Abstract
During the years or decades of prion disease incubation, at-risk individuals are certain to encounter diverse pathological insults, such as viral and bacterial infections, autoimmune diseases, or inflammatory processes. Whether prion disease incubation time and clinical signs or otherwise the pathology of intercurrent diseases can be affected by the coinfection process is unknown. To investigate this possibility, mice infected with the scrapie agent at both high and low titers were subsequently induced for experimental autoimmune encephalomyelitis, an immune system-mediated model of central nervous system (CNS) inflammation. We show here that coinduced mice died from a progressive neurological disease long before control mice succumbed to classical scrapie. To investigate the mechanism of the coinduced syndrome, we evaluated biochemical and pathological markers of both diseases. Brain and spleen PrPSc levels in the dying coinduced mice were comparable to those observed in asymptomatic scrapie-infected animals, suggesting that coinduced disease is not an accelerated form of scrapie. In contrast, inflammatory markers, such as demyelination, immune cell infiltrates, and gliosis, were markedly increased in coinduced mouse spinal cords. Activated astrocytes were especially elevated in the medulla oblongata. Furthermore, PrPsc depositions were found in demyelinated white matter areas in coinduced mouse spinal cords, suggesting the presence of activated infected immune cells that infiltrate into the CNS to facilitate the process of prion neuroinvasion. We hypothesize that inflammatory processes affecting the CNS may have severe clinical implications in subjects incubating prion diseases.
Transmissible and genetic prion diseases are characterized by long incubation times, which in humans can sometimes amount to decades (7, 13). Although the exact biological steps leading to death by prions are unknown, a general sequence of events has been outlined and is consistent with most of the available data (1). First, regardless of the site of entry, prions replicate in lymphoid organs, as shown by the fact that both infectivity and accumulation of PrPSc are initially detected in the spleens of infected animals (16, 19, 27, 39). PrPSc, a protease-resistant isoform of the host protein PrPC, was shown repeatedly to mark the presence of prions and is believed to be the only component of these infectious agents (30, 40). Prions invade the nervous system by a mechanism believed to involve mostly transmigration of infected lymphoid cells as well as retrograde transport in ascending peripheral tracts (3, 29, 35, 36, 39, 41). Once prions enter the brain, replication of infectivity and PrPSc accumulation occur faster than those in the lymphoid system, subsequently leading to the death of the affected organism (15, 20, 42).
In the presence of inflammatory conditions affecting peripheral organs, activated lymphoreticular cells induce unexpected deposits of PrPSc and prion infectivity in the sites of infiltration (22). For example, mastitis in sheep resulted in the deposition of PrPSc in the inflamed mammary glands (31). Furthermore, prion infectivity could be detected in urine from transgenic mice suffering from a kidney inflammation at higher titers than those obtained for scrapie-infected hamsters. Surprisingly, no difference in prion disease incubation time or clinical symptoms was reported for these animal models.
In this work, we examined whether central nervous system (CNS) inflammation, as opposed to peripheral inflammation, can affect the clinical outcome for mice infected with prions. To this end, we infected mice with the scrapie agent by either the intracerebral (i.c.) or the intraperitoneal (i.p.) route and, 30 days later, induced them for experimental autoimmune encephalomyelitis (EAE), an immune system-mediated model of CNS inflammation (21). At this time in prion disease incubation, infectivity and PrPSc accumulation are easily detected in the spleen, particularly in mouse prion models (5, 6, 23). To mimic conditions where individuals infected with low titers of prions sustain a subclinical form of prion disease, we also infected mice with a 10−8 dilution of the initial prion homogenate.
EAE is an inflammatory response to myelin proteins injected with adjuvants (21). The cell-mediated immune response is directed mainly towards the white matter, causing demyelination and axonal loss. The pathology of EAE is characterized by infiltration of lymphoid cells, mainly T cells, but also B cells, macrophages, dendritic cells, and neutrophils, which release cytokines, chemokines, metalloproteinases, and free radicals. Depending on the genetic background of the affected animal and on the regimen of immunization, EAE presents as an acute, chronic, or relapsing-remitting disease (21).
To induce an inflammatory CNS condition that would persist throughout prion disease incubation, we injected an encephalitogenic peptide of myelin oligodendrocyte glycoprotein (MOG35-55) into mice of the susceptible C57BL/6J strain (4). MOG immunization causes a chronic form of EAE, first affecting the peripheral immune system as the antigen is taken up by subcutaneous dendritic cells (32) and then passing through the regional lymph nodes and spleen, where T cells are extensively activated (12, 38); finally, the activated cells transmigrate into the CNS to cause a delayed-hypersensitivity type of inflammation (17, 43).
Mice were followed closely for signs of EAE, which presents as an acute syndrome 10 to 30 days after immunization and then transforms into a light chronic disease (4). Next, the coinduced mice and the control scrapie or EAE groups were observed daily for neurological symptoms. We show here that while control EAE mice sustained very mild chronic signs for the remainder of the experiment and control scrapie-infected mice succumbed to prion disease at the expected time for i.p. or i.c. inoculation, the coinduced mice died of severe neurological disease at dispersed time points, but always much earlier than the mice infected only with scrapie. In most cases, PrPSc levels in the brains of the dying coinduced mice were comparable to those apparent in asymptomatic mice incubating prion disease for the same periods, suggesting that induction of EAE does not accelerate typical prion disease. Pathological examinations of coinduced mouse spinal cords compared to those of controls revealed extensive demyelination in white matter areas concomitant with immune cell infiltrates and PrPSc depositions. Brains and spinal cords of the coinduced mice also presented massive gliosis, in contrast to moderate levels observed for both chronic EAE and asymptomatic scrapie. We hypothesize that infection with prions may increase the susceptibility of mice to CNS inflammatory insults.
