Abstract
Recent studies have established a protective role for T cells during primary West Nile virus (WNV) infection. Binding of CD40 by CD40 ligand (CD40L) on activated CD4+ T cells provides an important costimulatory signal for immunoglobulin class switching, antibody affinity maturation, and priming of CD8+ T-cell responses. We examined here the function of CD40-dependent interactions in limiting primary WNV infection. Compared to congenic wild-type mice, CD40−/− mice uniformly succumbed to WNV infection. Although CD40−/− mice produced low levels of WNV-specific immunoglobulin M (IgM) and IgG, viral clearance from the spleen and serum was not altered, and CD8+ T-cell priming in peripheral lymphoid tissues was normal. Unexpectedly, CD8+ T-cell trafficking to the central nervous system (CNS) was markedly impaired in CD40−/− mice, and this correlated with elevated WNV titers in the CNS and death. In the brains of CD40−/− mice, T cells were retained in the perivascular space and did not migrate into the parenchyma, the predominant site of WNV infection. In contrast, in wild-type mice, T cells trafficked to the site of infection in neurons. Beside its role in maturation of antibody responses, our experiments suggest a novel function of CD40-CD40L interactions: to facilitate T-cell migration across the blood-brain barrier to control WNV infection.
West Nile virus (WNV) is a single-stranded, positive-sense, enveloped RNA virus of the Flaviviridae family that is endemic in North America, Africa, Israel, parts of Asia, and eastern Europe (9, 20). Although WNV infections in humans is usually asymptomatic or self-limiting with a mild febrile illness, disease may progress to encephalitis or death in the elderly and immunocompromised, suggesting an important role for immune system control of WNV infection (43).
Studies in immunodeficient mice have shown increased tissue viral loads and mortality after WNV infection (42, 56, 57, 60, 75). Interferons have an early antiviral role and control initial WNV infection in peripheral tissues, thus limiting viremia and dissemination to the central nervous system (CNS) (56, 61, 62, 74). The induction of WNV-specific immunoglobulin M (IgM) coincides with the clearance of WNV from the bloodstream (12). CD8+ T cells control WNV infection in neurons as CD8-deficient or depleted mice develop elevated virus titers and persistence in the CNS (60, 75). Recent studies have established that CD4+ T cells promote efficient WNV-specific IgM and IgG production and sustain CD8+ T-cell responses in the CNS to allow viral clearance (63).
CD4+ T helper cells contribute to the clearance of acute viral infections through several mechanisms, including activation and priming of B cells and CD8+ T cells, production of inflammatory and antiviral cytokines, and direct cytotoxic effects on infected cells. B and CD8+ T cells require costimulatory signals from CD4+ T cells to elicit mature humoral and cellular responses. CD40, a member of the tumor necrosis factor alpha gene family, is expressed on B cells, some antigen-presenting cells, activated T cells, and endothelial cells and provides key activation signals (7, 23, 38, 45, 52, 71). The ligand for CD40, CD40L or CD154, is a transmembrane protein expressed on activated T cells, granulocytes, macrophages, endothelial cells, vascular smooth muscle cells, and activated platelets (6, 29, 72). Interaction of CD40 on B cells with CD40L on CD4+ T cells triggers immunoglobulin class switching, somatic hypermutation and affinity maturation, and proliferation (21, 29, 45). Moreover, CD40-CD40L interaction between CD4+ and CD8+ T cells may bypass antigen-presenting cell interactions to license memory CD8+ T cells (7). Humans who are functionally deficient in CD40-CD40L interactions have markedly elevated IgM levels and show increased susceptibility to opportunistic bacterial and parasitic infections (17, 27, 78). Studies in CD40 and CD40L deficient mice have also demonstrated a role for CD40 in priming naive T cells and cross-priming of cytotoxic T lymphocytes (CTLs) by dendritic cells (4, 10, 22, 54, 58).
Experiments in mice have shown that CD40-dependent interactions are essential during many viral infections. For example, a lack of CD40-CD40L interactions altered B-cell responses after infection with lymphocytic choriomeningitis and vesicular stomatitis viruses (49) and led to the establishment of latency in B cells by gammaherpesvirus 68 (77). The role of CD40 in priming antiviral CD8+ T cells, however, remains controversial. Although no defect was observed in primary CD8+ T-cell responses after lymphocytic choriomeningitis virus infection in mice lacking CD40 (49, 64, 76), impaired memory CD8+ CTL responses were observed (5, 6). In contrast, in studies with influenza A virus or polyomavirus, CD40-CD40L interactions were required for generation of both optimal primary and memory CD8+ T-cell responses (32, 35).
