Abstract
In demyelinating diseases such as multiple sclerosis (MS), myelin membrane structure is destabilized as myelin proteins are lost. Calcium-activated neutral proteinase (calpain) is believed to participate in myelin protein degradation because known calpain substrates [myelin basic protein (MBP); myelin-associated glycoprotein] are degraded in this disease. In exploring the role of calpain in demyelinating diseases, we examined calpain expression in Lewis rats with acute experimental allergic encephalomyelitis (EAE), an animal model for MS. Using double-immunofluorescence labeling to identify cells expressing calpain, we labeled rat spinal cord sections for calpain with a polyclonal millicalpain antibody and with mAbs for glial (GFAP, OX42, GalC) and inflammatory (CD2, ED2, interferon γ) cell-specific markers. Calpain expression was increased in activated microglia (OX42) and infiltrating macrophages (ED2) compared with controls. Oligodendrocytes (galactocerebroside) and astrocytes (GFAP) had constitutive calpain expression in normal spinal cords whereas reactive astrocytes in spinal cords from animals with EAE exhibited markedly increased calpain levels compared with astrocytes in adjuvant controls. Oligodendrocytes in spinal cords from rats with EAE expressed increased calpain levels in some areas, but overall the increases in calpain expression were small. Most T cells in grade 4 EAE expressed low levels of calpain, but interferon γ-positive cells demonstrated markedly increased calpain expression. These findings suggest that increased levels of calpain in activated glial and inflammatory cells in EAE may contribute to myelin destruction in demyelinating diseases such as MS.
Keywords: multiple sclerosis, calcium-activated neutral proteinase, neutral protease, demyelination
Multiple sclerosis (MS), a chronic inflammatory disease of the central nervous system (CNS), frequently causes human CNS demyelination. Although the etiology of MS is not completely understood, studies of human patients and animal models suggest CNS demyelination is a result of a T cell-mediated autoimmune response (1). The oligodendrocyte and/or myelin sheath are damaged while most axons remain morphologically intact (2). The resulting impairment of saltatory nerve conduction is responsible for symptoms (limb paralysis, loss of coordination, visual impairment, etc.) observed in patients with MS (3, 4).
The degradation of myelin proteins in MS has directed attention to proteinases believed to play a role in autoimmune demyelinating diseases. One of the most abundant neutral proteases in the CNS is calcium-activated neutral proteinase (calpain). This ubiquitous endopeptidase exists as proenzyme micro- and millicalpain isoforms (distinguished by μM and mM calcium activation requirements, respectively) when associated with a specific endogenous inhibitor, calpastatin. In the presence of increased calcium levels, calpain becomes activated via autoproteolytic cleavage of N-terminal peptides (5). Cytoskeletal and myelin proteins including myelin basic protein (MBP), myelin-associated glycoprotein, and neurofilament proteins are calpain substrates (6, 7).
Because these proteins are degraded in experimental allergic encephalomyelitis (EAE), investigators have attempted to determine whether glial and inflammatory cells express and/or secrete neutral proteinases such as calpain. In vitro studies have shown secretion of neutral proteinases by microglia, up-regulation and secretion of calpain in activated T cells, and increased expression of calpain and other proteases in reactive astrocytes (8–12).
In this study, calpain expression was examined in spinal cords of Lewis rats with EAE, an animal model for MS (13–15). Immunoperoxidase and double-immunofluorescence staining were used to determine which cells were responsible for calpain expression in acute EAE. Compared with normal controls, we found increased calpain expression in reactive astrocytes, activated T cells, activated microglia, and activated macrophages in the spinal cords of rats with EAE. A preliminary report of this work has been presented previously (16).
MATERIALS AND METHODS
EAE Induction and Tissue Preparation.
Male Lewis rats (6 weeks) were purchased from Charles River Breeding Laboratories and provided water and food pellets ad libitum. We immunized the animals subcutaneously with purified guinea pig MBP (25 μg/rat) in PBS emulsified with an equal volume of complete Freund’s adjuvant (CFA) containing Mycobacterium tuberculosis H37Ra (Difco). Controls were injected with PBS/CFA only. We monitored and weighed the animals daily after inoculation until the tail and hind limbs showed paralysis 9–12 days postinoculation. Spinal cords were collected after the rats were anesthetized, sacrificed, and perfused intracardially with 100 ml PBS. The spinal cords were frozen in Tissue-Tek O.C.T. Compound (Miles), and 5-μm cross-sections were cut by using a Reichert–Jung cryostat. We stained the sections with hematoxylin and eosin as described by Kiernan (17).