MATERIALS AND METHODS
Animal experiments.
All groups of mice comprised 8 to 10 animals, with the exception of the scrapie-EAE group infected i.p., which comprised 16 mice.
Scrapie infection.
Four- to 5-week-old female C57BL/6J mice (Harlan, Israel) were inoculated with 50 or 100 μl of 1% scrapie-infected brain homogenate (i.c. or i.p., respectively). Control mice were inoculated with saline or with 1% normal brain homogenate. For low-titer infection, mice were inoculated i.c. with 50 μl of 10−8-fold diluted scrapie-infected or normal brain homogenate. Throughout the experiment, mice were kept under specific-pathogen-free conditions, with food and water provided ad libitum. All animal experiments were done according to our institutional guidelines and National Institutes of Health regulations.
EAE induction.
Eight- to 9-week-old female C57BL/6J mice were immunized with an emulsion containing 300 μg of MOG35-55 (70% purified; synthesized at Hebrew University, Jerusalem, Israel) in phosphate-buffered saline and an equal volume of complete Freund's adjuvant containing 5 mg/ml H37RA (Difco Laboratories, Detroit, MI). The inoculum (0.2 ml) was injected subcutaneously into the left flank. One hundred nanograms of pertussis toxin (List Biological Labs) in 0.1 ml phosphate-buffered saline was also injected i.p. on day 0 and 48 h later. Mice were observed daily for the appearance of neurological symptoms, which were scored as follows: 0, asymptomatic; 1, partial loss of tail tonicity; 2, limp tail; 3, impaired righting reflex; 4, hind limb weakness (ataxia); 5, complete hind limb paralysis; 6, moribund or dead.
Pathological examinations.
Histological evaluations were performed on paraffin-embedded sections of brains and spinal cords sampled at various time points after prion infection and MOG immunization. Sections were stained with hematoxylin-eosin (H&E), Luxol fast blue/periodic acid-Schiff stain (PAS), and Bielschowsky silver impregnation (26) to assess cell infiltrations, demyelination, axonal loss, and spongiform changes. In consecutive sections, immunohistochemistry was performed with antibodies against the following targets: macrophages/activated microglia (anti-MAC3; BD Pharmingen, San Diego, CA), T cells (anti-CD3; Serotec, Oxford, United Kingdom), astrocytes (anti-GFAP; DAKO, Glostrup, Denmark), and prion protein (anti-PrP 6H4; Prionics, Schlieren, Switzerland). Immunohistochemistry was performed according to previously described protocols (44). The amount of inflammation was determined by counting the number of inflammatory cells expressing MAC3 and CD3 per mm2 of white and gray matter on an average of five spinal cord cross/longitudinal sections. The degree of demyelination was determined as the percentage of demyelinated area in comparison to the total area of white matter in spinal cord sections.
PrP immunoblots of brains and spleens.
Brains and spleens from mice in all experimental groups were homogenized in 10 volumes of 10 mM Tris-HCl (pH 7.5) containing 300 mM sucrose. Homogenates were normalized for protein level, digested with 50 μg/ml proteinase K (PK; Sigma), and immunoblotted with anti-PrP monoclonal antibody (MAb) IPC1 (Sigma) (40 μl in each lane for brains and 100 μl in each lane for spleen homogenates). The IPC1 antibody was developed in our lab and found to recognize the moiety DWEDRYYR, an epitope comprised of amino acids 144 to 152 of the mouse or hamster PrP sequence, and it is about 2 log more sensitive than PrP MAb 6H4 (Prionics) (R. Engelstein et al., unpublished data). An anti-mouse Fcγ fragment secondary antibody (Jackson) was used to develop the blots of spleen samples in order to avoid interference with mouse immunoglobulin proteins. To enrich PrPSc, 1 ml of an individual mouse brain or spleen homogenate was extracted with 2% Sarkosyl and ultracentrifuged (1 h, 4°C, 100,000 × g). Subsequently, the pellet was digested with PK and immunoblotted as described previously (34).
Statistical analysis.
Statistical evaluation of significant differences between groups with EAE was performed by using the Student t test and one-way analysis of variance. The comparison between survival time curves was performed by using the chi-square test. All statistical analyses were done by SigmaStat for Windows, version 3.11 (Systat Software, Inc.).
RESULTS
Early death of scrapie-infected mice following induction of EAE.
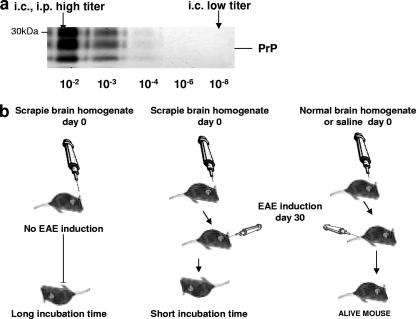
The design of the experiments is outlined in Fig. 1a and b. Briefly, mice were inoculated either i.c. or i.p. with 1% scrapie-infected or normal brain homogenate. Other groups of animals were inoculated i.c. with a 10−8 dilution of the initial scrapie-infected brain homogenate (Fig. 1a). Control groups of mice were inoculated either with saline or with normal brain homogenate. Thirty days later, designated groups of mice were induced for EAE with MOG. Starting on day 10 after induction, all EAE-induced mice were scored for clinical signs as described previously (4).
FIG. 1.
