Abstract
Lentiviruses, including human immunodeficiency virus type 1 (HIV-1), typically encode fusion glycoproteins with long cytoplasmic tails (CTs). We previously reported that immature HIV-1 particles are inhibited for fusion with target cells by a mechanism requiring the 152-amino-acid CT of gp41. The gp41 CT was also shown to mediate the detergent-resistant association of the HIV-1 envelope glycoprotein complex with immature HIV-1 particles, indicating that the gp41 CT forms a stable complex with Gag in immature virions. In the present study, we analyzed the effects of progressive truncations and point mutations in the gp41 CT on the fusion of mature and immature HIV-1 particles with target cells. We also determined the effects of these mutations on the detergent-resistant association of gp41 with immature HIV-1 particles. Removal of the C-terminal 28 amino acids relieved the dependence of HIV-1 fusion on maturation. However, a mutant Env protein lacking this region remained associated with immature HIV-1 particles treated with nonionic detergent. Further mutational analysis of the C-terminal region of gp41 revealed two specific sequences required for maturation-dependent HIV-1 fusion. Collectively, our results demonstrate that the extreme C terminus of gp41 plays a key role in coupling HIV-1 fusion competence to virion maturation. They further indicate that the stable association of gp41 with Gag in immature virions is not sufficient for inhibition of immature HIV-1 particle fusion.
Human immunodeficiency virus type 1 (HIV-1) virions assemble and bud from the plasma membrane of infected cells as immature particles that must undergo proteolytic maturation to become infectious (14, 15). During budding, the viral protease (PR) cleaves the Gag structural polyprotein precursor into MA (matrix), CA (capsid), NC (nucleocapsid), and p6 proteins (30). These proteins rearrange to form the mature conical core with a metastable capsid shell that is required for completion of the early phase of the virus life cycle in the target cell (reviewed in reference 33). Particles that are arrested in various stages of maturation are noninfectious, most likely due to impaired uncoating in target cells (36).
HIV-1 entry into target cells is mediated by interactions of viral Env proteins with CD4 and chemokine coreceptors on target cells. Binding to CD4 induces exposure of a coreceptor binding site on gp120, triggering conformational changes in the transmembrane protein gp41 leading to membrane fusion (reviewed in reference 23). In addition to the gp41 ectodomains that catalyze fusion, gp41 also possesses a 150-amino-acid cytoplasmic tail (CT). The CTs of lentiviral transmembrane fusion proteins are significantly longer than those of other retroviruses (16), suggesting that this domain has one or more functions specific to lentivirus replication or persistence.
Although no high-resolution structural data are available for the gp41 CT, this region contains several predicted structural features, including the following: three predicted α-helical regions, designated “lentivirus lytic peptides” (LLP-1, LLP-2, and LLP-3) because of their cytolytic effects on cell membranes (24, 25, 42); a membrane-proximal tyrosine-based endocytic motif, Y710XXL (21, 35); a palmitylated Cys at position 762, implicated in Env protein association with lipid rafts (3, 43); a C-terminal calmodulin-binding dileucine endocytic motif (7, 32, 40); and a diaromatic motif, Y802W803, required for association with the cellular protein TIP47 (4, 22).
The CT of HIV-1 gp41 appears to serve several functions in the virus life cycle. Most notably, the CT is required for Env incorporation during assembly of HIV-1 particles in a cell-type-dependent manner (11-13). Particle incorporation of Env appears to involve interactions between Gag and the gp41 CT, as indicated by several lines of evidence. First, Env promotes the selective budding of HIV-1 particles from the basolateral surfaces of polarized epithelial cells by a mechanism that requires the CT (21, 29). Second, incorporation of Env into HIV-1 particles is inhibited by mutations in the CT, and this defect was rescued by a mutation in the MA region of Gag (27). Evidence has also been reported for exposure of a proximal region of the gp41 CT on the outside of the viral/cellular membrane, where it forms a neutralization-sensitive epitope (9). More recently, a ternary interaction between the gp41 CT, the cellular protein TIP47, and the Gag polyprotein has been implicated in HIV-1 Env trafficking and particle incorporation (4, 22).
We have reported biochemical evidence for a stable interaction between Pr55Gag and the cytoplasmic tail of gp41 in immature HIV-1 particles (39). Both gp120 and gp41 remained associated with immature (i.e., protease-defective) virions after treatment with a nonionic detergent. This association was dependent on the gp41 CT, suggesting that the CT is tightly associated with Gag in immature HIV-1 particles. Subsequently, we and others showed that immature HIV-1 particles are impaired for fusion with target cells by a mechanism requiring the gp41 CT (26, 38). Based on these findings, we concluded that fusion of HIV-1, mediated by gp41 on the viral surface, is inhibited by an interaction of the gp41 CT with Gag within immature virions (38).
In the present study, we tested the hypothesis that the association of gp41 with Gag is responsible for the impaired fusion of immature particles. We analyzed the effects of truncations and point mutations in the gp41 CT on fusion of mature and immature particles and on the association of Env proteins with immature HIV-1 particles following treatment with nonionic detergent. The results revealed that a distal C-terminal region of gp41 is required for repressing the fusion activity of immature HIV-1 particles. They further indicate that the detergent-resistant association of Env with immature HIV-1 particles is not sufficient for coupling HIV-1 fusion to maturation.
MATERIALS AND METHODS
Cells and culture conditions.
293T and P4 cells were cultured at 37°C in 5% CO2 in Dulbecco's modification of Eagle's medium (Cellgro) supplemented with fetal bovine serum (10% [vol/vol]), penicillin (50 IU/ml), and streptomycin (50 μg/ml). SupT1 cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum and penicillin plus streptomycin.
HIV-1 proviral constructs.
Wild-type HIV-1 particles were generated from the pNL4-3 clone, which carries full-length open reading frames for all HIV-1 genes. pNL-CTdel144-2, encoding an Env protein lacking the C-terminal 144 amino acids, was previously described (13). The pNL-MA/p6 clone produces immature HIV-1 particles containing mutations inactivating all of the known Gag cleavage sites and was previously described (38). To construct novel mutations in the gp41 CT, PCR products were generated by segment overlap PCR using primers containing the desired mutations. Primer sequences for the truncation mutants are shown in Table 1. The products were digested with restriction enzymes and used to replace the corresponding NheI-XhoI or BamHI-XhoI restriction fragment in pNL4-3 and pNL-MA/p6 to generate mutant proviruses. pNL-CT42 was transferred using a HpaI-XhoI fragment. A series of triple alanine substitutions spanning V810 to L854 in the C terminus of the gp41 CT were created; the primer sequences are shown in Table 2. In addition to these coding changes, silent mutations were also included so as to generate a novel PstI restriction site to facilitate identification of mutant clones. All constructs were confirmed by DNA sequencing. MA/p6 mutant viruses containing Env mutations were created by transfer of the BssHI-EcoRI fragment from pNL-MA/p6 into the pNL4-3-based mutant viruses. pNL4-3.Env− and pNL4-3.MA/p6.Env− were constructed by replacing the 2.6-kbp SalI-BamHI fragment with the corresponding fragment from R9.Env− (2).
