 2004. Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J. Neurovirol. 10(Suppl. 1):15-20. [DOI] [PubMed] [Google Scholar]
- 6.Bodaghi, B., O. Goureau, D. Zipeto, L. Laurent, J. L. Virelizier, and S. Michelson. 1999. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J. Immunol. 162:957-964. [PubMed] [Google Scholar]
- 7.Brack-Werner, R. 1999. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1-22. [DOI] [PubMed] [Google Scholar]
- 8.Burudi, E. M., M. C. Marcondes, D. D. Watry, M. Zandonatti, M. A. Taffe, and H. S. Fox. 2002. Regulation of indoleamine 2,3-dioxygenase expression in simian immunodeficiency virus-infected monkey brains. J. Virol. 76:12233-12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpentier, P. A., W. S. Begolka, J. K. Olson, A. Elhofy, W. J. Karpus, and S. D. Miller. 2005. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 49:360-374. [DOI] [PubMed] [Google Scholar]
- 10.Chartrain, N. A., D. A. Geller, P. P. Koty, N. F. Sitrin, A. K. Nussler, E. P. Hoffman, T. R. Billiar, N. I. Hutchinson, and J. S. Mudgett. 1994. Molecular cloning, structure, and chromosomal localization of the human inducible nitric oxide synthase gene. J. Biol. Chem. 269:6765-6772. [PubMed] [Google Scholar]
- 11.Chin, K. C., and P. Cresswell. 2001. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc. Natl. Acad. Sci. USA 98:15125-15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu, Y. L., and W. C. Greene. 2006. Multifaceted antiviral actions of APOBEC3 cytidine deaminases. Trends Immunol. 27:291-297. [DOI] [PubMed] [Google Scholar]
- 13.Cosenza-Nashat, M. A., Q. Si, M. L. Zhao, and S. C. Lee. 2006. Modulation of astrocyte proliferation by HIV-1: differential effects in productively infected, uninfected, and Nef-expressing cells. J. Neuroimmunol. 178:87-99. [DOI] [PubMed] [Google Scholar]
- 14.Daubener, W., and C. R. MacKenzie. 1999. IFN-gamma activated indoleamine 2,3-dioxygenase activity in human cells is an antiparasitic and an antibacterial effector mechanism. Adv. Exp. Med. Biol. 467:517-524. [DOI] [PubMed] [Google Scholar]
- 15.Daubener, W., B. Spors, C. Hucke, R. Adam, M. Stins, K. S. Kim, and H. Schroten. 2001. Restriction of Toxoplasma gondii growth in human brain microvascular endothelial cells by activation of indoleamine 2,3-dioxygenase. Infect. Immun. 69:6527-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daubener, W., N. Wanagat, K. Pilz, S. Seghrouchni, H. G. Fischer, and U. Hadding. 1994. A new, simple, bioassay for human IFN-gamma. J Immunol. Methods 168:39-47. [DOI] [PubMed] [Google Scholar]
- 17.DeFilippis, V. R., B. Robinson, T. M. Keck, S. G. Hansen, J. A. Nelson, and K. J. Fruh. 2006. Interferon regulatory factor 3 is necessary for induction of antiviral genes during human cytomegalovirus infection. J. Virol. 80:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denis, M. 1994. Human monocytes/macrophages: NO or no NO? J. Leukoc. Biol. 55:682-684. [DOI] [PubMed] [Google Scholar]
- 19.Depboylu, C., T. A. Reinhart, O. Takikawa, Y. Imai, H. Maeda, H. Mitsuya, D. Rausch, L. E. Eiden, and E. Weihe. 2004. Brain virus burden and indoleamine-2,3-dioxygenase expression during lentiviral infection of rhesus monkey are concomitantly lowered by 6-chloro-2′,3′-dideoxyguanosine. Eur. J. Neurosci. 19:2997-3005. [DOI] [PubMed] [Google Scholar]