Antibodies.
The polyclonal millicalpain antibody (1:200 dilution) was raised in rabbits and characterized (18, 19). mAbs were used as described below: ED2, specific for a macrophage membrane glycoprotein at 1:200; OX42, for complement receptor type 3 (20, 21) on mononuclear phagocytes (including microglia) at 1:150 dilution; glial fibrillary acidic protein (GFAP) MIG-G2 clone, for astrocytic intermediate filament protein at 1:100 and interferon γ (IFN-γ) at 1:200 were purchased from BioSource International (Camarillo, CA). Monoclonal CD2, T cell marker at 1:200 (PharMingen); monoclonal galactocerebroside (GalC) antibody, oligodendrocyte marker at 1:10 (Boehringer Mannheim) as well as an aliquot donated by Narayan Bhat (Medical University of South Carolina); affinity-purified goat anti-rabbit IgG secondary antibody at 1:100 (Jackson Laboratories); and fluorescent secondary anti-mouse and anti-rabbit antibodies at 1:75 (Vector Laboratories) were also used.
Immunoperoxidase Staining.
Spinal cord sections were incubated in blocking solution (2% normal goat serum with 5% nonfat dry milk in PBS) for 20 min, calpain antibody for 45 min, methanol peroxide solution (0.01% H2O2) for 30 min, goat anti-rabbit IgG for 30 min, avidin-biotin solution (Vectastain ABC kit, Vector Laboratories) for 30 min, and 3–3′ diaminobenzidine tetrahydrochloride solution (Sigma Fast DAB tablets, Sigma) for 20 min, and were mounted after dehydration (22).
Fluorescent Antibody Labeling.
Spinal cord sections were blocked for 30 min with 2% horse and goat serum in PBS, incubated for 1 hr with the polyclonal calpain antibody and cell-specific mAb, and incubated with anti-rabbit FITC and anti-mouse Texas red conjugated secondary antibodies for 30 min. Spinal cord sections were rinsed in PBS and distilled water, mounted with a glycerol solution (pH 8.0) containing 10% p-phenylenediamine (Sigma) in PBS, and examined under a fluorescent microscope (Olympus) with camera attachment (22).
RESULTS
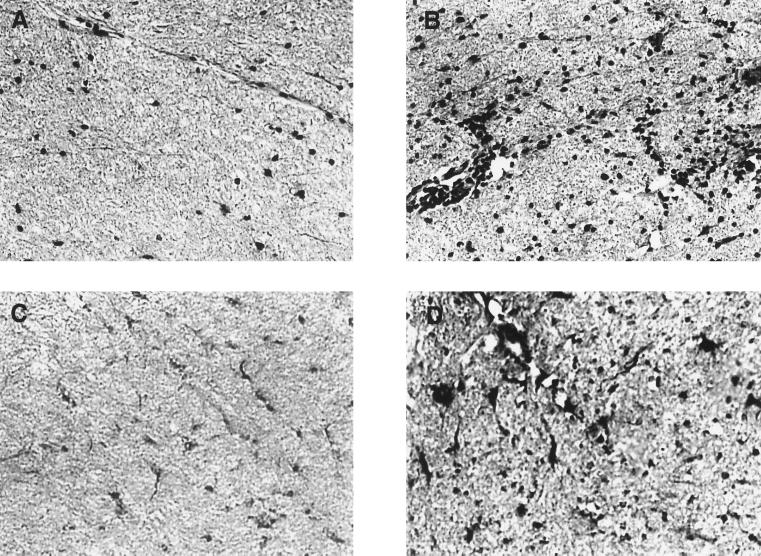
Glial cells were observed in the white matter of normal rat spinal cords by hematoxylin and eosin (H&E) staining at ×200 magnification (Fig. 1A). In spinal cord white matter from animals with acute EAE, perivascular cuffing and inflammatory cell infiltrates were widely distributed (Fig. 1B). Using immunoperoxidase staining, calpain-positive cells were observed throughout spinal cord sections from normal control rats (Fig. 1C). In acute EAE, calpain immunoreactivity was markedly increased in cell bodies and processes (Fig. 1D). Greater numbers of calpain-positive cells were observed in spinal cords from animals with EAE—often in clusters as noted with H&E staining.
