Abstract
Hepatitis C virus (HCV) is a cause of chronic liver disease, with more than 170 million persistently infected individuals worldwide. Although the combination therapy of alpha interferon (IFN-α) and ribavirin is effective for chronic HCV infection, around half of all patients infected with HCV genotype 1 fail to show sustained virologic responses and remain chronically infected. Previously, we demonstrated that bile acids were essential for growth of porcine enteric calicivirus in cell culture in association with down-regulation of IFN responses. Because hepatocytes are exposed to high concentrations of bile acids in the liver, we hypothesized that bile acids have similar effects on HCV replication. We incubated HCV replicon-harboring cells (genotype 1b, Con1) in the presence of various bile acids and monitored the expression of HCV RNA and protein (NS5B). The addition of an individual bile acid (deoxycholic acid, chenodeoxycholic acid, ursodeoxycholic acid, or glycochenodeoxycholic acid) in the medium increased the levels of HCV RNA and proteins up to fivefold at 48 h of incubation. An antagonist of bile acid receptor farnesoid X receptor (FXR), Z-guggulsterone, reduced the bile acid-mediated increase of HCV RNA. When IFN (α or γ) and each bile acid were incubated together, we observed that bile acid significantly reduced the anti-HCV effect of IFN. These results indicated that bile acids are factors in the failure of IFN treatment for certain patients infected with HCV genotype 1. Our finding may also contribute to the establishment of better regimens for treatment of chronic HCV infections by including agents altering the bile acid-mediated FXR pathway.
Hepatitis C virus (HCV) is a small, enveloped virus with single-stranded and positive-sense RNA and a genome size of ∼10 kb in the family Flaviviridae (15). Based on genetic variations among HCV isolates, HCV is classified into six genotypes (1 to 6), with several subtypes within each genotype (17). Genotype 1 is the most common in the United States, Europe, and most parts of Asia (17). HCV is a major public health problem as a cause of chronic liver disease, with more than 170 million persistently infected individuals worldwide (15, 17). HCV causes chronic infections in hepatocytes, with continued virus multiplication, while spontaneous clearance of the disease and the virus is rare (15, 17). Chronic HCV infection frequently results later in liver cirrhosis and liver cancer. Currently, the most effective treatment for chronic HCV infection is combination therapy with alpha interferon (IFN-α) and ribavirin. Both IFN-α and ribavirin are nonspecific antiviral agents effective against various DNA and RNA viruses. However, despite the improved efficacy of combined therapy with pegylated IFN-α and ribavirin, around half of all patients infected with HCV genotype 1 fail to show sustained virologic responses and remain chronically infected (17). The mechanisms underlying this limitation are not well understood. In the absence of an effective HCV vaccine, understanding factors that promote HCV replication and compromise the anti-HCV effect of IFN would be a significant advancement in the development of an effective treatment for HCV infections.
As a consequence of being unable to efficiently grow HCV in cell culture, understanding virus replication and developing improved therapeutics have been severely hampered. The development of HCV replicon-harboring cells has provided a valuable tool for studying the basic biology of the virus and new approaches for specific antivirals (16). The HCV replicon system also contributed to the recent success of isolating an HCV strain (genotype 2a) in cell culture (14, 35, 40). Previously, we demonstrated that the bile acids were essential for the growth of porcine enteric calicivirus (PEC) in cell culture and the replication was associated with the down-regulation of the IFN responses (1). The presence of bile acids at high concentrations in the small intestine, where PEC replicates in vivo, suggests a novel mechanism for host-virus interaction influenced by ubiquitous molecules in the environment. Because hepatocytes are exposed to high concentrations of bile acids in the liver, we hypothesized that bile acids have similar effects on HCV replication. We used replicon-harboring cells with HCV (genotype 1b, Con1) to study the effects of bile acids on virus replication. Here, we report that in the presence of bile acids, genome and protein expressions of HCV were significantly increased in replicon-harboring cells. Using an antagonist of the bile acid receptor, the farnesoid X receptor (FXR), we found that FXR plays a role in the bile acid-mediated promotion of HCV replication. Furthermore, we discovered that bile acids compromised the anti-HCV effect of IFN in the cells. These data suggest a novel mechanism for bile acid-mediated gene regulations at virus and host levels. These findings also suggest a mechanism for persistent infections of HCV in hepatocytes and the failure of IFN-based treatment for certain HCV patients. Importantly, these studies may contribute to the finding of better regimens for the treatment of chronic HCV infections by including agents altering the bile acid-mediated FXR pathway.
MATERIALS AND METHODS
Cells, antisera, and reagents.