Outline of scrapie and EAE coinduction experiments. (a) Prion inocula used in coinduction experiments. A 1% brain homogenate (10−2) was inoculated i.c. or i.p. into mice to represent a high-titer prion inoculum. A 10−8 dilution of the initial inoculum was used as the low-titer inoculum. (b) Scheme of coinduction experiments. Mice were first inoculated with the designated prion inoculum (i.c., low and high titers; i.p., high titer). Thirty days later, the same mice were either induced for EAE or left untreated. Control mice were induced for EAE 30 days after inoculation with saline or normal brain homogenate.
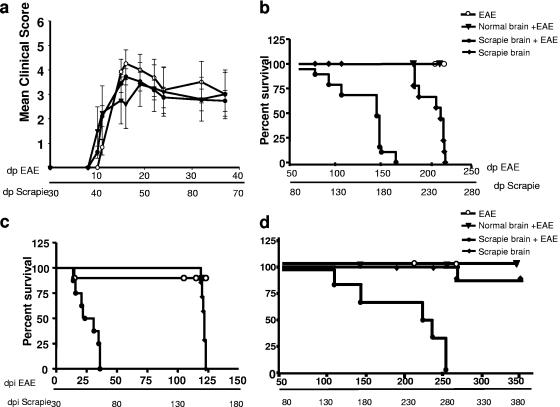
Figure 2a and b depict the results of the i.p. experiment. All mice immunized with MOG developed typical EAE (acute phase), with similar time courses and severity of clinical signs (Fig. 2a). About 30 days after the induction of EAE, mice inoculated with saline or normal brain homogenate recovered from the acute symptoms and sustained mild chronic signs (loss of righting reflex and distal limp tail) throughout the experiment. Around 50 days after induction of EAE, mice previously infected with prions presented new neurological symptoms, such as tail rigidity, kyphosis, ataxia, tremor, and bradykinesia, all of which are mostly characteristic of prion disease (37), and in particular paralysis of the lower limbs, which resembles a neurological sign of EAE (4). The intensity of symptoms increased gradually up to a moribund state, at a different rate for each individual mouse. Figure 2b depicts the survival time curves for the different experimental groups. The median survival time for the coinduced mice was 170 days post-EAE induction, while control prion-infected mice succumbed to typical prion disease much later (median survival time, 250 days). In contrast, EAE-induced mice remained in a light chronic state for the duration of the experiment. The effect of EAE on mice infected i.c. with prions was even more dramatic, as shown in the survival time curves in Fig. 2c. Mice in this group never recovered totally from the acute phase of EAE and had to be sacrificed early in the experiment (median survival time, 55 days). Control scrapie-infected mice succumbed to prion disease much later (median survival time, 150 days).
FIG. 2.
Clinical outcomes for scrapie-infected, EAE-induced, and coinduced mice. (a) EAE clinical scores. Mice induced for EAE 30 days after i.p. inoculation with saline or normal or scrapie-infected brain homogenate were scored for clinical signs as described in Materials and Methods. Each point represents the mean score ± standard deviation for all mice within the same group on the indicated days postinfection. (b) Survival time curves showing effect of i.p. (high-titer) scrapie infection. Following the acute phase of EAE, coinduced mice and scrapie-infected mice were observed for neurological signs and sacrificed when severe symptoms were apparent. The difference between both groups was highly significant (P < 0.001). (c) Survival time curves showing effect of i.c. (high-titer) scrapie infection. Mice were observed for EAE and other neurological symptoms from the day of EAE induction and sacrificed in a severe moribund state. The difference in survival times between the coinduced and scrapie-infected groups was highly significant (P < 0.001). (d) Survival time curves showing effect of i.p. (high-titer) coinduction. Following the acute phase of EAE, coinduced mice and scrapie-infected mice were observed for neurological signs and sacrificed when severe symptoms were apparent (moribund). The difference between both groups was highly significant (P < 0.001). dpi, days postinfection.
Figure 2d shows the survival time curves for mice infected i.c. with a 10−8 dilution of the initial brain homogenate before induction of EAE. The rationale of this experiment was to establish whether inflammation can also be deleterious to individuals suffering from subclinical prion disease. As expected from the high dilution of the inoculum (close to the end point of titration), only two mice infected with low prion titers succumbed to disease (at around 270 days), while four others remained asymptomatic at the termination of the experiment (>360 days). As for the coinduced experimental group, all mice first developed acute EAE (at 10 to 30 days postimmunization) and then developed a fatal progressive neurological disease (median survival time, 230 days), indicating that inflammatory insults can be deleterious to animals infected with marginal doses of prions. The difference in incubation times between coinduced mice and scrapie-infected mice in all three experiments was highly significant (P < 0.001).
PrPSc accumulation in brains of scrapie-EAE coinduced mice.
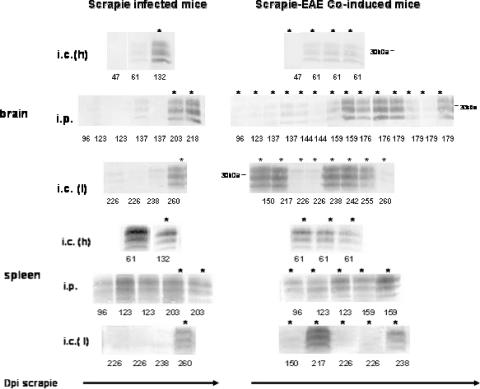
Samples containing comparable protein concentrations of brain extracts from the sick coinduced mice, asymptomatic mice incubating prion disease for the same periods of time, and control mice induced for EAE after inoculation of either normal brain homogenate or saline were digested with PK and immunoblotted with anti-PrP MAb IPC1 (Fig. 3). Although not totally quantitative, such immunoblots represent quite fairly the relative accumulation of PrPSc in the different samples. PrPSc levels in coinduced mice infected i.c. or i.p. with a high titer of prions were similar to those observed in asymptomatic mice incubating prion disease for the same periods of time. PrPSc could not be detected in any of the mice induced for EAE in the absence of prion infection (data not shown). Our results therefore indicate that clinical disease in the coinduced mice was mostly independent of PrPSc accumulation, suggesting that the coinduced disease was not an accelerated form of scrapie.