The function of CD40-CD40L interactions during in vivo infection with WNV and other encephalitic flaviviruses has not been studied. In the present study, we used CD40−/− mice to dissect the mechanisms by which this costimulatory molecule regulates WNV infection. As anticipated, CD40-CD40L interactions were required for efficient antibody production by B cells. Surprisingly, in the brains of CD40−/− mice, T cells were retained in the perivascular space and did not migrate into parenchyma, thus preventing control of WNV infection in the CNS.
MATERIALS AND METHODS
Cells and viruses.
BHK-21 cells were cultured as previously described (11). The WNV strain 3000.0259 was isolated in New York in 2000 from mosquitoes and amplified once in C6/36 Aedes albopictus cells (13). Virus was diluted to 102 PFU in 50 μl in Hanks balanced salt solution containing 1% heat-inactivated fetal bovine serum for injection into mice.
Mouse experiments.
Wild-type mice (CD45.1 and CD45.2), and CD40−/− (CD45.2) mice (29) that were backcrossed 10 generations onto the C57BL/6 (H-2KbDb) background were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were genotyped and bred in the animal facility of Washington University School of Medicine. Experiments were performed in accordance with Washington University Animal Studies guidelines. Eight- to twelve-week-old mice were inoculated subcutaneously with WNV by footpad injection after sedation with xylazine and ketamine.
Quantification of viral burden.
For analysis of viral burden in tissues of infected mice, organs were recovered after cardiac perfusion with 20 ml of phosphate-buffered saline (PBS) and dissection. Tissues were placed on ice, weighed, and stored at −80°C. To determine virus titers, tissues were homogenized by using a bead beater apparatus (BioSpec Products, Inc.) and titrated for virus by plaque assay on BHK21 cells as described previously (11). Serum was obtained from whole blood by phlebotomy of the axillary vein immediately prior to euthanasia. Viral RNA was extracted from 50 μl of serum by using the QIAamp viral RNA extraction kit (QIAGEN, Palo Alto, CA). Primers corresponding to the 1,160- to 1,229-nucleotide sequence of the WNV E gene were used to perform real-time reverse transcriptase PCR (RT-PCR) according to a previously described protocol (11, 34).
Measurement of WNV-specific antibodies.
The levels of WNV-specific IgM and IgG were determined by using an enzyme-linked immunosorbent assay with purified WNV E protein, as described previously (12). A plaque reduction neutralization test was used to determine the neutralizing activity of serum from infected mice (11). The results were plotted, and the neutralization titer for 50% inhibition was calculated. For some experiments, IgM was inactivated after treatment of serum with 0.05 M β-mercaptoethanol at 37°C for 1 h (11, 59).
Immunohistochemistry.
Tissue staining was performed as described previously (56, 60). Briefly, 9 days after WNV infection, moribund wild-type or CD40−/− mice were perfused sequentially with iced PBS and PBS with 4% paraformaldehyde (PFA). Brains were harvested and either embedded in paraffin or cryoprotected in 30% sucrose for generation of frozen sections. After deparaffinization, tissue sections were stained with hematoxylin and eosin or for WNV by using a cocktail of monoclonal antibodies (MAbs; E18, E22, and E31 (46) against WNV E protein and an antigen-enhancing staining kit (DakoCytomation). Frozen sections were washed with PBS and permeabilized with 0.1% Triton X-100 (Sigma), and nonspecific antibody was blocked with 10% normal goat serum for 1 h at room temperature. Monoclonal or polyclonal primary antibodies specific for CD3 (DakoCytomation), CD45.1 (Abcam, Inc.), and CD31 (BD Biosciences) were applied at 1 μg/ml in PBS containing 10% goat serum and 0.1% Triton X-100 overnight at 4°C. Primary antibodies were detected with secondary antibodies to Alexa Fluor 488 and Alexa Fluor 555. Nuclei were counterstained with ToPro3 (Molecular Probes, Inc.).
Intracellular IFN-γ staining.