- 20.Dong, Y., and E. N. Benveniste. 2001. Immune function of astrocytes. Glia 36:180-190. [DOI] [PubMed] [Google Scholar]
- 21.Edelmann, K. H., S. Richardson-Burns, L. Alexopoulou, K. L. Tyler, R. A. Flavell, and M. B. Oldstone. 2004. Does Toll-like receptor 3 play a biological role in virus infections? Virology 322:231-238. [DOI] [PubMed] [Google Scholar]
- 22.Farina, C., M. Krumbholz, T. Giese, G. Hartmann, F. Aloisi, and E. Meinl. 2005. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J. Neuroimmunol. 159:12-19. [DOI] [PubMed] [Google Scholar]
- 23.Federico, M., Z. Percario, E. Olivetta, G. Fiorucci, C. Muratori, A. Micheli, G. Romeo, and E. Affabris. 2001. HIV-1 Nef activates STAT1 in human monocytes/macrophages through the release of soluble factors. Blood 98:2752-2761. [DOI] [PubMed] [Google Scholar]
- 24.Fensterl, V., D. Grotheer, I. Berk, S. Schlemminger, A. Vallbracht, and A. Dotzauer. 2005. Hepatitis A virus suppresses RIG-I-mediated IRF-3 activation to block induction of beta interferon. J. Virol. 79:10968-10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujigaki, H., K. Saito, F. Lin, S. Fujigaki, K. Takahashi, B. M. Martin, C. Y. Chen, J. Masuda, J. Kowalak, O. Takikawa, M. Seishima, and S. P. Markey. 2006. Nitration and inactivation of IDO by peroxynitrite. J. Immunol. 176:372-379. [DOI] [PubMed] [Google Scholar]
- 26.Geller, D. A., M. E. de Vera, D. A. Russell, R. A. Shapiro, A. K. Nussler, R. L. Simmons, and T. R. Billiar. 1995. A central role for IL-1β in the in vitro and in vivo regulation of hepatic inducible nitric oxide synthase. J. Immunol. 155:4890-4898. [PubMed] [Google Scholar]
- 27.Gonzalez-Scarano, F., and J. Martin-Garcia. 2005. The neuropathogenesis of AIDS. Nat. Rev. Immunol. 5:69-81. [DOI] [PubMed] [Google Scholar]
- 28.Grant, R. S., H. Naif, S. J. Thuruthyil, N. Nasr, T. Littlejohn, O. Takikawa, and V. Kapoor. 2000. Induction of indolamine [sic] 2,3-dioxygenase in primary human macrophages by human immunodeficiency virus type 1 is strain dependent. J. Virol. 74:4110-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guillemin, G. J., S. J. Kerr, G. A. Smythe, D. G. Smith, V. Kapoor, P. J. Armati, J. Croitoru, and B. J. Brew. 2001. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J. Neurochem. 78:842-853. [DOI] [PubMed] [Google Scholar]
- 30.Guillemin, G. J., G. Smythe, O. Takikawa, and B. J. Brew. 2005. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 49:15-23. [DOI] [PubMed] [Google Scholar]
- 31.Heyes, M. P., B. J. Brew, A. Martin, R. W. Price, A. M. Salazar, J. J. Sidtis, J. A. Yergey, M. M. Mouradian, A. E. Sadler, and J. Keilp. 1991. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann. Neurol. 29:202-209. [DOI] [PubMed] [Google Scholar]
- 32.Hiscott, J., R. Lin, P. Nakhaei, and S. Paz. 2006. MasterCARD: a priceless link to innate immunity. Trends Mol. Med. 12:53-56. [DOI] [PubMed] [Google Scholar]
- 33.Hucke, C., C. R. MacKenzie, K. D. Adjogble, O. Takikawa, and W. Daubener. 2004. Nitric oxide-mediated regulation of gamma interferon-induced bacteriostasis: inhibition and degradation of human indoleamine 2,3-dioxygenase. Infect. Immun. 72:2723-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack, C. S., N. Arbour, J. Manusow, V. Montgrain, M. Blain, E. McCrea, A. Shapiro, and J. P. Antel. 2005. TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 175:4320-4330. [DOI] [PubMed] [Google Scholar]