FIG. 3.
PrPSc levels in brains and spleens of scrapie-infected and coinduced mice. Brain and spleen extracts from individual mice suffering from coinduced disease, scrapie-infected sick mice, or asymptomatic mice incubating scrapie for the designated times were digested with PK and immunoblotted with anti-PrP MAb IPC1. Each lane represents a 40-μl aliquot of a 10% brain homogenate or a 100-μl aliquot of a 10% spleen homogenate. For spleen samples, anti-mouse Fcγ fragment was used as a secondary antibody. The number under each lane designates the time of sacrifice for sick or asymptomatic mice. Asterisks represent individual terminally sick mice (coinduced or scrapie-infected mice), while the others were sacrificed in an asymptomatic state. i.c. (h), high-titer i.c. experiment; i.p., high-titer i.p. experiment; i.c. (l), low-titer i.c. experiment. dpi, days postinfection.
Puzzling results were obtained for mice infected with very low prion titers. While in the control, scrapie-only group, brain PrPSc was present at high levels in the two animals that developed disease (but not in the asymptomatic mice), only five of the eight dying coinduced mice accumulated detectable levels of brain PrPSc. In addition, there was no correlation between PrPSc levels and the time of death in the coinduced mice. The PrPSc level in the brain of the mouse sacrificed first (at 150 days postinfection) was comparable to those observed for sick scrapie-infected mice, but PrPSc could not be detected in the brain of the last coinduced mouse (sacrificed at 260 days postinfection). We therefore concluded that in the low-prion-titer-EAE experiment, there was no simple correlation between the accumulation of PrPSc and the clinical outcome of mice coinduced for scrapie and EAE.
PrPSc accumulation in spleens of scrapie-EAE coinduced mice.
The accumulation of PrPSc and prion infectivity in the spleen has been investigated extensively, in particular in the mouse model (15, 18). It is widely accepted that infectivity and PrPSc can be detected in the spleen very soon after prion infection. Subsequently, prion titers and PrPSc accumulation in the spleen reach a plateau and remain stable thereafter (5, 27). Although the function of PrPC is as yet unknown, it has been proposed that the normal prion protein plays a role in the activation of T cells (11, 24), which features in the induction of EAE (28). However, since it is not known whether the activation of immune cells can affect the rate of PrPC-to-PrPSc conversion, we tested the accumulation of PrPSc in the spleens of coinduced animals.
Spleen homogenates from scrapie-infected, coinduced, and EAE-induced mice were digested with PK and immunoblotted with anti-PrP MAb IPC1 (Fig. 3). As expected from the observations described above, PrPSc levels in spleens of all mice infected with high levels of prions, i.c. or i.p., were comparable and independent of the disease incubation time. No difference was observed for the accumulation of spleen PrPSc in the coinduced mice, indicating that the activation of immune cells by EAE had no effect on the conversion of PrPC to PrPSc. As expected, PrPSc was not detected in spleens from control mice with EAE (data not shown).
The association of prion disease incubation time with the accumulation of PrPSc in the spleen for low-titer prion infection has never been reported. Figure 3 shows that when mice were inoculated with a 10−8 dilution of infected brain homogenate, PrPSc was detected only in the spleens of those mice (two of six mice) affected with prion disease, consistent with the notion that spleen PrPSc correlates with prion infection (5). Interestingly, PrPSc was detected in only two of the eight mice dying of the coinduced disease.
Histopathological findings in CNS of mice suffering from coinduced disease.
Brain and spinal cord sections from moribund coinduced mice were examined for markers of inflammation and prion disease and compared to those of control mice (asymptomatic scrapie-infected and EAE-induced mice) sacrificed at the same time points. In addition to H&E staining, sections were stained for demyelination (Luxol fast blue/PAS), axonal damage (Bielschowsky silver impregnation), infiltrating T cells (CD3), activated macrophages/activated microglia (MAC3), reactive astrocytes (GFAP), and PrPSc.
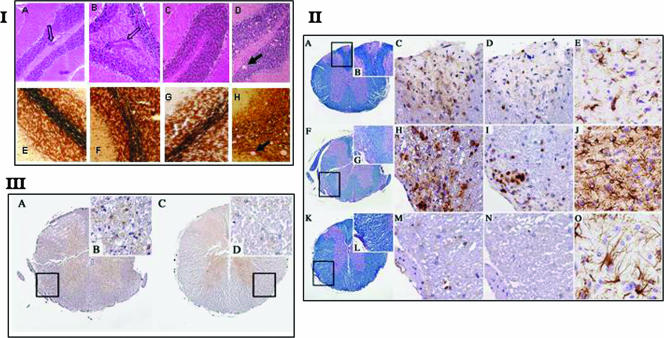
Figure 4, panel I, shows cerebellar sections from coinduced mice and controls. As shown, the numbers of perivascular infiltrations were similar for both coinduced and EAE-induced mice. However, no significant axonal damage or vacuolation was observed in the brain sections of the scrapie-infected mice at several time points (Fig. 4, panel I, images A to C and E to G [137 days postinfection]), in the presence or absence of EAE induction, in contrast to the visible vacuolation and axonal damage of the samples from scrapie-infected sick mice (Fig. 4, panel I, images D and H).