Intracellular gamma interferon (IFN-γ) staining was performed on splenocytes obtained at day 7 after infection as previously described (41, 63). Briefly, splenocytes were stimulated with 1 μg of an immunodominant Db-restricted WNV NS4B peptide/ml or 50 ng of phorbol myristic acid (Sigma)/ml and 1 μM ionomycin (Sigma) for 4 h at 37°C with the addition of Golgi plug (1 μl/ml; BD Biosciences), washed, and stained with fluorescein isothiocyanate (FITC)-conjugated MAbs to CD8, CD4, or a FITC-conjugated isotype control MAb (BD Biosciences) at 4°C for 30 min. After being washed and fixed with 1% PFA in PBS, cells were permeabilized with saponin and stained with an allophycocyanin (APC)-conjugated IFN-γ antibody or an isotype control (BD Biosciences) at 4°C for 30 min, washed, and analyzed by flow cytometry.
Quantification of brain leukocytes.
Quantitation of CNS lymphocytes by flow cytometry was performed as described previously (60). Whole brains were harvested from wild-type and CD40−/− mice 9 days after WNV infection. Prior to harvest, extensive cardiac perfusion was performed with PBS (20 ml) to deplete intravascular leukocytes. Brains were homogenized through a 70-μm-pore-size filter, and CNS leukocytes were isolated by Percoll gradient centrifugation. After the total number of cells was counted, the leukocytes were incubated with FITC-conjugated anti-CD3 antibody and APC-conjugated anti-CD4, anti-CD8, or isotype control antibody or FITC-conjugated anti-CD45 antibody (BD Biosciences) at 4°C for 30 min, washed, fixed with 1% PFA in PBS, and analyzed by flow cytometry.
Measuring brain chemokine levels by real-time quantitative RT-PCR.
Total RNA was prepared from the brain cortex of WNV-infected wild-type and CD40−/− mice 9 days after infection by using the RNeasy kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. After extensive DNase I treatment, total RNA was quantitated by spectrophotometry (Eppendorf AG), followed by cDNA (50 μl) synthesis using the Superscript III first-strand synthesis system (Invitrogen, Carlsbad, CA). All samples were reverse transcribed from a single reverse transcription master mix to minimize differences in reverse transcription efficiency. All oligonucleotide primers, reverse transcription and PCR, and data analysis were as published previously (33).
Chemokine receptor staining.
Spleens were isolated from wild-type or CD40−/− mice 7 days after WNV infection and single-cell suspensions were obtained. After erythrocyte lysis, splenocytes were washed in medium containing 5% fetal bovine serum, counted, and incubated with phycoerythrin-conjugated rat anti-mouse CCR5, a rabbit anti-mouse CXCR3 MAb, or a rabbit control antibody (Zymed) at 4°C for 30 min. After two washes, splenocytes were incubated with FITC-conjugated goat anti-rabbit antibody and APC-conjugated anti-mouse CD8 or isotype control antibody (BD Pharmingen) at 4°C for 30 min. After being washed and fixed with 1% PFA in PBS, the cells were analyzed by flow cytometry.
Adoptive transfer of CD8+ T cells.
Spleens were isolated from CD45.1+ mice 7 days after WNV infection, and single-cell suspensions were obtained by disruption through a 40-μm-pore-size filter. After erythrocyte lysis, CD8+ T cells were purified (93 to 96%) by negative selection using magnetic beads (Miltenyi Biotec) and resuspended in endotoxin-free sterile PBS. Four days after footpad infection with 102 PFU of WNV, 5 × 106 CD45.1+CD8+ T cells were adoptively transferred into CD45.2+ CD40−/− or wild-type mice via retro-orbital injection. Five days later, the brains were harvested, and leukocytes were isolated and characterized by flow cytometry for CD8 and CD45.1 expression.
Statistical analysis.
All data were analyzed with Prism software (GraphPad Software, San Diego, CA). For survival analysis, Kaplan-Meier survival curves were analyzed by the log-rank test. For virus burden and antibody experiments, statistical significance was determined by using the Mann-Whitney test.
RESULTS
CD40−/− mice are more susceptible to WNV infection.