TABLE 1.
Primers used for construction of gp41 CT truncation mutants
| CT mutation | Primer sequencea |
|---|---|
| CT126 | (S) 5′-AAGGTGGTTAACGAGACTGAGACAG |
| (A) 5′-GTCTCGTTAACCACCTTCTTCTTCTATTCC | |
| CT104 | (S) 5′-GAACGGATCCTAGGCATAAATCTGGG |
| (A) 5′-TTTATGCCTAGGATCCGTTCACTAATCG | |
| CT93 | (S) 5′-ATCTGCGGAGCCTGTGAGCTCTCAGCTACC |
| (A) 5′-TGAGAGCTCACAGGCTCCGCAGATCGTCCC | |
| CT90 | (S) 5′-CTCTTCTAGAACCACCGCTTGAGAGAC |
| (A) 5′-GCGGTGGTTCTAGAAGAGGCACAGGCTCCG | |
| CT66 | (S) 5′-GTGGTAAGCTTTCAAATATTGGTGG |
| (A) 5′-CCACCAATATTTGAAAGCTTACCACC | |
| CT59 | (S) 5′-TATTGGTGGTAGATCTTACAGTATTGG |
| (A) 5′-ATACTGTAAGATCTACCACCAATATTTG | |
| CT49 | (S) 5′-GGAACTATGATATCGTGCTGTTAACTTGC |
| (A) 5′-CAGCACGATATCATAGTTCCTGACCCA | |
| CT42b | (S) 5′-TGCTGTTAACTAGTTAAATGCCACAGCC |
| CT28 | (S) 5′-ACAGATCGCTAGCTAGAAGTATTACAA |
| (A) 5′-TACTTCTAGCTAGCGATCTGTCCCCTC |
S, sense primer; A, antisense primer.
A single mutagenic primer was used for this mutant (see Materials and Methods).
TABLE 2.
Primers used for construction of alanine-scanning CT mutants
| Mutation | Primer sequencea |
|---|---|
| VNL812AAA | (S) 5′-GAAAGGGCTGCAGCCTAAGATGGGTGGCAAGTGG |
| (A) 5′-ATCTTAGGCTGCAGCCCTTTCCAAGCCCTGTCTTATTC | |
| LN814AA | (S) 5′-CAGGGCGCTGCAGCGATTTTGCTATAAGATGGGTGG |
| (A) 5′-GCAAAATCGCTGCAGCGCCCTGTCTTATTCTTCTAGG | |
| TAI818AAA | (S) 5′-AGAATAGCTGCAGCCTTGGAAAGGATTTTGCTATAAG |
| (A) 5′-TTCCAAGGCTGCAGCTATTCTTCTAGGTATGTGGCG | |
| V820A | (S) 5′-ATACCTGCTGCAGCCAGACAGGGCTTGGAAAGGATT |
| (A) 5′-CTGTCTGGCTGCAGCAGGTATGTGGCGAATAGCTC | |
| EGT824AAA | (S) 5′-ATTCGCGCTGCAGCCAGAAGAATAAGACAGGGCTTGG |
| (A) 5′-TCTTCTGGCTGCAGCGCGAATAGCTCTATAAGCTGC | |
| DRV827AAA | (S) 5′-TATAGAGCTGCAGCCCACATACCTAGAAGAATAAGAC |
| (A) 5′-ATGTGGGCTGCAGCTCTATAAGCTGCTTGTAATAC | |
| IEV830AAA | (S) 5′-CAAGCAGCTGCAGCCGCTATTCGCCACATACCTAG |
| (A) 5′-AATAGCGGCTGCAGCTGCTTGTAATACTTCTATAACC | |
| LQ832AA | (S) 5′-GAAGTAGCTGCAGCGGCTTATAGAGCTATTCGCC |
| (A) 5′-ATAAGCCGCTGCAGCACTTCTATAACCCTATCTGTCC | |
| YR836AA | (S) 5′-AGGGTTGCTGCAGCCTTACAAGCAGCTTATAGAGC |
| (A) 5′-TTGTAAGGCTGCAGCAACCCTATCTGTCCCCTCAGC | |
| IR839AA | (S) 5′-GGGACAGCTGCAGCGATAGAAGTATTACAAGCAGC |
| (A) 5′-TCTATCGCTGCAGCTGTCCCCTCAGCTACTGCTATGG | |
| HIP842AAA | (S) 5′-GTAGCTGCTGCAGCCGATAGGGTTATAGAAGTATTAC |
| (A) 5′-CCTATCGGCTGCAGCAGCTACTGCTATGGCTGTGGC | |
| RRI845AAA | (S) 5′-GCCATAGCTGCAGCCGAGGGGACAGATAGGGTTATAG |
| (A) 5′-CCCCTCGGCTGCAGCTATGGCTGTGGCATTGAGC | |
| RQG848AAA | (S) 5′-AATGCCGCTGCAGCGGCAGTAGCTGAGGGGACAG |
| (A) 5′-GGCTGTGGCATTGAGCAAGTTAACAGCACTATTCTT | |
| LER851AAA | (S) 5′-AACTTGGCTGCAGCCACAGCCATAGCAGTAGCTGAGG |
| (A) 5′-GGCTGTGGCATTGAGCAAGTTAACAGCACTATTCTT | |
| ILL854AAA | (S) 5′-AGTGCTGCTGCAGCACTCAATGCCACAGCCATAGC |
| (A) 5′-ATTGAGTGCTGCAGCAGCACTATTCTTTAGTTCC |
S, sense primer; A, antisense primer.
Production of virus stocks.
To produce HIV-1 reporter viruses for quantifying virus-cell fusion, we transfected 293T cells in 100-mm-diameter dishes with 20 μg of proviral DNA and 7 μg of pMM310, encoding a BlaM-Vpr fusion protein, as previously described (38). Culture supernatants were harvested 36 to 48 h after transfection and clarified through 0.45-μm-pore-size syringe filters, and aliquots were buffered with HEPES (10 mM) prior to storage at −80°C. Samples were assayed for p24 by enzyme-linked immunosorbent assay (ELISA) (34) after incubation for 10 min at 95°C in the presence of 1% sodium dodecyl sulfate (SDS) to disrupt immature particles. Samples were subsequently diluted by at least 100-fold prior to assay to prevent SDS interference with antigen-antibody interactions in the ELISA.
Virus-cell fusion assay.
The HIV-1 virus-cell fusion assay was performed using BlaM-Vpr-bearing HIV-1 particles and SupT1 target cells as previously reported (18). All of the experiments were performed with Env-defective particles in parallel as a control to quantify the background in the assay. In this way, the potential for artifacts resulting from low signal/noise ratios was minimized. For quantitative comparison of fusion between viruses, the fusion was expressed as a function of the amount of p24 in the respective inoculum. All of the fusion assays were performed using a range of virus dilutions to obtain signals at which the assay was not saturated. For each virus, the value used in the calculation of fusion efficiency was obtained from the maximal virus dose that gave a fusion signal that was within the linear range of the titration curve. These values were obtained from duplicate samples (which were normally within 10% of one another), and signals obtained with Env-defective virus were subtracted from the results to determine the specific fusion efficiencies, which were then used to calculate the ratio of mature to immature fusion efficiency. The results from several experiments were used to calculate means and standard deviations for the ratios of the mature and immature HIV-1 fusion efficiencies.