FIG. 4.
(I) H&E and Bielschowsky staining of cerebella from coinduced mice and controls. Infiltrates of mononuclear cells were detected in cerebella of all mice immunized with MOG. Axonal loss was also detected in scattered areas. There was no statistical difference in the numbers of infiltrates or the loss of axons in both EAE-induced and coinduced groups of mice. In scrapie-infected cerebellum, we could detect some degree of axonal loss and no cellular infiltrates. (II) Demyelination and inflammation in spinal cords. (A to E) EAE-induced animal. (A) Spinal cord shows small areas of demyelination after immunization with MOG (Luxol fast blue/PAS stain) (rectangle in panel A is enlarged in panel B). Original magnification, ×40 (A) or ×200 (B). (C) Scattered macrophages (MAC3 stain). Original magnification, ×400. (D) Lymphocytes (CD3 stain). Original magnification, ×400. (E) Moderate gliosis is visible in gray matter of the medulla oblongata (GFAP stain). Original magnification, ×400. (F to J) Coinduced animal. (F) Spinal cord shows more prominent and florid demyelination after PrPSc inoculation and MOG immunization (Luxol fast blue/PAS stain) (rectangle in panel F is enlarged in panel G). Original magnification, ×40 (F) or ×200 (G). (H) Numerous macrophages (MAC3 stain). Original magnification, ×400. (I) Lymphocytes (CD3 stain). Original magnification, ×400. (J) Profound gliosis is visible in gray matter of the medulla oblongata (GFAP stain). Original magnification, ×400. (K to O) Scrapie-incubating animal. (K) Spinal cord does not show any demyelination after PrPSc inoculation (Luxol fast blue/PAS stain) (rectangle in panel K is enlarged in panel L). Original magnification, ×40 (K) or ×200 (L). (M and N) No inflammation (MAC3 stain [M] and CD3 stain [N]). Original magnification, ×400. (O) Moderate gliosis is visible in gray matter of the medulla oblongata (GFAP stain). Original magnification, ×400. (III) PrPSc deposition in coinduced and scrapie-incubating mice. A spinal cord after PrPSc inoculation (homogenate, 10−2) and immunization with MOG shows PrPSc deposition in gray matter (A) and in the demyelinated white matter (B) (rectangle in panel A is enlarged in panel B) (6H4 stain). Original magnification, ×40 (A) or ×600 (B). A spinal cord after PrPSc inoculation (diluted 10−8) shows PrPSc deposition in gray matter (C) and unaffected white matter (D) (rectangle in panel C is enlarged in panel D) (6H4 stain). Original magnification, ×40 (C) or ×600 (D).
Consistent with the severe lower limb paralysis, coinduced mouse spinal cords revealed higher levels of pathological inflammatory markers than those of EAE- and scrapie-induced controls (Fig. 4, panel II, and Table 1). This was the case for the more severe demyelination of the white matter (Fig. 4, panel II, images F and G for coinduced mice, A and B for EAE-induced mice, and K and M for asymptomatic scrapie-infected mice) as well as for the increased numbers of immune cell infiltrates (H and I) compared to those in the EAE sections (C and E). This was true both for T cells and for macrophages/activated microglia. The pathological examination of coinduced mouse spinal cords, together with PrPsc immunoblotting of brain samples, suggests that the coinduced disease is more certainly an exacerbated CNS inflammation, not an accelerated form of prion disease.
TABLE 1.
Quantitative evaluation of inflammation in coinduced mouse spinal cordsb
| Experimental group | Inoculum dilutiona | Demyelination (%) | No. of C3-stained cells/mm3
|
No. of MAC-stained cells/mm3 | Time of sacrifice (days postinfection)
|
||
|---|---|---|---|---|---|---|---|
| White matter | Gray matter | Scrapie | EAE | ||||
| EAE | 10−2 (NB) | 5 ± 4.7 | 80.5 ± 25.5 | 6 ± 3 | 42 ± 13 | 66 | |
| 10−8 (NB) | 2.5 ± 1.6 | 26.7 ± 4.4 | 11.7 ± 4.4 | 12.5 ± 1.3 | >170 | ||
| Scrapie | 10−2 (SB) | 0 | 4 ± 1 | 5 ± 2 | 0 | 96 | |
| 10−8 (SB) | 0 | 2.7 ± 1.7 | 2.3 ± 1.45 | 0 | >200 | ||
| Scrapie plus EAE | 10−2 (SB) | 19.06 ± 0.09* | 137 ± 36 | 29.5 ± 2.5* | 141.5 ± 45.5* | 96 | 66 |
| 10−8 (SB) | 6.6 ± 2.96 | 42.7 ± 14 | 25.3 ± 7.7 | 29 ± 9.86 | >200 | >170 | |
NB, normal brain homogenate; SB, scrapie-infected brain homogenate.
Data are means ± standard deviations. *, P < 0.05. Statistical evaluation was done using the t test and one-way analysis of variance to compare treatment groups.