Figure 1.
H&E and calpain immunoperoxidase staining of Lewis rat spinal cord (×200). (A) Normal rat spinal cord white matter with H&E stain. (B) Spinal cords of Lewis rats with acute EAE demonstrated perivascular cuffing and increased numbers of glial and inflammatory cells with H&E staining. (C) Immunoperoxidase staining of control spinal cords showed some calpain expression in glial cells of white matter. (D) In white matter of spinal cords from animals with EAE, a greater number of cells demonstrated markedly increased calpain expression with immunoperoxidase staining.
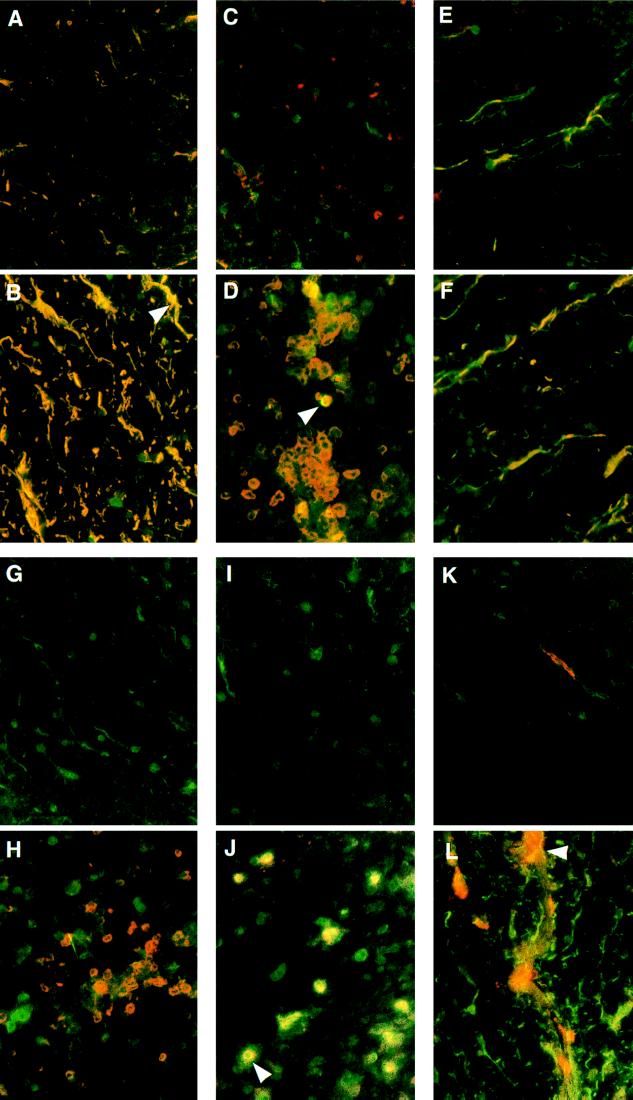
To identify calpain-positive cell types, we employed double-immunofluorescence staining. Calpain expression in resident glial cells was examined by double-labeling spinal cord sections with a polyclonal calpain antibody (fluorescein isothiocyanate secondary-green) and mAbs for cell-specific markers (Texas red secondary-red). Cells positive for calpain and the cell-specific marker appeared yellow when viewed with a dual-pass fluorescence filter.
Astrocytes (identified by antibody specific for GFAP) in normal rat spinal cords were observed throughout the gray and white matter. Calpain was expressed in astrocytic cell bodies and in proximal processes (Fig. 2A). Hence, some calpain expression seen in immunoperoxidase controls was astrocytic. In EAE, reactive astrocytes demonstrated increased GFAP expression and enlarged astrocytic processes. Calpain expression in reactive astrocytes was markedly increased in both the cell bodies and processes (Fig. 2B, arrow). Astrocytic foot processes, which often surrounded clusters of inflammatory cells, also were characterized by elevated calpain expression.