Immunoblotting of viral lysates and cell lysates.
Immunoblotting of virus and cell lysates was performed as previously described (10). Briefly, virus stocks were normalized by p24 ELISA and pelleted through a cushion of 20% sucrose in STE buffer in a Beckman TLA-55 rotor at 45,000 rpm for 30 min and the supernatants removed by aspiration. Proviral DNA-transfected cells were lysed with NP-40 at a final concentration of 0.5% in STE buffer for 30 min on ice and centrifuged to remove nuclei. The protein concentrations were measured in a bicinchoninic acid assay (Pierce). The viral pellets and cell lysates were dissolved in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, heated at 95°C for 5 min, and subjected to electrophoresis onto 4 to 20% polyacrylamide gels containing SDS (Bio-Rad). Proteins were transferred to polyvinylidene difluoride, and the blots were probed with rabbit antibody to CypA (from Louis Henderson) and mouse monoclonal antibodies to CA (from hybridoma 183-H12-5C; obtained via the NIH AIDS Research and Reference Reagent Program) and BlaM (QED Bioscience Inc.). The gp41 protein was detected with human monoclonal antibody 246-D, which recognizes the gp41 ectodomain (NIH AIDS Research and Reference Reagent Program). Protein bands were detected using the LI-COR Odyssey imaging system after probing with the appropriate infrared dye-conjugated secondary antibodies. Protein bands were visualized using the Odyssey infrared imaging system (LI-COR) and quantified using the instrument software. Background signals were determined from a uniform area near the relevant protein band and were subtracted manually.
Treatment of immature HIV-1 particles with nonionic detergent.
Immature cores were isolated as previously described (39). The filtered supernatants (60 ml) from transfected 293T cells were pelleted through a cushion of 20% sucrose in STE (10 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1 mM EDTA). Pellets were dissolved in a buffer containing 0.5% Triton X-100 at either 4° or 37°C and subsequently repelleted by centrifugation for 30 min in a Beckman TLA-55 rotor at 45,000 rpm. The pellets were dissolved in a total volume of 30 μl of SDS-PAGE sample buffer, and the proteins were analyzed by immunoblotting.
β-Lactamase (BlaM) assay.
To control for potential variations in BlaM levels in virus stocks, a correction factor for the fusion assay was generated by calculating the relative BlaM/p24 ratio in the pelleted virions. For quantitative comparison of BlaM activity between viruses, virus particles were concentrated by centrifugation through a cushion of 20% sucrose in STE buffer in a Beckman TLA-55 rotor at 45,000 rpm for 30 min. The viral pellets were lysed in phosphate-buffered saline buffer containing 0.01% Triton X-100, and the fluorescent BlaM substrate CCF2-FA (Invitrogen) was added to a final concentration of 0.8 μM. Reaction mixtures were incubated for 14 h at room temperature. Fluorescence was measured at 447 and 520 nm in a microplate fluorometer (BMG Fluostar). The β-lactamase activity levels in the samples were normalized for p24 content following ELISA quantification of p24 in the actual samples. The resulting values were then used to normalize the fusion signals.
RESULTS
Design and construction of CT truncations.
Immature HIV-1 particles are repressed for fusion with target cells by a mechanism that depends on the gp41 CT. To identify regions of the gp41 CT required for inhibition of immature HIV-1 fusion, we engineered proviruses encoding gp41 proteins truncated in the C-terminal region of Env in the full-length HIV-1 clone pNL4-3. The mutations were designed to progressively remove the reported conserved domains or motifs including the tyrosine-based sorting signal (Y710XXL; numbering based on the HIV-1NL4-3 provirus sequence), Cys762, three amphipathic helical segments (LLPs), and two inhibitory sequences (is1 and is2). The locations of the mutations are depicted in Fig. 1. To determine the effects of the mutations on the fusion of immature HIV-1 particles, we also transferred these mutations into the pNL-MA/p6 clone (38), which produces immature HIV-1 particles due to the presence of mutations at the Gag cleavage sites, which block cleavage of the protein by the viral PR. This approach was taken because of the requirement for active PR in the BlaM-Vpr virus-cell fusion assay (38).
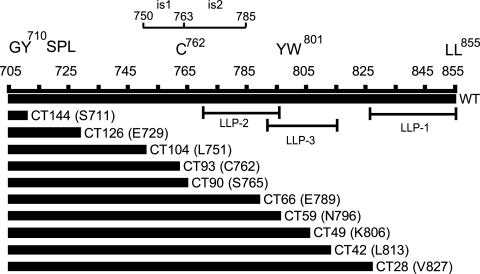
FIG. 1.
Construction of HIV-1 cytoplasmic tail truncation mutants. The mutants were named according to the number of amino acids removed from the C terminus of gp41. The codon that was altered is shown in parentheses. For example, CT28 refers to a loss of 28 amino acids resulting from the replacement of V827 with a stop codon. Regions corresponding to known and predicted functional sequence elements are also indicated in the diagram.
Truncation of the gp41 CT affects Env expression, incorporation, and stability.
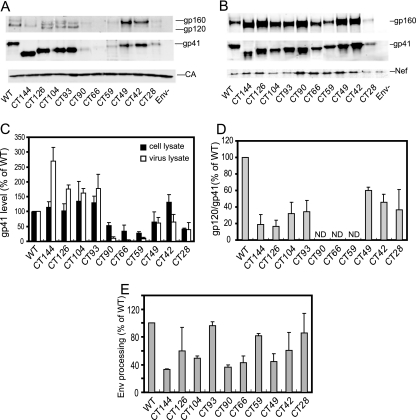
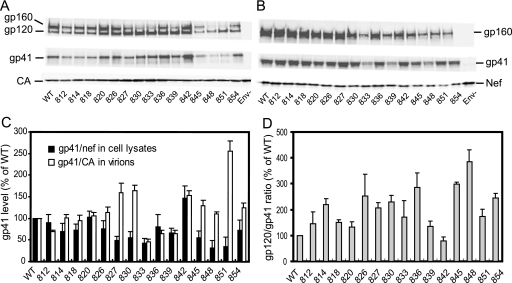
Previous studies have indicated that truncations of gp41 CT can lead to impaired incorporation of Env; however, removal of the entire CT can be tolerated when the virus is produced in a few cell lines, such as 293T, HeLa, and MT-4 (28). We therefore sought to more precisely localize regions of the CT that modulate Env incorporation. For Env expression and particle incorporation studies, the CT mutant proviral clones were transfected into 293T cells, and the resulting virus particles and cell lysates were analyzed by immunoblotting for gp41 and CA (Fig. 2). Band intensities were determined directly from the integrated signal intensities using the LI-COR Odyssey system, thereby avoiding the pitfalls associated with chemiluminescent detection and imaging with film. For analysis of Env expression, the Env glycoprotein (gp160 plus gp41) signals in cell lysates were normalized by the Nef band to control for transfection efficiency (Fig. 2C). Likewise, the virion-associated Env levels were determined by normalizing the gp41 intensities versus the CA intensities in virus lysates (Fig. 2C). Overall, the mutants constituted three classes. Mutants CT144, CT126, CT104, and CT93 exhibited roughly normal Env levels in cell lysates, and the levels of virion-associated gp41 were greater than or equal to that of the wild type. The second group, including CT90, CT66, and CT59, exhibited reduced Env expression (i.e., 30 to 50% of wild type) and low levels of particle-associated gp41 (5 to 15% of wild type). In the third group, which included mutants CT49, CT42, and CT28, Env expression and incorporation were only moderately affected (i.e., 40 to 140% of wild type). CT49 and CT42 exhibited normal levels of particle-associated gp41, while that of CT28 was moderately reduced (40%). Furthermore, comparison of the particle-associated gp120 and gp41 levels revealed that all truncated Env proteins (except CT90, CT66, and CT42, for which the gp120/gp41 ratio could not be accurately determined due to low levels of virion-associated Env) exhibited a reduced gp120/gp41 ratio relative to that of the wild type (Fig. 2D), suggesting that the CT regulates the stability of the gp41-gp120 association. Analysis of the gp160 and gp41 levels in cell lysates also revealed Env processing defects for several of the mutants, including CT144, CT104, CT90, CT66, and CT49 (Fig. 2E), suggesting that the gp41 cytoplasmic tail contains determinants important for efficient processing of gp160 to gp120 and gp41.