Huh-7, GS4.1 (replicon-harboring cells with HCV genotype 1b, provided by C. Seeger at Fox Chase Cancer Center, Philadelphia, PA) (7, 8), and HG23 (Norwalk virus [NV] replicon-harboring cells) (2) were maintained in Dulbecco's minimum essential medium containing 10% fetal bovine serum and antibiotics. Both GS4.1 and HG23 cells were maintained in the presence of G418 (0.5 μg/ml) in the medium. The monoclonal antibody specific for HCV NS5B or human β-actin was obtained from ViroStat (Portland, ME) or Cell Signaling Technology (Davers, MA), respectively. Type I IFN (human IFN-αA plus IFN-αD fusion protein) or recombinant human IFN-γ was obtained from Sigma (St. Louis, MO) or Serotec Inc. (Raleigh, NC), respectively. The bile acids in this study included chenodeoxycholic acid (CDCA), glycochenodeoxycholic acid (GCDCA), deoxycholic acid (DCA), and ursodeoxycholic acid (UDCA), and they were obtained from Sigma. The conjugated bile acid GCDCA was prepared in sterile water, while the other unconjugated bile acids (CDCA, DCA, and UDCA) were prepared in dimethyl sulfoxide (DMSO) as a 50 mM stock solution. An antagonist of FXR (a nuclear receptor of bile acids), Z-guggulsterone, and an antagonist of various nuclear receptors, mifepristone (RU486), were purchased from Sigma and prepared in DMSO as 15 mM and 10 mM stock solutions, respectively.
Treatment of GS4.1 cells with various bile acids.
First, we examined the cytotoxic effects of each bile acid on GS4.1 cells by using a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI) to obtain the maximum concentration of each bile acid with minimum cell toxicity. For the treatment of bile acids, 1- or 2-day-old semiconfluent GS4.1 cells were incubated with 1, 10, 20, 50, or 100 μM of CDCA or DCA for 24 or 48 h. For UDCA or GCDCA, the semiconfluent cells were incubated at concentrations of 1, 10, 50, 100, or 200 μM for 24 or 48 h. As controls, we used DMSO (solvent) for CDCA, DCA, or UDCA or mock medium for GCDCA in the cells. After incubation, total RNA was extracted for real-time quantitative reverse transcription-PCR (qRT-PCR) and cell lysates were prepared for Western blot analysis as described below. For comparative study, we also examined the effects of bile acids on NV replicon-harboring cells (HG23 cells), with similar treatment and detection of the NV genome by real-time qRT-PCR (2).
IFN-α or IFN-γ treatment on GS4.1 cells with or without bile acids.
To examine the effect of bile acids on the anti-HCV effect of IFN-α or IFN-γ, GS4.1 cells were treated with IFN-α or IFN-γ in the presence or absence of various bile acids. One- or 2-day-old semiconfluent GS4.1 cells were incubated with 50 units/ml of IFN-α alone or IFN-α and various concentrations of bile acids for 24 or 48 h. For IFN-γ, GS4.1 cells were incubated with 100 units/ml of IFN-γ alone or IFN-γ and CDCA or DCA for 48 h. In these studies, we used 10, 50, and 100 μM of CDCA or DCA or 50, 100, and 200 μM of UDCA or GCDCA. Negative controls included mock medium or solvent (DMSO). After incubation, total RNA was extracted for real-time qRT-PCR and cell lysates were prepared for Western blot analysis. The relative expression levels of the HCV protein or genome affected by IFN with or without bile acids were compared to those for mock treatment. Previously, we demonstrated that IFN-α or IFN-γ inhibited the expression of NV RNA and protein in Huh-7-based NV replicon-harboring cells (HG23 cells) (2). We used these cells to examine whether bile acids affected the anti-NV effect of IFN-α or IFN-γ. In this study, we applied various bile acids and IFN-α or IFN-γ in a manner similar to that described for GS4.1 cells above or preincubated various concentrations of bile acids for up to 24 h before adding mock medium, IFN-α, or IFN-γ and then incubated them for an additional 24 or 48 h. The levels of NV RNA were measured by real-time qRT-PCR (2).
Effects of Z-guggulsterone on bile acid-mediated promotion of HCV replication.
We incubated Z-guggulsterone at concentrations ranging from 0 to 60 μM for this experiment. First, GS4.1 cells were incubated with Z-guggulsterone alone to examine whether it affected the levels of HCV RNA. Next, GS4.1 cells were treated with Z-guggulsterone (30 μM) and various concentrations of CDCA or various concentrations of Z-guggulsterone and CDCA (100 μM) for 24 or 48 h. After incubation, the expression of HCV RNA was measured by real-time qRT-PCR. As a control, we tested mifepristone, which is an antagonist for various nuclear receptors, including progesterone receptor, with conditions similar to those for Z-guggulsterone.
Detection of HCV protein (NS5B), HCV RNA, or NV RNA.
For the Western blot analysis, protein samples of GS4.1 with or without various treatments were prepared in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer containing 1% β-mercaptoethanol and sonicated for 20 s. The proteins were resolved in a 10% Novex Tris-bis gel (Invitrogen, Carlsbad, CA) and transferred to a nitrocellulose membrane. The membranes were probed with the monoclonal antibody specific for the NS5B protein, and the binding of the antibodies was detected with peroxidase-conjugated goat anti-mouse immunoglobulin G (Sigma). In addition, membranes were probed with rabbit antiserum specific for β-actin and peroxidase-conjugated goat anti-rabbit immunoglobulin G as a loading control. Following incubation with a chemiluminescent substrate (SuperSignal West Pico chemiluminescent substrate; Pierce Biotechnology, Rockford, IL), signals were detected with X-ray film.