Figure 2.
Double-immunofluorescent staining of Lewis rat spinal cord for calpain (green) and cell-specific markers (red) (×200). (A) Calpain and GFAP (astrocytes) were colocalized (yellow) in normal spinal cord white matter. (B) Markedly increased calpain expression in reactive astrocytes of EAE (arrow). (C) Microglia (red) in normal spinal cord white matter. (D) Spinal cords from animals with EAE showed markedly increased calpain expression in reactive microglia (arrow). (E) Normal spinal cords stained for calpain and galactocerebroside (oligodendrocytes). (F) Oligodendrocytes in EAE showed a small increase in calpain immunofluorescence. (G) Calpain and CD2 (T cells) antibodies in controls often detected no T cells. (H) Spinal cords from EAE animals contained many T cells with limited calpain expression. (I) Normal spinal cords labeled with calpain and IFN-γ antibodies. (J) In EAE, calpain and IFN-γ expression were colocalized as both proteins appear inside and outside the activated T cells (arrow). (K) Macrophage (red) in normal spinal cord white matter. (L) Spinal cords of rats with EAE showed calpain expression in an activated macrophage (arrow).
Using a mAb specific for complement receptor type 3 found on mononuclear phagocytes, we observed quiescent, ramified microglia in both the gray and white matter of spinal cords of control rats. These cells expressed little calpain (Fig. 2C). In EAE, activated microglia were observed with larger cell bodies and a more rounded appearance than those identified in normal controls. Activated microglia often were seen in clusters. All activated microglia showed calpain expression whereas certain activated cells demonstrated much more intense calpain immunoreactivity than others (Fig. 2D, arrow). It was noted that activated microglia with large increases in calpain expression often were encased by astrocytic foot processes in inflammatory foci as described by Matsumoto et al. (23).
With the monoclonal galactocerebroside (GalC) antibody, oligodendrocyte cell bodies stained poorly, but myelinated processes were visible (Fig. 2E). In spinal cords from control rats, calpain expression was observed in the cell bodies and processes at expression levels similar to those of control astrocytes. In EAE, greater numbers of GalC-positive processes were visible, sometimes exhibiting increased calpain expression as well (Fig. 2F). Most often, however, oligodendrocyte calpain expression in EAE was not increased compared with normal controls.
In addition to glial cells, calpain expression was also observed in infiltrating inflammatory cells including T cells and macrophages. In normal spinal cords, T cells were rarely observed using an antibody specific for the pan-T cell marker CD2, and those present did not express detectable levels of calpain (Fig. 2G). In EAE, large numbers of T cells were observed both in clusters and individually. As with microglia, T cells expressed varying amounts of calpain, but most demonstrated little calpain expression (Fig. 2H). In control spinal cords, no cells were colocalized with a monoclonal IFN-γ antibody (Fig. 2I). In contrast, activated cells in spinal cords from rats with EAE expressed large amounts of calpain. This calpain immunoreactivity was not confined to the cell borders but appeared as a halo surrounding the cells (Fig. 2J, arrow).
Very few macrophages were observed in normal rat spinal cords. These often were found in vessels or subdural spaces and did not appear to express calpain (Fig. 2K). In acute EAE, activated macrophages were observed in large numbers in subdural spaces and were also present in the parenchyma. Activated macrophages expressed calpain, and portions of these cells showed more immunoreactivity than other areas within the cell (Fig. 2L, arrow). Macrophages also were observed in inflammatory foci.
Thus, qualitative immunocytochemical studies revealed increased calpain expression in reactive astrocytes, activated microglia, activated T cells, and activated macrophages in spinal cords from rats with EAE compared with Freund’s adjuvant controls.
DISCUSSION
Because myelin proteins are lost in demyelinating diseases, proteolytic enzymes have been suggested to participate in myelin degradation. Calpain, a ubiquitous, cytosolic proteinase active at physiological pH, also has been implicated (7, 24). Recent studies quantifying total calpain expression in the spinal cords of normal controls and animals with EAE have shown significantly increased calpain expression in rats with EAE (25). This finding agrees with immunoperoxidase data presented above in Fig. 1 C and D. Thus, double-immunofluorescent labeling was employed to determine cell phenotypes responsible for increased calpain expression in EAE.