FIG. 2.
Analysis of the effects of the CT truncation mutations on Env expression, processing, and incorporation into particles. Provirus-transfected 293T cells were harvested, and virions were pelleted from the culture supernatants. (A) Samples of the indicated virus lysates were diluted to equivalent p24 concentrations and analyzed by SDS-PAGE and immunoblotting. (B) Cell lysates were normalized for total protein. Immunoblots were probed with antibodies to the indicated proteins. Band intensities were integrated with the LI-COR Odyssey software. (C) The efficiency of Env expression was calculated as the ratio of gp41/Nef in cell lysates; Env incorporation was quantified as the gp41/CA ratio in viral lysates. Values are expressed as a percentage of those corresponding to the wild-type virus. (D) The ratio of the gp120 to gp41 band signals in cell lysates was calculated and expressed as a percentage of that of wild-type HIV-1. Results shown are representative of three independent experiments. ND, not determined. (E) The extent of Env processing was determined as the ratio of gp41 to the sum of gp160 plus gp41 band intensities in cell lysates and was expressed as a percentage of the value obtained for wild-type Env. Shown are the mean values and standard deviations obtained from three independent experiments.
Identification of a gp41 CT domain regulating fusion of immature HIV-1 particles.
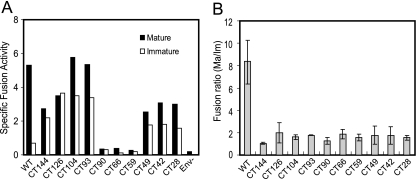
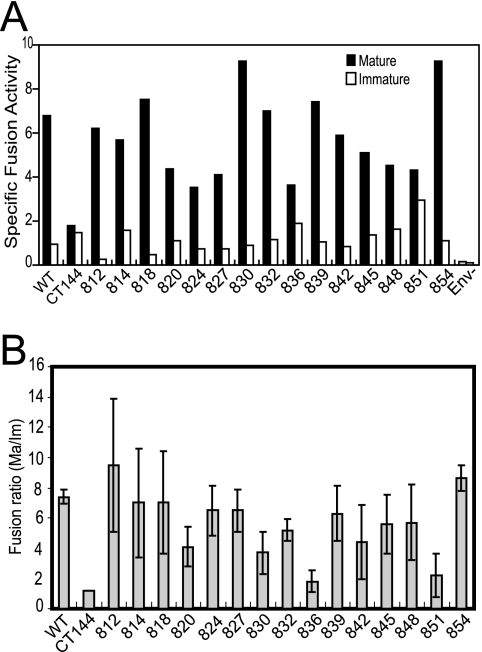
Using a specific assay of HIV-1 virus-cell fusion, we previously demonstrated that the impaired fusion capacity of immature HIV-1 particles is due to a repressive activity of the gp41 CT (38). To determine the role of conserved gp41 CT sequences in fusion repression, we tested a panel of mature and immature HIV-1 particles bearing the truncated Env proteins for fusion with target cells. The fusion signals were normalized for virus input (by p24 ELISA) and for virion-associated BlaM activity to control for variations in BlaM incorporation. Although the poor Env incorporation in some of the mutant viruses resulted in lower fusion signals, the results revealed that the fusion of HIV-1 particles bearing truncated Env proteins was less dependent on maturation than particles with full-length Env (Fig. 3). The dependencies of the fusion of each the CT mutant viruses on maturation were only slightly greater than that of the CT144 mutant (Fig. 3B), which lacks all but six cytoplasmic amino acids and whose fusion is essentially independent of maturation (26, 38). The CT28 mutant removed only 28 residues from the C terminus of gp41, resulting in the loss of the sequence corresponding to LLP-1. These results thus implicated sequences within LLP-1 in the maturation-dependent regulation of HIV-1 fusion.
FIG. 3.
Analysis of mature and immature virus particles encoding truncated Env proteins for the ability to fuse with cells. (A) BlaM-containing mature and immature HIV-1 particles were assayed for fusion with SupT1 cells. The relative fusion signals were normalized by p24 and BlaM activity as described in Materials and Methods. (B) Ratio of fusion efficiency of mature to immature virus particles. Shown are the mean values and standard deviations of three independent determinations.
Identification of gp41 CT sequences required for Env association with Gag in immature HIV-1 particles.
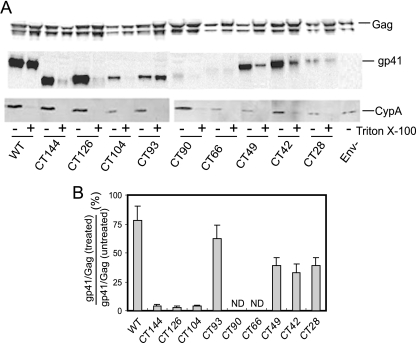
We previously showed that the stable interaction of gp41 with Pr55Gag in immature HIV-1 particles requires the HIV-1 CT (39). To identify regions of the CT required for association with Gag in truncated CT immature virions, we assayed the levels of Env proteins on immature particles following treatment with nonionic detergent (0.5% Triton X-100). Treated and untreated particles were pelleted, and proteins were analyzed by immunoblotting with antibodies to gp41, Gag, and CypA (Fig. 4A). CypA is specifically incorporated into HIV-1 virions but is removed upon detergent treatment of HIV-1 particles (39). As previously observed, immature particles bearing wild-type Env retained a majority of the gp41 protein following detergent exposure. By contrast, the Env proteins were removed from the CT-deficient virions (CT144) upon detergent treatment. Quantification of the band signals revealed a striking difference between the CT104 and CT93 mutants. Upon treatment with detergent, immature particles retained less than 5% of the CT104 mutant gp41 protein but nearly 80% of the CT93 mutant glycoprotein (Fig. 4B). Additional studies demonstrated that treatment of the CT93 immature particles with Triton X-100 at 37°C did not remove the Env protein (unpublished results). Treatment at 37°C is known to disrupt membrane microdomains known as lipid rafts (5); this result therefore indicates that the detergent-resistant association of the CT93 Env protein with immature particles is probably not mediated by lipid rafts. Although some of the mutants exhibited inefficient Env expression and/or incorporation, overall the results indicated that mutants bearing gp41 proteins corresponding to CT93 or longer remained associated with immature particles following detergent treatment. Thus, a gp41 CT as short as 57 amino acids is apparently sufficient for detergent-resistant association with immature virions. By contrast, Env proteins with CTs of 46 amino acids or less, including CT104, CT126, and CT144, were removed from immature HIV-1 particles upon detergent treatment. These results suggest that a region of the CT between 46 and 57 amino acids is necessary to link gp41 to Gag in immature HIV-1 particles.