In addition to the immunoblotting of total brain and spleen homogenates, which shows total PrPSc accumulation, we tested the specific locations of PrPSc depositions by immunocytochemistry. To this end, spinal cord sections from scrapie-infected and coinduced mice were immunostained for PrPSc with anti-PrP MAB 6H4 (Prionics). Figure 4, panel III (images A and B), shows that in addition to the presence of PrPSc in gray matter, coinduced mice were also positive for PrPSc depositions in their demyelinated white matter areas. These findings are consistent with the possibility that during the coinduced disease, activated prion-infected immune cells infiltrate white matter areas. Sections (Fig. 4, panel III, images C and D) depict PrPSc immunostaining in the spinal cord of one of the two mice affected with prion disease after infection with a low prion titer. This mouse was the only scrapie-infected control which presented low levels of PrPSc aggregates in the spinal cord white matter (without apparent demyelination), albeit at much lower levels than those in all coinduced mice (Fig. 4, panel III, images A and B). Whether this is a rare feature of low-titer prion infection remains to be established.
In prion disease, astrocytosis, as measured by the number of cells expressing GFAP, was shown to be associated with the accumulation of PrPSc (14, 45). Astrocytosis is also a feature of CNS inflammation, as is the case for EAE (2, 33). Figure 4, panel II, shows massive immunostaining for GFAP in coinduced mouse brain stems (medulla oblongata) compared to the moderate GFAP immunostaining in both EAE- and scrapie-induced controls. As for the clinical status of the animals, and as opposed to the case for scrapie-infected mice, astrogliosis in coinduced mice was independent of the rate of PrPSc accumulation. We hypothesize that this massive astrogliosis may constitute an additive effect of both the prion infection and the inflammatory EAE process.
DISCUSSION
We have shown here that when scrapie-infected mice were challenged with EAE, an autoimmune CNS inflammatory insult, they succumbed to a progressive neurological disease long before control scrapie-infected mice perished of typical prion disease. This was true for both i.c. and i.p. inoculation of prions. Most important, the induction of EAE also resulted in early fatal neurological disease in all mice infected with very low prion titers, while inoculation of such a low titer (without EAE) transmitted scrapie to a small fraction of the infected animals. Biochemical and pathological assessments of prion and EAE markers suggested that the rate of PrPSc accumulation in coinduced mice did not correlate with the aggravated clinical disease, as is the case for classical scrapie. In contrast, coinduced mice did present exacerbated inflammation, as seen by the more extensive demyelination and larger number of immune cell infiltrates than in classical EAE, suggesting that incubation of prion disease rendered the mice susceptible to inflammatory insults. In addition, the massive levels of gliosis present in the coinduced mouse brains and spinal cords suggest an additive deleterious effect of both prion disease and inflammation that may consequently have caused the death of these mice.
Interestingly, while the total levels of PrPSc in both brains and spleens did not correlate with the clinical outcome of the coinduced animals, immunostaining experiments with the moribund coinduced mice revealed PrPSc depositions in demyelinated white matter spinal cord areas, probably colocalized with hematopoietic cell infiltrates. Such PrPSc deposits may result from the activation by EAE of prion-infected spleen cells which subsequently infiltrate into these unusual areas, consistent with the observation that peripheral inflammation can redirect PrPSc accumulation to otherwise PrPSc-negative peripheral organs (22). PrPSc aggregates in activated cell infiltrates may be neurotoxic and may contribute to the clinical deterioration of the coinduced mice. Such an effect has never been observed for PrPSc in nonactivated cells (8-10). Another possibility is that infiltration of activated and scrapie-infected immune cells into the CNS can circumvent and therefore facilitate prion disease neuroinvasion and, subsequently, prion replication in the CNS. Both mechanisms, facilitated neuroinvasion and toxicity, may occur in parallel. This may explain why some of the sick coinduced mice infected with low-titer prions, as opposed to others in the same group with similar pathology and clinical symptoms, presented high PrPSc levels. It is also possible that prion-infected spleen cells react to inflammation differently than do uninfected spleen cells, at least in the relapsing phases of EAE. We have shown here that the length and severity of the EAE acute phase were similar for controls and prion-infected mice.