Calpain-positive astrocytes in spinal cords from rats with EAE often were observed adjacent to inflammatory foci. Reactive astrocytes encased the inflammatory foci or even surrounded single, activated microglia, in which case both cells expressed markedly increased levels of calpain. This finding suggests that cell–cell interactions can modulate calpain expression during an immune-mediated inflammatory response. In normal spinal cords, astrocytes were responsible for a majority of the calpain expression. Calpain expressed in normal astrocytes may be involved in cytoskeletal remodeling of astrocytic processes because spectrin and other cytoskeletal proteins are known calpain substrates. However, increased calpain expression in reactive astrocytes in EAE also may play a role in tissue destruction if the calpain–calpastatin ratio is too large to allow calpastatin regulation of calpain activation.
Activated mononuclear phagocytes (including microglia) in EAE expressed significantly more calpain than quiescent microglia in controls. If calpain is secreted by activated microglia before or during symptomatic EAE, the extracellular calcium concentration would be sufficient to activate this proteinase at physiological pH. Because myelin proteins (including MBP) are calpain substrates used for EAE induction, we hypothesize that extracellular calpain may degrade myelin proteins to produce immunogenic fragments engulfed by antigen-presenting cells for major histocompatibility complex class II antigen presentation (26–28). A small percentage of activated mononuclear phagocytes, often observed in clusters in or adjacent to inflammatory foci, demonstrated large increases in calpain expression. Activated calpain from these cells may participate in myelin protein degradation for antigen presentation (possibly contributing to epitope spreading) because macrophages and T cells also are present in inflammatory foci.
Because calpain present in oligodendrocytes is capable of degrading myelin proteins when activated, calpain-mediated intracellular myelinolysis may follow if cellular calcium levels are increased within the myelin sheath (29). If oligodendrocyte cell membranes are compromised during demyelinating diseases, extracellular calcium is free to enter the cell. At this point, calpain present in oligodendrocytes may be activated and capable of degrading myelin intracellularly. Previous studies have suggested that oligodendrocytes are susceptible to complement cascade progression when the blood–brain barrier is compromised to allow plasma into the CNS (30). If the complement membrane attack complex imparts sufficient structural damage to the myelin membrane, increased calcium levels may be sufficient to activate this neutral proteinase because the intracellular pH would still be in the physiological range. In vitro studies have demonstrated vesicular disruption of the myelin sheath, as seen in demyelinating diseases, when rat sciatic nerves are exposed to calcium ionophores at physiological pH (31). Activated calpain also participates in programmed cell death in certain cell types, but reports of oligodendrocyte death via apoptosis in demyelinating diseases are conflicting (32–35).
In addition to resident glial cells, inflammatory cells including activated T cells demonstrated increased calpain expression in spinal cords from animals with EAE compared with normal controls. Deshpande et al. (9, 10) found activated T cells show dramatically increased calpain expression compared with unstimulated T cells in vitro. Activated T cells also secrete calpain, which can degrade myelin proteins in vitro (10). Using fluorescent double-labeling techniques with both anti-CD2 and IFN-γ antibodies, we found little calpain expression in CD2-positive cells, but markedly increased calpain immunoreactivity in cells expressing IFN-γ, which is produced by activated T cells. In fact, the calpain and IFN-γ labeling appeared to obscure the cell borders. These observations suggest that activated T cells may secrete calpain in vivo, confirming previous studies demonstrating cellular release of calpain in the presence of stimulatory factors (10, 36, 37). Activated T cells therefore may secrete calpain in EAE.
In addition to T cells, macrophages were observed in rat spinal cords. The small number of macrophages found in control spinal cords did not have significant calpain expression, although activated macrophages in EAE did show increased calpain expression. Activated macrophages were observed in inflammatory foci, but calpain expression was not uniform throughout these cells. Calpain also may be secreted by activated macrophages as are other proteases (38, 39). However, intracellular calpain in activated macrophages, such as lipid-laden macrophages observed in MS plaques, may participate in the degradation of myelin engulfed via phagocytosis (40).