FIG. 4.
Analysis of the effects of CT truncations on the detergent-resistant association of Env with immature HIV-1 particles. Immature viruses were pelleted though a layer of 0.5% Triton X-100 at 4°C to remove the viral membrane. (A) Pellets were dissolved, and proteins were analyzed by immunoblotting with antibodies to Gag, gp41, and CypA. (B) To quantify the detergent-resistant association of gp41 with immature particles, gp41 and Gag band signal intensities were quantified and the gp41/Gag ratios in detergent-treated and untreated samples were calculated for each virus. The results are expressed as a percentage of gp41 retention following detergent treatment of immature particles containing the wild-type Env protein. Results shown are the mean values and standard deviations obtained in three independent experiments.
Analysis of point mutations in the C-terminal region of gp41.
To more precisely identify residues in LLP-1 required for inhibition of immature HIV-1 particle fusion, we generated alanine-scanning substitutions (CT-3A mutants) between V810 and L854 in the gp41 CT (Table 3). To assess the effects of the mutations on expression and particle incorporation of Env, we analyzed the levels of gp120, gp41, and CA in pelleted virions and gp120, gp41, and Nef in cell lysates by immunoblotting (Fig. 5A and B). Most of the mutants produced levels of cell-associated Env protein equivalent to or slightly higher than wild-type Env (Fig. 5B). Exceptions were the 832 and 839 mutants, which were found to express approximately one-half the normal level of cell-associated gp41 when normalized for Nef expression (Fig. 5C). Two of the mutants, 848 and 851, exhibited markedly reduced levels of particle-associated gp41 despite efficient cellular expression of Env (Fig. 5C). Thus, the efficiency of Env incorporation in the mutant was not solely determined by the level of cellular expression of Env. Comparison of the levels of gp120 and gp41 in viral lysates revealed elevated gp120/gp41 ratios in several of the mutants, including 848 and 851, relative to wild type (Fig. 5D). Collectively, the results indicate that point mutations near the end of gp41 can alter Env expression, incorporation, and stability. Analysis of the viruses in single-cycle infection assays revealed approximately fourfold differences in infectivity among the mutants (unpublished data). Two of the mutants (832 and 839) were highly infectious despite exhibiting reduced Env incorporation. Explanations for this discrepancy may include the elevated gp120/gp41 ratio observed with these mutants (Fig. 5D) as well as a possible nonlinear relationship between infectivity and the level of particle-associated Env protein. In agreement with our observations, other investigators have also reported that point mutations and deletions in LLP-1 can reduce HIV-1 fusion and infectivity (19, 31).
TABLE 3.
Alanine-scanning gp41 mutants
| Virus | Sequence of gp41 C terminus |
|---|---|
| WT | YWSQELKNSAVNLLNATAIAVAEGTDRVIEVLQAAYRAIRHIPRRIRQGLERILL |
| VNL812AAA | YWSQELKNSAAAALNATAIAVAEGTDRVIEVLQAAYRAIRHIPRRIRQGLERILL |
| LN814AA | YWSQELKNSAVNLAAATAIAVAEGTDRVIEVLQAAYRAIRHIPRRIRQGLERILL |
| TAI818AAA | YWSQELKNSAVNLLNAAAAAVAEGTDRVIEVLQAAYRAIRHIPRRIRQGLERILL |
| V820A | YWSQELKNSAVNLLNATAIAAAEGTDRVIEVLQAAYRAIRHIPRRIRQGLERILL |
| EGT824AAA | YWSQELKNSAVNLLNATAIAVAAAADRVIEVLQAAYRAIRHIPRRIRQGLERILL |
| DRV827AAA | YWSQELKNSAVNLLNATAIAVAEGTAAAIEVLQAAYRAIRHIPRRIRQGLERILL |
| IEV830AAA | YWSQELKNSAVNLLNATAIAVAEGTDRVAAALQAAYRAIRHIPRRIRQGLERILL |
| LQ832AA | YWSQELKNSAVNLLNATAIAVAEGTDRVIEVAAAAYRAIRHIPRRIRQGLERILL |
| YR836AA | YWSQELKNSAVNLLNATAIAVAEGTDRVIEVLQAAAAAIRHIPRRIRQGLERILL |
| IR839AA | YWSQELKNSAVNLLNATAIAVAEGTDRVIEVLQAAYRAAAHIPRRIRQGLERILL |
| HIP842AAA | YWSQELKNSAVNLLNATAIAVAEGTDRVIEVLQAAYRAIRAAARRIRQGLERILL |
| RRI845AAA | YWSQELKNSAVNLLNATAIAVAEGTDRVIEVLQAAYRAIRHIPAAARQGLERILL |
| RQG848AAA | YWSQELKNSAVNLLNATAIAVAEGTDRVIEVLQAAYRAIRHIPRRIAAALERILL |
| LER851AAA | YWSQELKNSAVNLLNATAIAVAEGTDRVIEVLQAAYRAIRHIPRRIRQGAAAILL |
| ILL854AAA | YWSQELKNSAVNLLNATAIAVAEGTDRVIEVLQAAYRAIRHIPRRIRQGLERAAA |
| CT28 | YWSQELKNSAVNLLNATAIAVAEGTDR |
FIG. 5.
Immunoblot analysis of mutant HIV-1 particles encoding amino acid substitutions in the gp41 CT. Virus lysates (A) and cell lysates (B) were normalized by p24 ELISA and total protein levels, respectively, and subjected to SDS-PAGE and immunoblotting with the indicated antibodies. (C) Relative Env expression was determined as the ratio of gp41 to Nef in cell lysates, and Env incorporation into particles was determined as the ratio of gp41 to CA in viral lysates. (D) The gp120/gp41 ratios were calculated and expressed as a percentage of the wild-type value. Data shown in panels C and D are the mean values and standard deviations obtained from three independent experiments.
Mutation at the C terminus of HIV-1 Env uncouple virus entry from maturation.