To examine HCV or NV genome levels in cells with various treatments, real-time qRT-PCR was performed with a one-step Platinum qRT-PCR kit (Invitrogen), following an established protocol, with HCV-specific primers (S130/AS311) which are specific for recognition of the highly conservative 5′ noncoding region in the HCV genome and FAM (6-carboxyfluorescein) probes (molecular beacon) (37, 39) or with norovirus genogroup 1-specific primers and FAM-labeled genogroup 1 probes (11), respectively. As a quantity control for cellular RNA levels, qRT-PCR for β-actin was performed as described previously (30). For qRT-PCR, the total RNA of cells (in six-well plates) was extracted with an RNeasy kit (QIAGEN). The qRT-PCR amplification was performed with a Cepheid SmartCycler with the following parameters: 45°C for 30 min and 95°C 10 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 50°C for 1 min, and elongation at 72°C for 30 s. For HCV, a standard concentration curve was generated in each experiment by serial dilution of samples to calculate the comparable genome levels in various treatment and nontreatment groups. For NV, a standard concentration curve was generated with serial dilutions of RNA transcripts derived from pNV-Neo in each experiment to calculate the total number of genome copies present (2). The relative genome levels in cells with various treatments were calculated after the RNA levels were normalized to those of β-actin.
Promoter-luciferase assay for IFN response elements.
One-day-old semiconfluent GS4.1 cells in six-well plates were transfected with 3 μg each of pISRE-TA-Luc (Clontech, Palo Alto, CA) and pRL-CMV (Promega) by using Lipofectamine 2000 (Invitrogen). After 4 h of transfection, the medium was replaced with fresh medium containing various concentrations of DCA, CDCA, GCDCA, or UDCA, ranging from 1 to 200 μM, and incubated for an additional 8 h. Mock medium or IFN-α (50 units/ml) was then added to the medium and incubated for an additional 12 h. pRL-CMV (for renillar luciferase under the cytomegalovirus [CMV] promoter) (Promega) served as a control to measure the efficiency of the transfection and to standardize luciferase expression levels. The cells were then lysed with 0.2 ml of lysis buffer, and luciferase levels were assayed with the Dual Glo luciferase assay system (Promega) in a 20/20N luminometer (Promega). The level of luciferase (firefly luciferase) expression from each reporter plasmid was normalized against the expression level of the Renilla luciferase encoded by pRL-CMV.
RESULTS
Cytotoxic effects of bile acids on GS4.1 or HG23 cells.
Cell viability in the presence of CDCA or DCA was over 90% at a concentration of less than 100 μM for 24 or 48 h of incubation. However, cell viability dropped to below 80% at 200 μM of CDCA or DCA for 24 or 48 h of incubation. UDCA and GCDCA showed less cytotoxic effect in GS4.1 or HG23 cells than CDCA or DCA, with little cytotoxic effect (over 90% cell viability) at up to 200 μM for up to 48 h of incubation.
Bile acids promote HCV replication.
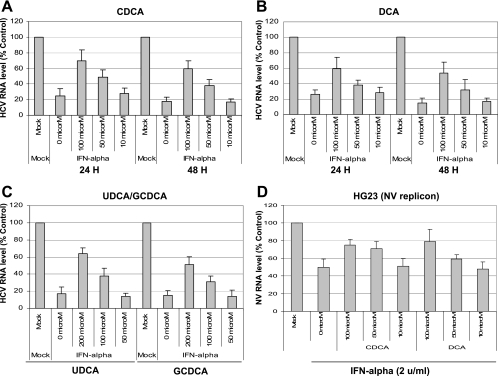
The effects of bile acids on HCV replication were examined by adding individual bile acids in the medium and incubating them for 24 or 48 h. HCV replication was measured by the expression levels of HCV RNA and protein (NS5B), using real-time qRT-PCR and Western blot analysis, respectively. All bile acids that we tested in this study (CDCA, DCA, UDCA, and GCDCA) increased the levels of the HCV genome up to fivefold at 24 or 48 h of incubation (Fig. 1A to C). Similar to those for the HCV genome, HCV protein (NS5B) levels increased up to 10-fold in the presence of CDCA or DCA at 48 h of incubation (Fig. 2). Unconjugated bile acids CDCA and DCA showed similar kinetics in promoting HCV replication: above 50 μM at 24 h and above 20 μM at 48 h, they increased the expression of HCV RNA in the cells in a dose-dependent manner (Fig. 1A and B). A hydrophilic unconjugated bile acid, UDCA, also promoted HCV replication but required a higher concentration (200 μM) for the effect at 48 h of incubation (Fig. 1C). Similarly, GCDCA, a conjugated bile acid, increased the levels of the HCV genome in the replicon-harboring cells at 200 μM (Fig. 1C). Western blot analysis of HCV NS5B also confirmed that CDCA or DCA had higher efficiency in promoting HCV replication in GS4.1 than UDCA or GCDCA (data not shown). When we examined whether bile acids also enhanced NV replication in similar NV replicon-harboring cells, we did not observe any difference in NV replication (Fig. 1D), indicating that bile acid-mediated promotion of HCV replication was specific to the virus.