Because calpain expression is increased in activated glial and infiltrating inflammatory cells in EAE, this proteinase may participate in myelinolysis. Myelin proteins are known calpain substrates, so once released, calpain may play a role in converting MBP and other myelin proteins into immunogenic peptides. Future studies using calpain-specific inhibitors may elucidate the involvement of calpain in myelin degeneration and/or antigenic peptide production in demyelinating diseases.
Acknowledgments
Technical assistance by George W. Ohlandt is greatly appreciated. This work was supported in part by Grants NS-31622 from the National Institutes of Health–National Institute of Neurological Disorders and Stroke, SCRF-1238 from the Paralyzed Veterans of America, and RG-2130B2 from the National Multiple Sclerosis Society, and by the MUSC Medical Scientist Training Program (D.C.S.).
ABBREVIATIONS
- EAE
experimental allergic encephalomyelitis
- MBP
myelin basic protein
- GFAP
glial fibrillary acidic protein
- GalC
galactocerebroside
- MS
multiple sclerosis
- CNS
central nervous system
- IFN-γ
interferon γ
- H&E
hematoxylin and eosin
References
- 1.Martin R, McFarland H F. Crit Rev Clin Lab Sci. 1995;32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- 2.Raine C S. In: Multiple Sclerosis. Hallpike J F, Adams C W, Tourtellotte W W, editors. Baltimore: Williams & Wilkins; 1983. pp. 413–478. [Google Scholar]
- 3.McFarlin D E, McFarland H F. N Eng J Med. 1982;307:1183–1188. doi: 10.1056/NEJM198211043071905. [DOI] [PubMed] [Google Scholar]
- 4.McFarlin D E, McFarland H F. N Eng J Med. 1982;307:1246–1251. doi: 10.1056/NEJM198211113072005. [DOI] [PubMed] [Google Scholar]
- 5.Hathaway D R, McClelland P. In: Intracellular Calcium-Dependent Proteolysis. Mellgren R L, Murachi T, editors. Boca Raton, FL: CRC; 1990. pp. 91–102. [Google Scholar]
- 6.Inuzuka T, Sato S, Baba H, Miyatake T. Acta Neurol Scand. 1987;76:18–23. doi: 10.1111/j.1600-0404.1987.tb03538.x. [DOI] [PubMed] [Google Scholar]
- 7.Banik N L. Crit Rev Neurobiol. 1992;16:257–271. [PubMed] [Google Scholar]
- 8.Banati R B, Rothe G, Valet G, Kreutzberg G W. Glia. 1993;7:183–191. doi: 10.1002/glia.440070208. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande R V, Goust J M, Chakrabarti A K, Barbosa E, Hogan E L, Banik N L. J Biol Chem. 1995;270:2497–2505. doi: 10.1074/jbc.270.6.2497. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande R V, Goust J M, Hogan E L, Banik N L. J Neurosci Res. 1995;42:259–265. doi: 10.1002/jnr.490420214. [DOI] [PubMed] [Google Scholar]
- 11.Siman R, Card J P, Davis L G. J Neurosci. 1990;10:2400–2411. doi: 10.1523/JNEUROSCI.10-07-02400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddleston M, Mucke L. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerlero de Rosbo N, Carnegie P R, Bernard C C. J Neurochem. 1986;47:1007–1012. doi: 10.1111/j.1471-4159.1986.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 14.Heber-Katz E. Ann NY Acad Sci. 1995;756:283–293. doi: 10.1111/j.1749-6632.1995.tb44525.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsunoda I, Fujinami R S. J Neuropath Exp Neurol. 1996;55:673–686. doi: 10.1097/00005072-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Shields D C, Deibler G E, Banik N L. Ann NY Acad Sci. 1997;825:128–130. doi: 10.1111/j.1749-6632.1997.tb48422.x. [DOI] [PubMed] [Google Scholar]