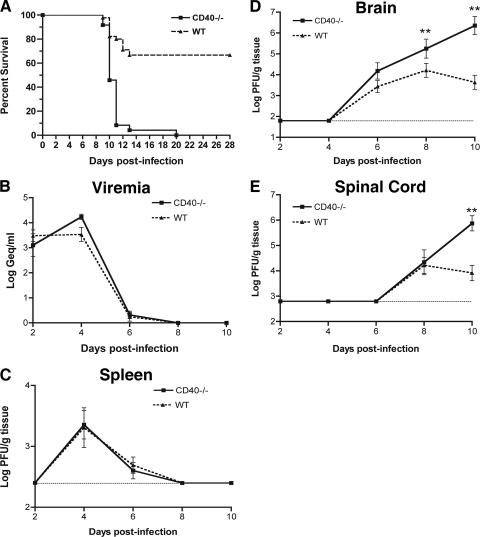
Ligation of CD40-CD40L is required for optimal T-dependent humoral responses (29) and possibly for maturation of antigen-specific CD8+ T-cell responses (4, 22). To assess the role of CD40 in controlling WNV infection, we analyzed survival in wild-type C57BL/6 mice and congenic CD40−/− mice. Mice in all groups exhibited clinical signs of infection, including weight loss, hunching, fur ruffling, and decreased activity. However, CD40−/− mice were significantly more vulnerable and displayed higher mortality rates compared to wild-type mice (Fig. 1A). Whereas only 30% of wild-type mice succumbed to subcutaneous infection with 102 PFU of WNV, 100% of CD40−/− mice (P < 0.0001) died. Thus, an absence of CD40 function increased lethality after WNV infection.
FIG. 1.
Survival data and WNV infection in wild-type and CD40−/− mice. (A) Wild-type (n = 45) and CD40−/− (n = 25) C57BL/6 mice were infected with 102 PFU WNV via a subcutaneous route in several independent experiments. A significant difference in survival between the two groups of mice was observed (P < 0.0001). (B to E) WNV burden in wild-type and CD40−/− mice at days 2, 4, 6, 8, and 10 after infection. (B) Serum WNV RNA levels were determined by quantitative RT-PCR and expressed as genomic equivalents of WNV per ml of serum. (C to E) WNV was measured from the spleen (C), brain (D), and spinal cord (E) by viral plaque assay. Samples were obtained from 4 to 10 mice per time point per group. The dotted line represents the limit of sensitivity of the assay. Asterisks indicate time points at which differences are statistically significant compared to wild-type mice.
WNV burden in CD40−/− mice.
To understand how an absence of CD40-CD40L interaction enhanced the susceptibility of mice to WNV infection, the levels of WNV RNA and infectious virus were measured by fluorogenic RT-PCR or viral plaque assay. Wild-type and CD40−/− mice were infected with 102 PFU of WNV, and the viral burden was assessed in the serum, spleen, brain, and spinal cord at days 2, 4, 6, 8, and 10 after infection (Fig. 1B to E).
(i) Viremia.
Throughout the time course of infection, viremia was below the limit of detection by direct plaque assay in all samples in both wild-type and CD40−/− mice (data not shown). However, using a more sensitive quantitative RT-PCR assay (11), we detected WNV RNA in the serum of both wild-type and CD40−/− mice. Notably, the kinetics and magnitude of viremia were virtually identical between wild-type and CD40-deficient mice, with viral RNA detected at days 2 (103.5 and 103.1 PFU/ml, respectively [P > 0.5]) and 4 (104.2 and 103.5 PFU/ml, respectively [P > 0.1]) after infection. In both wild-type and CD40−/− mice, viremia fell below the level of detection after day 6 (Fig. 1B).
(ii) Spleen.
In the spleens from both wild-type and CD40−/− mice, similar kinetics of WNV accumulation were observed by plaque assay at days 4 (103.4 PFU/g versus 103.3 PFU/g, respectively [P > 0.1]) and 6 (102.6 PFU/g versus 102.7 PFU/g [P > 0.1]) after infection. By day 8, infectious WNV was completely cleared from the spleen in both groups of mice (Fig. 1C).
(iii) CNS.
WNV levels in the brain and spinal cord were markedly increased in CD40−/− mice (Fig. 1D and E). Although wild-type and CD40−/− mice had similar levels of WNV in the brain on day 6 postinfection, increased viral burden in CD40−/− mice was observed on day 8 (105.3 PFU/g versus 104.2 PFU/g [P < 0.05]). By day 10, CD40−/− mice averaged almost 3-log-higher virus titers (106.4 PFU/g versus 103.6 PFU/g [P < 0.0001]) than wild-type mice, corresponding to an increased rate of death. A similar pattern was observed in the spinal cord, with a marked increase in WNV levels in CD40−/− mice compared to wild-type mice at day 10 postinfection (∼105 PFU/g versus 103 PFU/g [P < 0.01]). Overall, our data suggest that a deficiency of CD40 did not alter the kinetics of WNV dissemination to the CNS but rather was essential for the control of CNS infection.
Enhanced WNV antigen staining in the CNSs of CD40−/− mice.