FIG. 1.
Effects of bile acids on HCV or NV replication in GS4.1 or HG23 cells, respectively. (A, B) Unconjugated bile acid CDCA (A) or DCA (B) was incubated in 1- or 2-day-old semiconfluent GS4.1 cells for 24 or 48 h. After the incubation, HCV RNA levels were measured by real-time qRT-PCR. HCV RNA levels are expressed as percentages of levels for mock (DMSO, 0 μM)-treated cells. (C) A hydrophilic unconjugated bile acid (UDCA) or a conjugated bile acid (GCDCA) was incubated in 1- or 2-day-old GS4.1 cells for 48 h, and HCV RNA levels were measured to examine their effects on HCV replication. Viral RNA levels are presented as percentages of control (DMSO for UDCA or distilled water for GCDCA) levels. (D) Bile acid (CDCA, DCA, UDCA, or GCDCA) was incubated in 1- or 2-day-old semiconfluent HG23 cells for 48 h. After the incubation, NV RNA levels were measured by real-time qRT-PCR. NV RNA levels are expressed as a percentages of levels for mock (DMSO)-treated cells. Bars in all figures represent means ± standard deviations for at least three independent experiments.
FIG. 2.
Effects of bile acids on the expression of HCV NS5B in GS4.1 cells. Unconjugated bile acid CDCA or DCA was incubated in 1- or 2-day-old semiconfluent GS4.1 cells for 48 h. Cell lysates were prepared after the incubation, and Western blot analysis was performed for the expression of HCV NS5B. As a loading control, Western blot analysis of β-actin was performed with the same samples.
Bile acids compromised the anti-HCV effect of IFN-α or IFN-γ.
We next examined whether bile acids inhibited the anti-HCV effect of IFN-α or IFN-γ in GS4.1 cells. We incubated IFN-α or IFN-α with various concentrations of CDCA, DCA, UDCA, or GCDCA for 24 or 48 h. After the incubation, the expression of HCV protein or RNA was measured. Incubation with IFN-α alone (50 units/ml) reduced the HCV genome level to approximately 20% or 15% of that for mock-treated cells at 24 or 48 h (Fig. 3A to C), respectively. However, when bile acids were incubated together with IFN-α, the reduction rates were significantly reduced in a dose-dependent manner. Similar to the kinetics of bile acid-mediated promotion of HCV replication, UDCA and GCDCA required higher concentrations (200 μM) than DCA or CDCA (50 μM) for inhibition of IFN actions against HCV replication (Fig. 3A to C). Similarly, while IFN-γ (100 units/ml) alone reduced the HCV genome level to 25% at 48 h (Fig. 4A) in the presence of bile acids, CDCA, or DCA, the reduction rates were reduced at 48 h (Fig. 4A). This phenomenon was also observed in NV replicon-harboring cells, but only when bile acids were preincubated for up to 24 h before addition of IFN-α or IFN-γ. Incubation with IFN-α (2 units/ml) or IFN-γ (50 units/ml) reduced the NV genome level to approximately 50% of that for mock-treated cells at 48 h. When DCA or CDCA was incubated simultaneously with IFN-α or IFN-γ, NV RNA levels were similar among the groups with or without bile acids (data not shown). However, when DCA or CDCA was preincubated at concentrations above 50 μM for 24 h before addition of either IFN, the rates of reduction of NV RNA by IFN-α or IFN-γ were significantly reduced in the cells (Fig. 3D and 4B).
FIG. 3.
Effects of bile acids on the anti-HCV or anti-NV effect of IFN-α in GS4.1 or HG23 cells, respectively. (A to C) One- or 2-day-old semiconfluent GS4.1 cells were incubated with mock medium, IFN-α alone (50 units/ml), or IFN-α with various concentrations of bile acids for 24 (A and B) or 48 (A to C) h. For this study, we examined 0, 10, 50, and 100 μM of CDCA (A) or DCA (B) or 0, 50, 100, and 200 μM of UDCA or GCDCA (C). After the incubation, total RNA was extracted for real-time qRT-PCR. The relative expression levels of the HCV genome affected by IFN with or without bile acids were compared to those for mock (DMSO or medium) treatment. (D) One- or 2-day-old semiconfluent HG23 cells were incubated with various concentrations of CDCA or DCA for 24 h before addition of mock medium or IFN-α (2 units/ml); cells were further incubated for 48 h. The levels of the NV genome were measured by real-time qRT-PCR.
FIG. 4.
Effects of bile acids on the anti-HCV or anti-NV effect of IFN-γ in GS4.1 or HG23 cells, respectively. (A) One- or 2-day-old semiconfluent GS4.1 cells were incubated with mock medium, IFN-γ alone (100 units/ml), or IFN-γ with various concentrations of bile acids for 48 h. Bile acids, CDCA, or DCA was examined at 0, 10, 50, and 100 μM. After the incubation, total RNA was extracted for real-time qRT-PCR. The relative expression levels of the HCV genome affected by IFN with or without bile acids were compared to those for mock (DMSO) treatment. (B) One- or 2-day-old semiconfluent HG23 cells were incubated with various concentrations (0, 10, 50, and 100 μM) of CDCA or DCA for 24 h before addition of mock medium or IFN-γ (100 units/ml); cells were further incubated for 48 h. The levels of the NV genome were measured by real-time qRT-PCR.