To test the hypothesis that the HIV-1 C-terminal domain LLP-1 regulates immature HIV-1 fusion, we quantified the ability of mature and immature BlaM-Vpr reporter viruses bearing the point mutant Env proteins to fuse with target cells. The results revealed that all of the immature virions were less able to fuse with the cell than their corresponding mature virus particles (Fig. 6A). However, the fusion of two of the mutants, 836 and 851, was significantly less dependent on maturation than HIV-1 particles containing the wild-type Env protein (Fig. 6B). The ratio of gp120 to gp41 on 836 and 851 particles was equal to or greater than that of wild-type Env (Fig. 5D), suggesting that these mutations do not destabilize the gp120-gp41 association. This is in contrast to the shorter gp41 truncation mutants (e.g., CT144), which exhibited decreased gp120/gp41 ratios. Collectively, these results indicate that a small region of the distal C-terminal region of the gp41 CT determines the dependence of HIV-1 fusion competence on particle maturation. While the 851 mutant Env protein exhibited markedly reduced incorporation into HIV-1 particles, this was not the case with the 836 mutant, indicating that the reduction in Env incorporation is unlinked to maturation-dependent regulation of HIV-1 fusion. Immunoblot analysis of detergent-treated immature HIV-1 particles bearing the 836 and 851 mutant Env proteins revealed that the mutant gp41 proteins remain associated with the particles (data not shown). These results further reinforce the conclusion drawn from analysis of the gp41 truncation mutants that the detergent-stable association of Env with immature HIV-1 particles is not sufficient for efficient coupling of fusion to particle maturation.
FIG. 6.
Point mutations near the C terminus of gp41 reduce the dependence of HIV-1 fusion on particle maturation. (A) Mature and immature BlaM reporter particles bearing the indicated mutant Env proteins were assayed for fusion with SupT1 cells. The fusion of mature (filled symbols) and immature (open symbols) HIV-1 particles was expressed as the blue/green fluorescence ratio per nanogram of p24 in the inoculum. (B) Calculation of the ratio of the fusion of mature and immature particles. Shown are mean values and standard deviations from three independent experiments.
DISCUSSION
In this study, we tested the hypothesis that HIV-1 fusion is coupled to maturation by a physical link between the gp41 CT and the core within immature particles. We and others had previously shown that immature HIV-1 particles are repressed for fusion with target cells and that repression is not observed in particles lacking the gp41 CT (26, 38). Our prior observation that the full-length, but not CT-deficient, HIV-1 Env protein exhibits a detergent-resistant association with immature HIV-1 particles (39) suggested that the gp41 CT forms a stable link to a component of the viral core, most likely via the Gag polyprotein. We therefore sought to determine whether the interaction of Env with immature particles was sufficient and/or necessary for repressing fusion of immature HIV-1. Through analysis of viruses containing mutations in the gp41 CT, we observed that removal of 28 or more amino acids substantially relieved the impaired fusion of immature HIV-1 particles. By contrast, removal of as many as 93 amino acids from the carboxyl terminus of gp41 resulted in Env proteins that retained the detergent-resistant association with immature particles. Although some of the truncations resulted in a reduced gp120/gp41 ratio and/or reduced Env incorporation, the reduced dependence of fusion on particle maturation was not linked to either of these phenotypes. Collectively, our data indicate that formation of a stable link between gp41 and the immature core is not sufficient for coupling HIV-1 fusion to maturation.
Previous studies have shown that intermediate truncations of the gp41 CT result in a defect in HIV-1 particle incorporation of Env even in cells, such as HeLa and 293T, in which HIV-1 does not require the full-length CT for incorporation (17, 28, 37). In the present study, we further defined a region of the gp41 CT required for Env expression. Removal of 59 to 90 amino acids from the C terminus of gp41 resulted in a strong reduction in cell-associated Env protein, but a mutant Env lacking the C-terminal 93 amino acids was expressed well. This region encompasses a predicted amphipathic helical segment termed LLP-2; the apparent instability of these mutants suggests that the structure of LLP-2 may be improperly folded in the context of the truncated protein and may be degraded intracellularly. Two regions of the gp41 CT (spanning residues 750 to 763 and 764 to 785) have been shown to promote accumulation of Env in the Golgi complex and reduce Env expression at the cell surface (6). The CT93 mutation corresponds to a truncation at Env codon 762; thus, the difference in incorporation of CT90 and CT93 may be due to an inhibitory sequence retained in the CT90 mutant Env protein. Analysis of alanine-scanning mutants further identified a region near the extreme carboxyl terminus of gp41 required for efficient Env incorporation. Two such mutants exhibiting impaired Env incorporation, 845 and 848, encoded substitutions in arginine residues identified as critical for HIV-1 particle incorporation of Env in a previous study (19).
In this study, we identified two gp41 CT point mutants (836 and 851) that exhibited a markedly reduced dependence of HIV-1 fusion with target cells on particle maturation. The results establish a novel role of the extreme C-terminal gp41 domain known as LLP-1 in inhibiting the fusion of immature HIV-1 particles. LLP-1 is predicted to form an amphipathic helix that has the potential to interact with membranes, and peptides containing this sequence have been shown by circular dichroism spectroscopy to form helical structures in solution (19). LLP-1 has been reported to be involved in Env oligomerization, stability, membrane binding, cell surface expression, and incorporation of Env into virus particles (1). Structural modeling of LLP-1 suggests that Y835 and L849 are on the hydrophobic face of the amphipathic helix, while R836 and E850 lie on the hydrophilic face. It is thus possible that these mutations uncouple fusion from maturation by disrupting the amphipathic nature of LLP-1, resulting in its dissociation from the viral membrane.
The Env subunit gp120 is noncovalently bound to gp41; this heterodimeric complex can be dissociated, resulting in a selective reduction in gp120 on HIV-1 particles. In previous studies, we consistently observed a decrease in gp120 levels versus gp41 in particles lacking the C-terminal 144 amino acids of gp41 (38, 39). In the present study, we also observed a selective decrease in gp120 versus gp41 levels on all of the particles bearing truncated Env proteins (Fig. 2A). Thus, the gp41 CT appears to be important for maintaining the gp120-gp41 heterodimer. A similar conclusion was drawn in a recent study of gp41 mutations in SIV (1). We did not observe a selective reduction in gp120 on the alanine-scanning gp41 mutants, yet two of these mutants exhibited reduced dependence of fusion on particle maturation (Fig. 5 and 6; mutants 836 and 851). We conclude that the selective loss of gp120 from the virus particles is not necessary for uncoupling HIV-1 fusion activation from particle maturation. Furthermore, our results did not reveal a correlation between particle-associated Env levels and maturation-dependent fusion, thus arguing against an indirect effect of the mutation on maturation-dependent fusion via altered Env incorporation.