- 35.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 36.Jiang, Z., T. W. Mak, G. Sen, and X. Li. 2004. Toll-like receptor 3-mediated activation of NF-κB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-β. Proc. Natl. Acad. Sci. USA 101:3533-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.John, G. R., S. C. Lee, and C. F. Brosnan. 2003. Cytokines: powerful regulators of glial cell activation. Neuroscientist 9:10-22. [DOI] [PubMed] [Google Scholar]
- 38.John, G. R., S. C. Lee, X. Song, M. Rivieccio, and C. F. Brosnan. 2004. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia 49:161-176. [DOI] [PubMed] [Google Scholar]
- 39.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131-137. [DOI] [PubMed] [Google Scholar]
- 40.Kawai, T., and S. Akira. 2006. TLR signaling. Cell Death Differ. 13:816-825. [DOI] [PubMed] [Google Scholar]
- 41.Kim, M. O., Q. Si, J. N. Zhou, R. G. Pestell, C. F. Brosnan, J. Locker, and S. C. Lee. 2002. Interferon-beta activates multiple signaling cascades in primary human microglia. J. Neurochem. 81:1361-1371. [DOI] [PubMed] [Google Scholar]
- 42.Lee, S. C., M. A. Cosenza, Q. Si, M. Rivieccio, and C. F. Brosnan. 2005. The CNS: cells, tissues and reactions to insult, p. 1-22. In R. M. Ransohoff and E. N. Benveniste (ed.), Cytokines and the CNS. CRC Press, Boca Raton, FL.
- 43.Lee, S. C., D. W. Dickson, W. Liu, and C. F. Brosnan. 1993. Induction of nitric oxide synthase activity in human astrocytes by IL-1β and IFN-γ. J. Neuroimmunol. 46:19-24. [DOI] [PubMed] [Google Scholar]
- 44.Lee, S. C., W. Liu, C. F. Brosnan, and D. W. Dickson. 1992. Characterization of human fetal dissociated CNS cultures with an emphasis on microglia. Lab. Investig. 67:465-475. [PubMed] [Google Scholar]
- 45.Lee, S. C., W. Liu, D. W. Dickson, C. F. Brosnan, and J. W. Berman. 1993. Cytokine production by human fetal microglia and astrocytes: differential induction by LPS and IL-1β. J. Immunol. 150:2659-2667. [PubMed] [Google Scholar]
- 46.Levy, D. E., I. Marie, E. Smith, and A. Prakash. 2002. Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback. J. Interferon Cytokine Res. 22:87-93. [DOI] [PubMed] [Google Scholar]
- 47.Lin, R., C. Heylbroeck, P. Genin, P. M. Pitha, and J. Hiscott. 1999. Essential role of interferon-regulatory factor 3 in direct activation of RANTES chemokine transcription. Mol. Cell. Biol. 19:959-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu, J., M.-L. Zhao, C. F. Brosnan, and S. C. Lee. 1996. Expression of type II nitric oxide synthase in primary human astrocytes and microglia: role of IL-1β and IL-1 receptor antagonist. J. Immunol. 157:3569-3576. [PubMed] [Google Scholar]
- 49.Liu, J. S. H., T. Amaral, C. F. Brosnan, and S. C. Lee. 1998. Interferons are critical regulators of IL-1b and IL-1ra expression in human fetal microglia. J. Immunol. 161:1989-1996. [PubMed] [Google Scholar]
- 50.Mahanonda, R., N. Sa-Ard-Iam, P. Montreekachon, A. Pimkhaokham, K. Yongvanichit, M. M. Fukuda, and S. Pichyangkul. 2007. IL-8 and IDO expression by human gingival fibroblasts via TLRs. J. Immunol. 178:1151-1157. [DOI] [PubMed] [Google Scholar]
- 51.Mellor, A. 2005. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem. Biophys. Res. Commun. 338:20-24. [DOI] [PubMed] [Google Scholar]
- 52.Mellor, A. L., and D. H. Munn. 2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4:762-774. [DOI] [PubMed] [Google Scholar]