Effects of Z-guggulsterone on bile acid-mediated promotion of HCV replication.
To evaluate a potential mechanism for the bile acid-mediated promotion of HCV replication, we tested an antagonist of FXR, Z-guggulsterone, in replicon-harboring cells. Z-guggulsterone has been demonstrated to be an antagonist of bile acid receptor FXR and to inhibit the expression of small heterodimer partner (SHP) in hepatocytes (34). Treatment of Z-guggulsterone at up to 60 μM for 48 h did not change the levels of HCV RNA in GS4.1 cells (data not shown). Next, we examined the effects of Z-guggulsterone on bile acid-mediated promotion of HCV replication by incubating 100 μM of CDCA with various concentrations (1 to 60 μM) of Z-guggulsterone. Concentrations above 15 μM of Z-guggulsterone reduced CDCA (100 μM)-mediated increase of HCV RNA levels at 48 h of treatment. CDCA alone at 100 μM increased the level of HCV RNA up to fourfold, but in the presence of Z-guggulsterone at 30 or 60 μM, the increase in induction rates by CDCA (100 μM) was only approximately twofold (Fig. 5A). Also, when 30 μM of Z-guggulsterone was incubated with various concentrations of CDCA, ranging from 10 to 100 μM, inhibitory effects were observed at 50 and 100 μM of CDCA (Fig. 5B). However, mifepristone (a progesterone antagonist) did not affect HCV replication with or without bile acids at concentrations up to 20 μM (data not shown).
FIG. 5.
Effects of Z-guggulsterone on bile acid-mediated replication of HCV. (A) One- or 2-day-old semiconfluent GS4.1 cells were incubated with CDCA (100 μM) with various concentrations (0 to 60 μM) of Z-guggulsterone (30 μM) for 48 h. (B) GS4.1 cells were incubated with various concentrations (0 to 100 μM) of CDCA with or without of Z-guggulsterone (30 μM) for 48 h. After the incubation, the expression of the HCV genome was measured by real-time qRT-PCR.
Bile acids inhibited IFN responses in replicon-harboring cells.
We next examined whether bile acids could affect IFN responses in GS4.1 cells by using a reporter system. A reporter plasmid for luciferase expression under the control of the DNA promoter ISRE (pISRE-TA-luc) was transfected into GS4.1 cells, and expression of luciferase was measured after incubation with IFN-α alone (50 μM) or IFN-α and various bile acids (CDCA, DCA, UDCA, or GCDCA). Bile acids were either incubated with IFN-α simultaneously or preincubated for up to 8 h before addition of IFN-α. pRL-CMV (for renillar luciferase under the CMV promoter) was cotransfected in all experiments to control the efficiency of the transfection and standardize luciferase expression levels. When bile acids were incubated with IFN-α simultaneously, there was no consistent reduction of luciferase expression. However, when bile acids were preincubated for up to 8 h before addition of IFN-α, individual bile acids at concentrations of above 50 μM (CDCA), 100 μM (DCA), or 200 μM (UDCA or GCDCA) could inhibit IFN-α mediated luciferase expression by 20 to 50% (Fig. 6).
FIG. 6.
Promoter-luciferase assay for IFN response elements. One-day old GS4.1 cells in six-well plates were transfected with 3 μg each of pISRE-TA-Luc and pRL-CMV by using Lipofectamine 2000. After 4 h of transfection, the medium was replaced with fresh medium with various concentrations of DCA, CDCA, GCDCA, or UDCA, ranging from 0 to 200 μM, and incubated for an additional 8 h. Then, mock medium or IFN-α (50 units/ml) was added to the medium and incubated for an additional 12 h. The cells were then lysed and assayed using the Dual Glo luciferase assay system with a luminometer. The level of luciferase (firefly luciferase) expression from each reporter plasmid was normalized against the expression level of the Renilla luciferase encoded by pRL-CMV.
DISCUSSION
Primary bile acids (cholic acid and CDCA) are synthesized from cholesterol and conjugated with glycine or taurine in the liver. The conjugated or unconjugated bile acids are collected and stored in the gallbladder at concentrations as high as 320 mM. Their release into the duodenum is triggered primarily by hormones (cholecystokinin and secretin) in response to the presence of dietary fat in the duodenum (10). While the secreted bile acids travel through the intestinal tracts, they are reabsorbed into the blood system (through the portal vein) by passive diffusion or active mechanisms with the specific bile acid transporters in the ileum, which collects up to 95% of the secreted bile acids. Secondary bile acids (lithocholic acid and DCA) are converted from the primary bile acids by normal microflora in the small intestine and then enter the pool of bile acids and circulate in the system. In the portal vein, where the bile acids travel to the liver, the concentration of total bile acids can reach up to 80 μM (13). The concentration of bile acids in the systemic circulation is below 10 μM (13). This enterohepatic circulation is essential in maintaining an effective concentration of bile acids and cholesterol homeostasis. Interestingly, it was demonstrated that the cholesterol biosynthetic pathway and fatty acid contents in cells are important in HCV replication by regulating the cellular levels of protein geranylgeranylation and the proper formation of viral replicase complexes (12, 38). There are at least three different classes of bile acid receptors: bile acid transporters, nuclear receptors, and a G protein-coupled receptor of bile acids (TGR-5). Bile acid-specific transporters, including the ileal Na+-dependent bile salt transporter on intestinal cells and the hepatic sinusoidal Na+-taurocholate cotransporting polypeptide (NTCP) on hepatocytes, are essential in the circulation of conjugated bile acids. Most natural bile acids are in conjugated forms, and they require bile acid transporters for efficient internalization into intestine and liver cells.