Our results are therefore in accordance with the possibility that individuals incubating prion disease may be highly susceptible to CNS inflammation. Therefore, although the importance of this work vis à vis the pathogenesis of prion disease is clear, the immediate significance of these experiments remains in their implications for human and animal health. During the years or decades of prion disease incubation, especially with low or subclinical prion titers, at-risk individuals are certain to encounter diverse pathological insults, such as viral and bacterial infections, autoimmune disease, or neoplastic processes, all of which may have an inflammatory component. These individuals may develop severe neurological diseases not immediately associated with prion infections due to the dissociation of the putative clinical findings from the accumulation of PrPSc. If so, then the effects of the bovine spongiform encephalopathy epidemic on the population should be looked upon differently.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Aguzzi, A. 2003. Prions and the immune system: a journey through gut, spleen, and nerves. Adv. Immunol. 81:123-171. [DOI] [PubMed] [Google Scholar]
- 2.Bannerman, P., A. Hahn, A. Soulika, V. Gallo, and D. Pleasure. 2007. Astrogliosis in EAE spinal cord: derivation from radial glia, and relationships to oligodendroglia. Glia 55:57-64. [DOI] [PubMed] [Google Scholar]
- 3.Bartz, J. C., J. M. Aiken, and R. A. Bessen. 2004. Delay in onset of prion disease for the HY strain of transmissible mink encephalopathy as a result of prior peripheral inoculation with the replication-deficient DY strain. J. Gen. Virol. 85:265-273. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Nun, A., I. Mendel, R. Bakimer, M. Fridkis-Hareli, D. Teitelbaum, R. Arnon, M. Sela, and N. Kerlero de Rosbo. 1996. The autoimmune reactivity to myelin oligodendrocyte glycoprotein (MOG) in multiple sclerosis is potentially pathogenic: effect of copolymer 1 on MOG-induced disease. J. Neurol. 243:S14-S22. [DOI] [PubMed] [Google Scholar]
- 5.Beringue, V., K. T. Adjou, F. Lamoury, T. Maignien, J. P. Deslys, R. Race, and D. Dormont. 2000. Opposite effects of dextran sulfate 500, the polyene antibiotic MS-8209, and Congo red on accumulation of the protease-resistant isoform of PrP in the spleens of mice inoculated intraperitoneally with the scrapie agent. J. Virol. 74:5432-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beringue, V., F. Lamoury, K. T. Adjou, T. Maignien, M. Demoy, P. Couvreur, and D. Dormont. 2000. Pharmacological manipulation of early PrPres accumulation in the spleen of scrapie-infected mice. Arch. Virol. 2000(Suppl.):39-56. [DOI] [PubMed] [Google Scholar]
- 7.Bishop, M. T., P. Hart, L. Aitchison, H. N. Baybutt, C. Plinston, V. Thomson, N. L. Tuzi, M. W. Head, J. W. Ironside, R. G. Will, and J. C. Manson. 2006. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 5:393-398. [DOI] [PubMed] [Google Scholar]
- 8.Brandner, S., A. Raeber, A. Sailer, T. Blattler, M. Fischer, C. Weissmann, and A. Aguzzi. 1996. Normal host prion protein (PrPC) is required for scrapie spread within the central nervous system. Proc. Natl. Acad. Sci. USA 93:13148-13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bueler, H., A. Aguzzi, A. Sailer, R. A. Greiner, P. Autenried, M. Aguet, and C. Weissmann. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339-1347. [DOI] [PubMed] [Google Scholar]
- 10.Bueler, H., A. Raeber, A. Sailer, M. Fischer, A. Aguzzi, and C. Weissmann. 1994. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol. Med. 1:19-30. [PMC free article] [PubMed] [Google Scholar]
- 11.Cashman, N. R., R. Loertscher, J. Nalbantoglu, I. Shaw, R. J. Kascsak, D. C. Bolton, and P. E. Bendheim. 1990. Cellular isoform of the scrapie agent protein participates in lymphocyte activation. Cell 61:185-192. [DOI] [PubMed] [Google Scholar]
- 12.Chabannes, D., and J. F. Borel. 1990. Chronic-relapsing experimental allergic encephalomyelitis in Lewis rats: correlation between clinical state and antimyelin basic protein reactivity in draining lymph node cells. Transplant. Proc. 22:2591-2593. [PubMed] [Google Scholar]
- 13.Collinge, J., J. Whitfield, E. McKintosh, J. Beck, S. Mead, D. J. Thomas, and M. P. Alpers. 2006. Kuru in the 21st century—an acquired human prion disease with very long incubation periods. Lancet 367:2068-2074. [DOI] [PubMed] [Google Scholar]
- 14.Dandoy-Dron, F., F. Guillo, L. Benboudjema, J. P. Deslys, C. Lasmezas, D. Dormont, M. G. Tovey, and M. Dron. 1998. Gene expression in scrapie. Cloning of a new scrapie-responsive gene and the identification of increased levels of seven other mRNA transcripts. J. Biol. Chem. 273:7691-7697. [DOI] [PubMed] [Google Scholar]
- 15.Daude, N. 2004. Prion diseases and the spleen. Viral Immunol. 17:334-349. [DOI] [PubMed] [Google Scholar]
- 16.Eklund, C. M., R. C. Kennedy, and W. J. Hadlow. 1967. Pathogenesis of scrapie virus infection in the mouse. J. Infect. Dis. 117:15-22. [DOI] [PubMed] [Google Scholar]
- 17.Engelhardt, B., and R. M. Ransohoff. 2005. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 26:485-495. [DOI] [PubMed] [Google Scholar]
- 18.Flechsig, E., I. Hegyi, R. Leimeroth, A. Zuniga, D. Rossi, A. Cozzio, P. Schwarz, T. Rulicke, J. Gotz, A. Aguzzi, and C. Weissmann. 2003. Expression of truncated PrP targeted to Purkinje cells of PrP knockout mice causes Purkinje cell death and ataxia. EMBO J. 22:3095-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser, H., and A. G. Dickinson. 1978. Studies of the lymphoreticular system in the pathogenesis of scrapie: the role of spleen and thymus. J. Comp. Pathol. 88:563-573. [DOI] [PubMed] [Google Scholar]