- 17.Kiernan J A. Histological and Histochemical Methods: Theory and Practice. 2nd Ed. New York: Pergamon; 1990. [Google Scholar]
- 18.Chakrabarti A K, Yoshida Y, Powers J M, Singh I, Hogan E L, Banik N L. J Neurosci Res. 1988;20:351–358. doi: 10.1002/jnr.490200309. [DOI] [PubMed] [Google Scholar]
- 19.Banik N L, Chakrabarti A K, Hogan E L. J Neurosci Res. 1990;25:119–124. doi: 10.1002/jnr.490250115. [DOI] [PubMed] [Google Scholar]
- 20.Perry V H, Hume D A, Gordon S. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- 21.Giulian D, Baker T J. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyor W R, Glass J D, Griffin J W, Becker P S, McArthur J C, Bezman L, Griffin D E. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto Y, Ohmori K, Fujiwara M. J Neuroimmunol. 1992;37:23–33. doi: 10.1016/0165-5728(92)90152-b. [DOI] [PubMed] [Google Scholar]
- 24.Sato S, Quarles R H, Brady R O, Tourtellotte W W. Ann Neurol. 1984;15:264–267. doi: 10.1002/ana.410150310. [DOI] [PubMed] [Google Scholar]
- 25.Shields, D. C. & Banik, N. L. (1998) Brain Res., in press. [DOI] [PubMed]
- 26.Lassman H, Rinner W, Hickey W F. Neuropathol Appl Neurobiol. 1994;20:195–196. [PubMed] [Google Scholar]
- 27.Banik N L, McAlhaney W W, Hogan E L. J Neurochem. 1985;45:581–588. doi: 10.1111/j.1471-4159.1985.tb04026.x. [DOI] [PubMed] [Google Scholar]
- 28.Banik N L, Chou C H, Deibler G E, Krutzch H C, Hogan E L. J Neurosci Res. 1994;37:489–496. doi: 10.1002/jnr.490370408. [DOI] [PubMed] [Google Scholar]
- 29.Chakrabarti A K, Banik N L, Lobo D, Terry E. Trans Amer Soc Neurochem. 1990;21:259. (abstr.). [Google Scholar]
- 30.Compston A. Eye. 1992;6:123–128. doi: 10.1038/eye.1992.27. [DOI] [PubMed] [Google Scholar]
- 31.Schlaepfer W W. Nature (London) 1977;265:734–736. doi: 10.1038/265734a0. [DOI] [PubMed] [Google Scholar]
- 32.Squier M K T, Cohen J J. J Immunol. 1997;158:3690–3697. [PubMed] [Google Scholar]
- 33.Dowling P, Husar W, Menonna J, Donnenfeld H, Cook S, Sidhu M. J Neurol Sci. 1997;149:1–11. doi: 10.1016/s0022-510x(97)05213-1. [DOI] [PubMed] [Google Scholar]
- 34.Bonetti B, Raine C S. Ann Neurol. 1997;42:74–84. doi: 10.1002/ana.410420113. [DOI] [PubMed] [Google Scholar]
- 35.Barac-Latas V, Suchanek G, Breitschopf H, Stuehler A, Wege H, Lassmann H. Glia. 1997;19:1–12. doi: 10.1002/(sici)1098-1136(199701)19:1<1::aid-glia1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Adachi E, Mukaiyama T, Sasai K, Hayashi T, Kawashima S, Kasai Y, Hayashi M, Hashimoto P H. Arch Histol Cytol. 1990;53:413–422. doi: 10.1679/aohc.53.413. [DOI] [PubMed] [Google Scholar]
- 37.Saido T C, Suzuki H, Yamazaki H, Tanoue K, Suzuki K. J Biol Chem. 1993;268:7422–7426. [PubMed] [Google Scholar]
- 38.Dijkstra C S, De Groot C J A, Huitinga I. J Neuroimmunol. 1992;40:183–188. doi: 10.1016/0165-5728(92)90132-5. [DOI] [PubMed] [Google Scholar]
- 39.Cammer W, Bloom B R, Norton W T, Gordon S. Proc Natl Acad Sci USA. 1978;75:1554–1558. doi: 10.1073/pnas.75.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S C, Moore G R, Golenwsky G, Raine C S. J Neuropathol Exp Neurol. 1990;49:122–136. doi: 10.1097/00005072-199003000-00005. [DOI] [PubMed] [Google Scholar]