Because we have demonstrated that bile acids were essential in PEC replication in cells and hepatocytes are exposed to high concentrations of bile acids inside and outside, we hypothesized that bile acids affect HCV replication in replicon-harboring cells. All bile acids that we tested in this study (CDCA, DCA, UDCA, and GCDCA) were able to increase the expression levels of HCV protein and RNA in replicon-harboring cells (Fig. 1 and 2). Unconjugated bile acids (CDCA or DCA), which could penetrate cell membranes efficiently, showed higher efficiency in the promotion of HCV replication. On the other hand, conjugated bile acid (GCDCA) and hydrophilic bile acid (UDCA) required higher concentrations to increase the expression levels of HCV protein and RNA than CDCA or DCA. These results may be due to the facts that conjugated bile acids require bile acid transporters, which are lacking in replicon-harboring cells, for efficient internalization into cells and that hydrophobic properties of bile acids are important for efficient transport into cells. Also, these results indicated that bile acids promote HCV replication in the cytoplasm. It is known that although expression of NTCP in hepatocytes is abundant in vivo, it is limited on cultured hepatocytes, including continued cell lines (33). We are conducting similar experiments with the presence of bile acid transporters, such as NTCP, in GS4.1 cells. However, bile acids did not affect the replication of NV in similar Huh-7-based replicon-harboring cells. It would be interesting to examine whether bile acids promote HCV replication in cells other than hepatocytes.
Recent studies indicate that bile acids not only play an essential role as carriers of dietary lipids for absorption but may also function as hormonelike regulatory molecules with specific cellular receptors and signaling pathways (10). Bile acids regulate the expressions of various transport proteins and enzymes through binding and activation of nuclear receptors, such as FXR (18, 22) and pregnane X receptor (31). The activation of FXR by bile acids induces the expressions of various proteins, including SHP, which represses the expression of cholesterol 7-hydroxylase, the rate-limiting enzyme in bile acid synthesis (18, 22). This FXR/SHP pathway is well developed in hepatic, intestinal, and renal cells and participates in the regulation of fatty acid (including cholesterol) metabolism and glucose homeostasis (33, 36). We tested whether this bile acid-mediated FXR pathway is important in bile acid-mediated HCV replication by using a bile acid antagonist of FXR, Z-guggulsterone. In the presence of Z-guggulsterone (above 15 μM), the bile acid-mediated increase of HCV RNA was reduced up to 50% of that with bile acids alone (Fig. 4). This suggests that the FXR pathway is important for bile acid-mediated HCV replication. We also found that Z-guggulsterone inhibited bile acid-mediated PEC replication in LLC-PK cells (data not shown), indicating that the FXR pathway may be important in the replication of not only HCV but other viruses as well.
In 1990, the U.S. Food and Drug Administration (FDA) approved IFN therapy as a treatment for chronic HCV infection. Initial treatment with IFN-α was found effective in less than 20% of patients (9). The effectiveness of IFN-α treatment was improved up to 40% in conjunction with ribavirin (20, 28). Combination therapy has been improved further by using pegylated IFN, which enhances the stability of IFN in vivo (5, 19). Combination therapy is very effective with genotype 2 or 3, with a sustained-virological-response rate of around 80%, but it is not effective for HCV genotype 1, with a sustained-virological-response rate of only 40 to 50% (17). IFNs play a central role in innate immunity against virus infections before adapted immunity arises. The functions of type I IFN (such as alpha and beta) or type II IFN (gamma) are mediated through interaction with IFN receptors which are expressed in all nucleated cells (29). Reasons for the failed response to IFN therapy are not well understood. Both viral and host factors are likely involved in the mechanisms of IFN resistance. As for viral factors, it has been shown that viral proteins, such as core, E2, and NS5A, can antagonize the antiviral action of IFN through inhibition of PKR (6, 17, 23, 27, 32). In addition, the expression of viral protein (structural and nonstructural proteins) could inhibit the JAK-STAT pathway and prevent the antiviral actions of IFNs (4). As host factors, the genetic backgrounds of the patients (such as racial background) were shown to be a resistance factor for IFN treatment (21). Recently, Zhang et al. reported that alcohol could potentiate the replication of HCV and inhibit the anti-HCV effect of IFN through the activation of NF-κB and the endogenous opioid system (39). The authors concluded that alcohol may play an important role in vivo as a cofactor in HCV disease progression and compromise IFN-based therapy against HCV infection (39).