Recently, a requirement of the host cell protein TIP47 for HIV-1 particle incorporation of full-length Env protein into HIV-1 particles has been demonstrated (4, 22). TIP47 binds both Gag and Env, apparently promoting their interaction in cells. A diaromatic motif, YW, in the gp41 CT is required for TIP47 binding and Env incorporation. Our present studies suggest that TIP47 binding to gp41 is not sufficient for the maturation dependence of HIV-1 particle fusion, as the mutations in the C-terminal region of gp41 that uncouple fusion from maturation are not expected to reduce TIP47 binding to gp41. However, it remains possible that TIP47 binding to gp41 alters the conformation of the CT, thus allowing it to bind efficiently to Gag and locking Env into a fusion-inactive state. Mutations in the distal region of the CT may thus uncouple fusion from maturation by preventing formation of a specific structure induced by TIP47 binding. Testing this hypothesis may require structural studies of the gp41 CT in its free and TIP47-bound conformations.
We have previously proposed two models to explain the inhibitory effect of the gp41 CT on immature HIV-1 particle fusion (38). In the first, the binding of the gp41 CT to Gag inhibits the receptor-induced conformational changes in gp120 and/or gp41 required for fusion. Currently, there is no direct evidence supporting this model. Wyss et al. recently reported that truncation of the gp41 CT leads to enhanced cell-cell fusion and exposure of CD4-induced epitopes on gp120 (41), but these effects required a more extensive truncation than that which we observed to be sufficient to derepress the fusion of immature HIV-1 particles. Murakami and coworkers reported that immature HIV-1 particles exhibit efficient binding of a gp41 monoclonal antibody capable of recognizing gp41 in the postfusion conformation (26). This result suggested that the Env protein present on immature HIV-1 particles is capable of undergoing fusion-related conformational changes, thus arguing against the conformational model. In the second model, the Env conformation change required for fusion may be induced normally on immature HIV-1 particles, but the stable association of gp41 with Gag may constrain the ability of Env to move laterally within the viral membrane. Consequently, the impaired clustering of Env on the surface of immature virions could significantly inhibit their ability to undergo fusion. However, our present results indicate that a stable association of Env with Gag is not sufficient for inhibiting fusion of immature HIV-1 particles with target cells, arguing against the lateral diffusion model. Furthermore, infection analysis of HIV-1 particles containing mixed Env oligomers led Yang and coworkers to conclude that a single functional Env trimer was sufficient to mediate HIV-1 fusion and infection (44). Based on these observations, we favor a model in which the interaction of the LLP-1 segment of the gp41 CT with Gag within immature HIV-1 particles inhibits the receptor-induced conformational changes required for fusion. This hypothesis requires further study by assaying the binding of a panel of conformation-dependent HIV-1 Env antibodies to mature and immature HIV-1 particles. The mutants characterized in the present study will be useful for linking observations obtained in such experiments to maturation-dependent regulation of HIV-1 fusion.
In an interesting paper, Kol and coworkers recently reported evidence for a third mechanism by which the gp41 CT may inhibit immature HIV-1 fusion (20). Using atomic force microscopy to measure the stiffness of HIV-1 particles, they showed that immature HIV-1 particles are significantly more rigid than mature particles. Truncation of the gp41 CT, or pseudotyping by heterologous Env proteins with short CTs, reduced the stiffness of immature HIV-1 particles to a level similar to that of mature virions. These remarkable observations suggest the possibility that the gp41 CT inhibits fusion of HIV-1 particles by preventing deformation of the particle following lipid mixing between virus and cell membranes. It is therefore possible that interactions between the gp41 CT and Gag lock the immature particle into a structurally rigid sphere and that deformation of the particle is a specific requirement for fusion pore expansion. How this would occur with the relatively low number of Env trimers present on the surface of HIV-1 particles (8) remains to be determined.
Acknowledgments
We thank Louis Henderson for the rabbit antibody to human cyclophilin A. The following reagents were obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID: monoclonal antibody to HIV-1 gp41 (clone 246-D) from Susan Zolla-Pazner and monoclonal antibodies to CA and gp41 (clones 183-H12-5C and Chessie 8, respectively) from Bruce Chesebro.
This study was supported by NIH grant AI047056.
Footnotes
Published ahead of print on 3 July 2007.
REFERENCES
- 1.Affranchino, J. L., and S. A. Gonzalez. 2006. Mutations at the C-terminus of the simian immunodeficiency virus envelope glycoprotein affect gp120-gp41 stability on virions. Virology 347:217-225. [DOI] [PubMed] [Google Scholar]
- 2.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharya, J., P. J. Peters, and P. R. Clapham. 2004. Human immunodeficiency virus type 1 envelope glycoproteins that lack cytoplasmic domain cysteines: impact on association with membrane lipid rafts and incorporation onto budding virus particles. J. Virol. 78:5500-5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blot, G., K. Janvier, S. Le Panse, R. Benarous, and C. Berlioz-Torrent. 2003. Targeting of the human immunodeficiency virus type 1 envelope to the trans-Golgi network through binding to TIP47 is required for Env incorporation into virions and infectivity. J. Virol. 77:6931-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 6.Bultmann, A., W. Muranyi, B. Seed, and J. Haas. 2001. Identification of two sequences in the cytoplasmic tail of the human immunodeficiency virus type 1 envelope glycoprotein that inhibit cell surface expression. J. Virol. 75:5263-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byland, R., P. J. Vance, J. A. Hoxie, and M. Marsh. 2007. A conserved dileucine motif mediates clathrin and AP-2-dependent endocytosis of the HIV-1 envelope protein. Mol. Biol. Cell 18:414-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chertova, E., J. W. Bess, Jr., B. J. Crise, I. R. Sowder, T. M. Schaden, J. M. Hilburn, J. A. Hoxie, R. E. Benveniste, J. D. Lifson, L. E. Henderson, and L. O. Arthur. 2002. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J. Virol. 76:5315-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleveland, S. M., L. McLain, L. Cheung, T. D. Jones, M. Hollier, and N. J. Dimmock. 2003. A region of the C-terminal tail of the gp41 envelope glycoprotein of human immunodeficiency virus type 1 contains a neutralizing epitope: evidence for its exposure on the surface of the virion. J. Gen. Virol. 84:591-602. [DOI] [PubMed] [Google Scholar]
- 10.Davis, M. R., J. Jiang, J. Zhou, E. O. Freed, and C. Aiken. 2006. A mutation in the human immunodeficiency virus type 1 Gag protein destabilizes the interaction of the envelope protein subunits gp120 and gp41. J. Virol. 80:2405-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards, T. G., S. Wyss, J. D. Reeves, S. Zolla-Pazner, J. A. Hoxie, R. W. Doms, and F. Baribaud. 2002. Truncation of the cytoplasmic domain induces exposure of conserved regions in the ectodomain of human immunodeficiency virus type 1 envelope protein. J. Virol. 76:2683-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freed, E. O., and M. A. Martin. 1996. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70:341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freed, E. O., and M. A. Martin. 1995. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J. Virol. 69:1984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller, S. D., T. Wilk, B. E. Gowen, H. G. Krausslich, and V. M. Vogt. 1997. Cryo-electron microscopy reveals ordered domains in the immature HIV-1 particle. Curr. Biol. 7:729-738. [DOI] [PubMed] [Google Scholar]