- 20.Glatzel, M., O. Giger, H. Seeger, and A. Aguzzi. 2004. Variant Creutzfeldt-Jakob disease: between lymphoid organs and brain. Trends Microbiol. 12:51-53. [DOI] [PubMed] [Google Scholar]
- 21.Gold, R., C. Linington, and H. Lassmann. 2006. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 129:1953-1971. [DOI] [PubMed] [Google Scholar]
- 22.Heikenwalder, M., N. Zeller, H. Seeger, M. Prinz, P. C. Klohn, P. Schwarz, N. H. Ruddle, C. Weissmann, and A. Aguzzi. 2005. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science 307:1107-1110. [DOI] [PubMed] [Google Scholar]
- 23.Ierna, M., C. F. Farquhar, G. W. Outram, and M. E. Bruce. 2006. Resistance of neonatal mice to scrapie is associated with inefficient infection of the immature spleen. J. Virol. 80:474-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaacs, J. D., G. S. Jackson, and D. M. Altmann. 2006. The role of the cellular prion protein in the immune system. Clin. Exp. Immunol. 146:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Ketring, J. L., J. J. Davis, D. L. Feeback, R. W. Leech, and R. A. Brumback. 1989. Modification of the silver impregnation technique of Bielschowsky for use in glycol methacrylate-embedded brain tissue. Arch. Pathol. Lab. Med. 113:196-198. [PubMed] [Google Scholar]
- 27.Kimberlin, R. H., and C. A. Walker. 1979. Pathogenesis of mouse scrapie: dynamics of agent replication in spleen, spinal cord and brain after infection by different routes. J. Comp. Pathol. 89:551-562. [DOI] [PubMed] [Google Scholar]
- 28.Kuchroo, V. K., A. C. Anderson, H. Waldner, M. Munder, E. Bettelli, and L. B. Nicholson. 2002. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu. Rev. Immunol. 20:101-123. [DOI] [PubMed] [Google Scholar]
- 29.Lasmezas, C. I., J. Y. Cesbron, J. P. Deslys, R. Demaimay, K. T. Adjou, R. Rioux, C. Lemaire, C. Locht, and D. Dormont. 1996. Immune system-dependent and -independent replication of the scrapie agent. J. Virol. 70:1292-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legname, G., I. V. Baskakov, H. O. Nguyen, D. Riesner, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 2004. Synthetic mammalian prions. Science 305:673-676. [DOI] [PubMed] [Google Scholar]
- 31.Ligios, C., C. J. Sigurdson, C. Santucciu, G. Carcassola, G. Manco, M. Basagni, C. Maestrale, M. G. Cancedda, L. Madau, and A. Aguzzi. 2005. PrPSc in mammary glands of sheep affected by scrapie and mastitis. Nat. Med. 11:1137-1138. [DOI] [PubMed] [Google Scholar]
- 32.Link, H., Y. M. Huang, and B. G. Xiao. 1999. Dendritic cells in experimental allergic encephalomyelitis and multiple sclerosis. J. Neuroimmunol. 100:102-110. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto, Y., K. Ohmori, and M. Fujiwara. 1992. Microglial and astroglial reactions to inflammatory lesions of experimental autoimmune encephalomyelitis in the rat central nervous system. J. Neuroimmunol. 37:23-33. [DOI] [PubMed] [Google Scholar]
- 34.Meyer, R. K., M. P. McKinley, K. A. Bowman, M. B. Braunfeld, R. A. Barry, and S. B. Prusiner. 1986. Separation and properties of cellular and scrapie prion proteins. Proc. Natl. Acad. Sci. USA 83:2310-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohan, J., K. L. Brown, C. F. Farquhar, M. E. Bruce, and N. A. Mabbott. 2004. Scrapie transmission following exposure through the skin is dependent on follicular dendritic cells in lymphoid tissues. J. Dermatol. Sci. 35:101-111. [DOI] [PubMed] [Google Scholar]
- 36.Mohan, J., M. E. Bruce, and N. A. Mabbott. 2005. Follicular dendritic cell dedifferentiation reduces scrapie susceptibility following inoculation via the skin. Immunology 114:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Outram, G. W. 1976. The pathogenesis of scrapie in mice. Front. Biol. 44:325-357. [PubMed] [Google Scholar]
- 38.Ovadia, H., and P. Y. Paterson. 1982. Effect of indomethacin treatment upon actively-induced and transferred experimental allergic encephalomyelitis (EAE) in Lewis rats. Clin. Exp. Immunol. 49:386-392. [PMC free article] [PubMed] [Google Scholar]
- 39.Prinz, M., G. Huber, A. J. Macpherson, F. L. Heppner, M. Glatzel, H. P. Eugster, N. Wagner, and A. Aguzzi. 2003. Oral prion infection requires normal numbers of Peyer's patches but not of enteric lymphocytes. Am. J. Pathol. 162:1103-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosicarelli, B., B. Serafini, M. Sbriccoli, M. Lu, F. Cardone, M. Pocchiari, and F. Aloisi. 2005. Migration of dendritic cells into the brain in a mouse model of prion disease. J. Neuroimmunol. 165:114-120. [DOI] [PubMed] [Google Scholar]
- 42.Rubenstein, R., P. A. Merz, R. J. Kascsak, C. L. Scalici, M. C. Papini, R. I. Carp, and R. H. Kimberlin. 1991. Scrapie-infected spleens: analysis of infectivity, scrapie-associated fibrils, and protease-resistant proteins. J. Infect. Dis. 164:29-35. [DOI] [PubMed] [Google Scholar]
- 43.Skundric, D. S., K. Huston, M. Shaw, H. Y. Tse, and C. S. Raine. 1994. Experimental allergic encephalomyelitis. T cell trafficking to the central nervous system in a resistant Thy-1 congenic mouse strain. Lab. Investig. 71:671-679. [PubMed] [Google Scholar]
- 44.Unterberger, U., R. Hoftberger, E. Gelpi, H. Flicker, H. Budka, and T. Voigtlander. 2006. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J. Neuropathol. Exp. Neurol. 65:348-357. [DOI] [PubMed] [Google Scholar]
- 45.Xiang, W., O. Windl, G. Wunsch, M. Dugas, A. Kohlmann, N. Dierkes, I. M. Westner, and H. A. Kretzschmar. 2004. Identification of differentially expressed genes in scrapie-infected mouse brains by using global gene expression technology. J. Virol. 78:11051-11060. [DOI] [PMC free article] [PubMed] [Google Scholar]