In earlier reports, bile acids have been shown to counteract IFN pathways in liver cells and natural killer cells (24-26). Podevin et al. showed that bile acids inhibited IFN-induced 2′,5′ oligoadenylate synthetase (OAS) activity in different hepatoma cell lines, including Huh-7 (26). In addition, we demonstrated that bile acids inhibited STAT1 activation by IFN-α or -γ in LLC-PK cells (1). However, there is little information regarding the effects of bile acids on virus replication in hepatocytes. In this study, we discovered that bile acids compromised the anti-HCV effect of IFN in replicon-harboring cells. Bile acids also inhibited the anti-NV effect of IFN in NV replicon-harboring cells. Similar to the effects of bile acids on HCV replication, the effective concentrations of various bile acids for anti-IFN activity differed depending on their hydrophobicity and conjugation. We also demonstrated the anti-IFN activities of bile acids in a reporter system. In the presence of bile acids, induction of IFN responses was reduced in GS4.1 cells. In this study, reduction of IFN responses was achieved consistently when bile acids were incubated for 8 h before addition of IFN-α to the medium, which suggests that bile acids induce expression of certain proteins, resulting in the inhibition of IFN responses in the cells. For a potential mechanism for what we observed, as it was demonstrated that some Huh-7 clones (such as Huh-7.5) support higher levels of HCV replication than parental cells via mutations in the IFN-induction pathway (defective in retinoic acid-inducible gene-I) (3), we speculate that bile acids down-regulate the genes related to IFN, possibly through the FXR/SHP pathway, and consequently, they promote HCV replication in GS4.1 cells.
Although putative receptors of HCV, such as CD81 and human scavenger receptor class B1, are distributed throughout the body, HCV mainly replicates within hepatocytes in the liver. This suggests there could be hepatocyte-specific cofactors that determine HCV liver tropism. It is possible that bile acids or the bile acid-mediated FXR pathway could induce the cofactor for HCV replication in hepatocytes as we demonstrated in this study. Furthermore, our results suggest that bile acids may be important in the failure of IFN treatment for certain patients infected with HCV genotype 1. We are conducting similar studies with replicon-harboring cells containing HCV genotype 2. Our finding may contribute to the establishment of better regimens for treatment of chronic HCV infections by including agents altering the bile acid-mediated FXR pathway.
Acknowledgments
This work was supported by NIH COBRE grant 2 P20 RR016443-07 and the NIH Kansas IDeA Network of Biomedical Research Excellence (P20 RR16475).
We thank Sarah Strouse for technical assistance.
This paper is designated contribution no. 07-232-J from the Kansas Agricultural Experiment Station.
Footnotes
Published ahead of print on 3 July 2007.
REFERENCES
- 1.Chang, K. O., S. V. Sosnovtsev, G. Belliot, Y. Kim, L. J. Saif, and K. Y. Green. 2004. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc. Natl. Acad. Sci. USA 101:8733-8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, K. O., S. V. Sosnovtsev, G. Belliot, A. D. King, and K. Y. Green. 2006. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology 353:463-473. [DOI] [PubMed] [Google Scholar]
- 3.Foy, E., K. Li, R. Sumpter, Jr., Y. M. Loo, C. L. Johnson, C. Wang, P. M. Fish, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. USA 102:2986-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.François, C., G. Duverlie, D. Rebouillat, H. Khorsi, S. Castelain, H. E. Blum, A. Gatignol, C. Wychowski, D. Moradpour, and E. F. Meurs. 2000. Expression of hepatitis C virus proteins interferes with the antiviral action of interferon independently of PKR-mediated control of protein synthesis. J. Virol. 74:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 6.Gale, M. J., Jr., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 7.Guo, J. T., J. A. Sohn, Q. Zhu, and C. Seeger. 2004. Mechanism of the interferon alpha response against hepatitis C virus replicons. Virology 325:71-81. [DOI] [PubMed] [Google Scholar]
- 8.Guo, J. T., Q. Zhu, and C. Seeger. 2003. Cytopathic and noncytopathic interferon responses in cells expressing hepatitis C virus subgenomic replicons. J. Virol. 77:10769-10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoofnagle, J. H., and D. Lau. 1996. Chronic viral hepatitis—benefits of current therapies. N. Engl. J. Med. 334:1470-1471. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, L. R. 1998. Secretion, p. 445-472. In L. R. Johnson (ed.), Essential medical physiology, 2nd ed. Lippincott-Raven, New York, NY.
- 11.Kageyama, T., S. Kojima, M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, N. Takeda, and K. Katayama. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 41:1548-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapadia, S. B., H. Barth, T. Baumert, J. A. McKeating, and F. V. Chisari. 2007. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J. Virol. 81:374-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legrand-Defretin, V., C. Juste, T. Corring, and A. Rerat. 1986. Enterohepatic circulation of bile acids in pigs: diurnal pattern and effect of a reentrant biliary fistula. Am. J. Physiol. 250:G295-G301. [DOI] [PubMed] [Google Scholar]
- 14.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 15.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, 4 ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA.
- 16.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 17.Major, E. M., B. Rehermann, and S. M. Feinstone. 2001. Hepatitis C viruses, 4 ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA.