- 15.Gottlinger, H. G., J. G. Sodroski, and W. A. Haseltine. 1989. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:5781-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter, E., and R. Swanstrom. 1990. Retrovirus envelope glycoproteins. Curr. Top. Microbiol. Immunol. 157:187-253. [DOI] [PubMed] [Google Scholar]
- 17.Iwatani, Y., T. Ueno, A. Nishimura, X. Zhang, T. Hattori, A. Ishimoto, M. Ito, and H. Sakai. 2001. Modification of virus infectivity by cytoplasmic tail of HIV-1 TM protein. Virus Res. 74:75-87. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, J., and C. Aiken. 2006. Maturation of the viral core enhances the fusion of HIV-1 particles with primary human T cells and monocyte-derived macrophages. Virology 346:460-468. [DOI] [PubMed] [Google Scholar]
- 19.Kalia, V., S. Sarkar, P. Gupta, and R. C. Montelaro. 2003. Rational site-directed mutations of the LLP-1 and LLP-2 lentivirus lytic peptide domains in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41 indicate common functions in cell-cell fusion but distinct roles in virion envelope incorporation. J. Virol. 77:3634-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kol, N., Y. Shi, M. Tsvitov, D. Barlam, R. Z. Shneck, M. S. Kay, and I. Rousso. 2007. A stiffness switch in human immunodeficiency virus. Biophys. J. 92:1777-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodge, R., J.-P. Lalonde, G. Lemay, and E. A. Cohen. 1997. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 16:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Verges, S., G. Camus, G. Blot, R. Beauvoir, R. Benarous, and C. Berlioz-Torrent. 2006. Tail-interacting protein TIP47 is a connector between Gag and Env and is required for Env incorporation into HIV-1 virions. Proc. Natl. Acad. Sci. USA 103:14947-14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McManus, C. M., and R. W. Doms. 2000. Fusion mediated by the HIV-1 envelope protein. Subcell. Biochem. 34:457-481. [DOI] [PubMed] [Google Scholar]
- 24.Miller, M. A., M. W. Cloyd, J. Liebmann, C. R. Rinaldo, Jr., K. R. Islam, S. Z. Wang, T. A. Mietzner, and R. C. Montelaro. 1993. Alterations in cell membrane permeability by the lentivirus lytic peptide (LLP-1) of HIV-1 transmembrane protein. Virology 196:89-100. [DOI] [PubMed] [Google Scholar]
- 25.Miller, M. A., R. F. Garry, J. M. Jaynes, and R. C. Montelaro. 1991. A structural correlation between lentivirus transmembrane proteins and natural cytolytic peptides. AIDS Res. Hum. Retrovir. 7:511-519. [DOI] [PubMed] [Google Scholar]
- 26.Murakami, T., S. Ablan, E. O. Freed, and Y. Tanaka. 2004. Regulation of human immunodeficiency virus type 1 Env-mediated membrane fusion by viral protease activity. J. Virol. 78:1026-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami, T., and E. O. Freed. 2000. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and alpha-helix 2 of the gp41 cytoplasmic tail. J. Virol. 74:3548-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murakami, T., and E. O. Freed. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc. Natl. Acad. Sci. USA 97:343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owens, R. J., J. W. Dubay, E. Hunter, and R. W. Compans. 1991. Human immunodeficiency virus envelope protein determines the site of virus release in polarized epithelial cells. Proc. Natl. Acad. Sci. USA 88:3987-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng, C., B. K. Ho, T. W. Chang, and N. T. Chang. 1989. Role of human immunodeficiency virus type 1-specific protease in core protein maturation and viral infectivity. J. Virol. 63:2550-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piller, S. C., J. W. Dubay, C. A. Derdeyn, and E. Hunter. 2000. Mutational analysis of conserved domains within the cytoplasmic tail of gp41 from human immunodeficiency virus type 1: effects on glycoprotein incorporation and infectivity. J. Virol. 74:11717-11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tencza, S. B., T. A. Mietzner, and R. C. Montelaro. 1997. Calmodulin-binding function of LLP segments from the HIV type 1 transmembrane protein is conserved among natural sequence variants. AIDS Res. Hum. Retrovir. 13:263-269. [DOI] [PubMed] [Google Scholar]
- 33.Vogt, V. M. 1996. Proteolytic processing and particle maturation. Curr. Top. Microbiol. Immunol. 214:95-131. [DOI] [PubMed] [Google Scholar]
- 34.Wehrly, K., and B. Chesebro. 1997. p24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods 12:288-293. [DOI] [PubMed] [Google Scholar]
- 35.West, J. T., S. K. Weldon, S. Wyss, X. Lin, Q. Yu, M. Thali, and E. Hunter. 2002. Mutation of the dominant endocytosis motif in human immunodeficiency virus type 1 gp41 can complement matrix mutations without increasing Env incorporation. J. Virol. 76:3338-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiegers, K., G. Rutter, H. Kottler, U. Tessmer, H. Hohenberg, and H.-G. Krausslich. 1998. Sequential steps in human immunodeficiency virus particle maturation revealed by alterations of individual Gag polyprotein cleavage sites. J. Virol. 72:2846-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilk, T., T. Pfeiffer, and V. Bosch. 1992. Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology 189:167-177. [DOI] [PubMed] [Google Scholar]
- 38.Wyma, D. J., J. Jiang, J. Shi, J. Zhou, J. E. Lineberger, M. D. Miller, and C. Aiken. 2004. Coupling of human immunodeficiency virus type 1 fusion to virion maturation: a novel role of the gp41 cytoplasmic tail. J. Virol. 78:3429-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyma, D. J., A. Kotov, and C. Aiken. 2000. Evidence for a stable interaction of gp41 with Pr55Gag in immature human immunodeficiency virus type 1 particles. J. Virol. 74:9381-9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyss, S., C. Berlioz-Torrent, M. Boge, G. Blot, S. Honing, R. Benarous, and M. Thali. 2001. The highly conserved C-terminal dileucine motif in the cytosolic domain of the human immunodeficiency virus type 1 envelope glycoprotein is critical for its association with the AP-1 clathrin adaptor [correction of adapter]. J. Virol. 75:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wyss, S., A. S. Dimitrov, F. Baribaud, T. G. Edwards, R. Blumenthal, and J. A. Hoxie. 2005. Regulation of human immunodeficiency virus type 1 envelope glycoprotein fusion by a membrane-interactive domain in the gp41 cytoplasmic tail. J. Virol. 79:12231-12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu, Y., H. Lu, J. P. Kennedy, X. Yan, L. A. McAllister, N. Yamamoto, J. A. Moss, G. E. Boldt, S. Jiang, and K. D. Janda. 2006. Evaluation of “credit card” libraries for inhibition of HIV-1 gp41 fusogenic core formation. J. Comb. Chem. 8:531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, C., C. P. Spies, and R. W. Compans. 1995. The human and simian immunodeficiency virus envelope glycoprotein transmembrane subunits are palmitoylated. Proc. Natl. Acad. Sci. USA 92:9871-9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, X., S. Kurteva, X. Ren, S. Lee, and J. Sodroski. 2005. Stoichiometry of envelope glycoprotein trimers in the entry of human immunodeficiency virus type 1. J. Virol. 79:12132-12147. [DOI] [PMC free article] [PubMed] [Google Scholar]