- 53.Morgello, S., E.-S. Cho, S. Nielsen, O. Devinsky, and C. K. Petito. 1987. Cytomegalovirus encephalitis in patients with acquired immunodeficiency syndrome: an autopsy study of 30 cases and a review of the literature. Hum. Pathol. 18:289-297. [DOI] [PubMed] [Google Scholar]
- 54.Munn, D. H. 2006. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr. Opin. Immunol. 18:220-225. [DOI] [PubMed] [Google Scholar]
- 55.Navarro, F., and N. R. Landau. 2004. Recent insights into HIV-1 Vif. Curr. Opin. Immunol. 16:477-482. [DOI] [PubMed] [Google Scholar]
- 56.Netterwald, J., S. Yang, W. Wang, S. Ghanny, M. Cody, P. Soteropoulos, B. Tian, W. Dunn, F. Liu, and H. Zhu. 2005. Two gamma interferon-activated site-like elements in the human cytomegalovirus major immediate-early promoter/enhancer are important for viral replication. J. Virol. 79:5035-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oberdorfer, C., O. Adams, C. R. MacKenzie, C. J. de Groot, and W. Daubener. 2003. Role of IDO activation in anti-microbial defense in human native astrocytes. Adv. Exp. Med. Biol. 527:15-26. [DOI] [PubMed] [Google Scholar]
- 58.Obojes, K., O. Andres, K. S. Kim, W. Daubener, and J. Schneider-Schaulies. 2005. Indoleamine 2,3-dioxygenase mediates cell type-specific anti-measles virus activity of gamma interferon. J. Virol. 79:7768-7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park, C., S. Lee, I. H. Cho, H. K. Lee, D. Kim, S. Y. Choi, S. B. Oh, K. Park, J. S. Kim, and S. J. Lee. 2005. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia 53:248-256. [DOI] [PubMed] [Google Scholar]
- 60.Paulus, C., S. Krauss, and M. Nevels. 2006. A human cytomegalovirus antagonist of type I IFN-dependent signal transducer and activator of transcription signaling. Proc. Natl. Acad. Sci. USA 103:3840-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng, G., K. J. Lei, W. Jin, T. Greenwell-Wild, and S. M. Wahl. 2006. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J. Exp. Med. 203:41-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potula, R., L. Poluektova, B. Knipe, J. Chrastil, D. Heilman, H. Dou, O. Takikawa, D. H. Munn, H. E. Gendelman, and Y. Persidsky. 2005. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in animal model of HIV-1 encephalitis. Blood 106:2382-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rice, J., R. Connor, S. Worgall, J. P. Moore, P. L. Leopold, R. J. Kaner, and R. G. Crystal. 2002. Inhibition of HIV-1 replication in alveolar macrophages by adenovirus gene transfer vectors. Am. J. Respir. Cell Mol. Biol. 27:214-219. [DOI] [PubMed] [Google Scholar]
- 64.Rivieccio, M. A., G. R. John, X. Song, H. S. Suh, Y. Zhao, S. C. Lee, and C. F. Brosnan. 2005. The cytokine IL-1β activates IFN response factor 3 in human fetal astrocytes in culture. J. Immunol. 174:3719-3726. [DOI] [PubMed] [Google Scholar]
- 65.Rivieccio, M. A., H. S. Suh, Y. Zhao, M. L. Zhao, K. C. Chin, S. C. Lee, and C. F. Brosnan. 2006. TLR3 ligation activates an antiviral response in human fetal astrocytes: a role for viperin/cig5. J. Immunol. 177:4735-4741. [DOI] [PubMed] [Google Scholar]