- 18.Makishima, M., A. Y. Okamoto, J. J. Repa, H. Tu, R. M. Learned, A. Luk, M. V. Hull, K. D. Lustig, D. J. Mangelsdorf, and B. Shan. 1999. Identification of a nuclear receptor for bile acids. Science 284:1362-1365. [DOI] [PubMed] [Google Scholar]
- 19.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 20.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, J. K. Albrecht, et al. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 21.Muir, A. J., J. D. Bornstein, and P. G. Killenberg. 2004. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N. Engl. J. Med. 350:2265-2271. [DOI] [PubMed] [Google Scholar]
- 22.Parks, D. J., S. G. Blanchard, R. K. Bledsoe, G. Chandra, T. G. Consler, S. A. Kliewer, J. B. Stimmel, T. M. Willson, A. M. Zavacki, D. D. Moore, and J. M. Lehmann. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science 284:1365-1368. [DOI] [PubMed] [Google Scholar]
- 23.Paterson, M., C. D. Laxton, H. C. Thomas, A. M. Ackrill, and G. R. Foster. 1999. Hepatitis C virus NS5A protein inhibits interferon antiviral activity, but the effects do not correlate with clinical response. Gastroenterology 117:1187-1197. [DOI] [PubMed] [Google Scholar]
- 24.Podevin, P., M. C. Blanc, M. Vaubourdolle, C. Veyrunes, M. T. Bonnefis, and R. Poupon. 1997. Bile acid inhibition of interferon activity in human lymphocytes: no evidence of oxidative stress. Eur. J. Clin. Investig. 27:491-496. [DOI] [PubMed] [Google Scholar]
- 25.Podevin, P., Y. Calmus, M. T. Bonnefis, C. Veyrunes, C. Chereau, and R. Poupon. 1995. Effect of cholestasis and bile acids on interferon-induced 2′,5′-adenylate synthetase and NK cell activities. Gastroenterology 108:1192-1198. [DOI] [PubMed] [Google Scholar]
- 26.Podevin, P., O. Rosmorduc, F. Conti, Y. Calmus, P. J. Meier, and R. Poupon. 1999. Bile acids modulate the interferon signalling pathway. Hepatology 29:1840-1847. [DOI] [PubMed] [Google Scholar]
- 27.Podevin, P., A. Sabile, R. Gajardo, N. Delhem, A. Abadie, P. Y. Lozach, L. Beretta, and C. Brechot. 2001. Expression of hepatitis C virus NS5A natural mutants in a hepatocytic cell line inhibits the antiviral effect of interferon in a PKR-independent manner. Hepatology 33:1503-1511. [DOI] [PubMed] [Google Scholar]
- 28.Poynard, T., P. Marcellin, S. S. Lee, C. Niederau, G. S. Minuk, G. Ideo, V. Bain, J. Heathcote, S. Zeuzem, C. Trepo, J. Albrecht, et al. 1998. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 29.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spann, K. M., K. C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78:4363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staudinger, J. L., B. Goodwin, S. A. Jones, D. Hawkins-Brown, K. I. MacKenzie, A. LaTour, Y. Liu, C. D. Klaassen, K. K. Brown, J. Reinhard, T. M. Willson, B. H. Koller, and S. A. Kliewer. 2001. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. USA 98:3369-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan, S. L., and M. G. Katze. 2001. How hepatitis C virus counteracts the interferon response: the jury is still out on NS5A. Virology 284:1-12. [DOI] [PubMed] [Google Scholar]
- 33.Trauner, M., and J. L. Boyer. 2003. Bile salt transporters: molecular characterization, function, and regulation. Physiol. Rev. 83:633-671. [DOI] [PubMed] [Google Scholar]
- 34.Urizar, N. L., A. B. Liverman, D. T. Dodds, F. V. Silva, P. Ordentlich, Y. Yan, F. J. Gonzalez, R. A. Heyman, D. J. Mangelsdorf, and D. D. Moore. 2002. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 296:1703-1706. [DOI] [PubMed] [Google Scholar]
- 35.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamagata, K., H. Daitoku, Y. Shimamoto, H. Matsuzaki, K. Hirota, J. Ishida, and A. Fukamizu. 2004. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J. Biol. Chem. 279:23158-23165. [DOI] [PubMed] [Google Scholar]
- 37.Yang, J. H., J. P. Lai, S. D. Douglas, D. Metzger, X. H. Zhu, and W. Z. Ho. 2002. Real-time RT-PCR for quantitation of hepatitis C virus RNA. J. Virol. Methods 102:119-128. [DOI] [PubMed] [Google Scholar]
- 38.Ye, J., C. Wang, R. Sumpter, Jr., M. S. Brown, J. L. Goldstein, and M. Gale, Jr. 2003. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc. Natl. Acad. Sci. USA 100:15865-15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, T., Y. Li, J. P. Lai, S. D. Douglas, D. S. Metzger, C. P. O'Brien, and W. Z. Ho. 2003. Alcohol potentiates hepatitis C virus replicon expression. Hepatology 38:57-65. [DOI] [PubMed] [Google Scholar]
- 40.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 102:9294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]