- 66.Roberts, E. S., M. A. Zandonatti, D. D. Watry, L. J. Madden, S. J. Henriksen, M. A. Taffe, and H. S. Fox. 2003. Induction of pathogenic sets of genes in macrophages and neurons in neuroAIDS. Am. J. Pathol. 162:2041-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson, C. M., P. T. Hale, and J. M. Carlin. 2005. The role of IFN-γ and TNF-α-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J. Interferon Cytokine Res. 25:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanghavi, S. K., and T. A. Reinhart. 2005. Increased expression of TLR3 in lymph nodes during simian immunodeficiency virus infection: implications for inflammation and immunodeficiency. J. Immunol. 175:5314-5323. [DOI] [PubMed] [Google Scholar]
- 69.Schafer, S. L., R. Lin, P. A. Moore, J. Hiscott, and P. M. Pitha. 1998. Regulation of type I interferon gene expression by interferon regulatory factor-3. J. Biol. Chem. 273:2714-2720. [DOI] [PubMed] [Google Scholar]
- 70.Scholle, F., and P. W. Mason. 2005. West Nile virus replication interferes with both poly(I:C)-induced interferon gene transcription and response to interferon treatment. Virology 342:77-87. [DOI] [PubMed] [Google Scholar]
- 71.Schwarcz, R. 1993. Metabolism and function of brain kynurenines. Biochem. Soc. Trans. 21:77-82. [DOI] [PubMed] [Google Scholar]
- 72.Scumpia, P. O., K. M. Kelly, W. H. Reeves, and B. R. Stevens. 2005. Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes. Glia 52:153-162. [DOI] [PubMed] [Google Scholar]
- 73.Sen, G. C., and S. N. Sarkar. 2005. Hitching RIG to action. Nat. Immunol. 6:1074-1076. [DOI] [PubMed] [Google Scholar]
- 74.Sharma, S., B. R. TenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 75.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J. Clin. Investig. 100:3154-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suh, H. S., M. O. Kim, and S. C. Lee. 2005. Inhibition of GM-CSF signaling and microglial proliferation by anti-CD45RO: role of Hck tyrosine kinase and PI3K/Akt. J. Immunol. 174:2712-2719. [DOI] [PubMed] [Google Scholar]
- 77.Takikawa, O. 2005. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated l-tryptophan metabolism. Biochem. Biophys. Res. Commun. 338:12-19. [DOI] [PubMed] [Google Scholar]
- 78.Takikawa, O., T. Kuroiwa, F. Yamazaki, and R. Kido. 1988. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J. Biol. Chem. 263:2041-2048. [PubMed] [Google Scholar]
- 79.Tanabe, M., M. Kurita-Taniguchi, K. Takeuchi, M. Takeda, M. Ayata, H. Ogura, M. Matsumoto, and T. Seya. 2003. Mechanism of up-regulation of human Toll-like receptor 3 secondary to infection of measles virus-attenuated strains. Biochem. Biophys. Res. Commun. 311:39-48. [DOI] [PubMed] [Google Scholar]
- 80.Town, T., D. Jeng, L. Alexopoulou, J. Tan, and R. A. Flavell. 2006. Microglia recognize double-stranded RNA via TLR3. J. Immunol. 176:3804-3812. [DOI] [PubMed] [Google Scholar]
- 81.van den Pol, A. N., M. D. Robek, P. K. Ghosh, K. Ozduman, P. Bandi, M. D. Whim, and G. Wollmann. 2007. Cytomegalovirus induces interferon-stimulated gene expression and is attenuated by interferon in the developing brain. J. Virol. 81:332-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang, S., J. Netterwald, W. Wang, and H. Zhu. 2005. Characterization of the elements and proteins responsible for interferon-stimulated gene induction by human cytomegalovirus. J. Virol. 79:5027-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou, H., and S. Perlman. 2007. Mouse hepatitis virus does not induce beta interferon synthesis and does not inhibit its induction by double-stranded RNA. J. Virol. 81:568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]