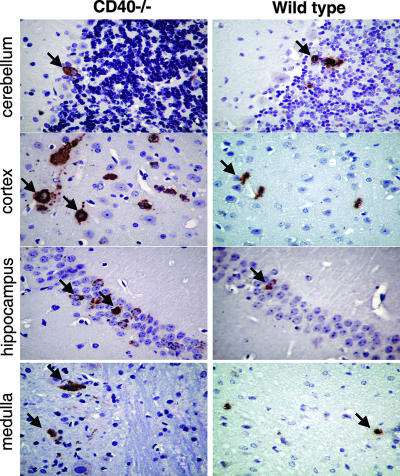
To understand the cellular basis for increased infection and mortality in CD40−/− mice, we examined brain tissues from CD40−/− and wild-type mice for WNV antigen staining and histopathological changes after infection. Brains were harvested from equivalently moribund CD40−/− and wild-type mice on day 10. In wild-type mice, scattered neurons in the cerebellum, cortex, hippocampus, and medulla stained positive for WNV antigen. In contrast, CD40−/− mice displayed enhanced WNV antigen staining, particularly in the cortex and hippocampus (Fig. 2). Neuronal damage and destruction, as evidenced by dysmorphic and/or degenerating cells, was increased among various neuronal subpopulations within the CNSs of CD40−/− mice, including Purkinje and granule cell neurons of the cerebellum and in hippocampal and brain stem neurons. These data are consistent with the increased level of WNV infection in the CNSs of CD40−/− mice.
FIG. 2.
WNV antigen staining in the brain. The brains of infected (left) CD40−/− and (right) wild-type mice were harvested 10 days after infection with WNV, sectioned, and stained with anti-WNV MAbs. Arrows indicate examples of infected cells. Typical sections from the cerebellum, cortex, hippocampus, and medulla at ×40 magnification are shown after staining samples from at least five mice per group.
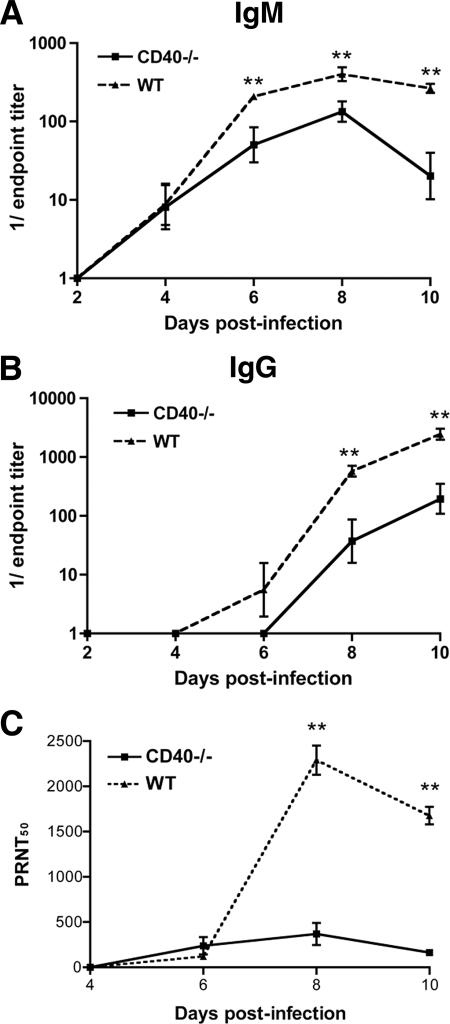
Reduced WNV-specific antibody responses in CD40−/− mice.
Although our studies demonstrated that CD40−/− mice had increased viral burdens in the CNS, the mechanism of control remained unclear. Because CD40 costimulates B-cell activation and antibody maturation (23, 25, 29), we evaluated how an absence of CD40-CD40L interactions affected the kinetics and magnitude of the WNV-specific IgM and IgG response.
(i) IgM.
Consistent with previous studies (11) in wild-type mice, WNV-specific IgM was detected by day 4 after infection (Fig. 3A), and WNV-specific IgG was detected by day 6 after infection (Fig. 3B). Equivalent levels of anti-WNV IgM were observed in wild-type and CD40−/− mice at day 4 after infection (geometric mean titer [GMT] 1/23, CD40−/−; GMT 1/24, wild type; P > 0.5). However, in CD40−/− mice, IgM titers were decreased significantly at day 6 (GMT 1/91, CD40−/−; GMT 1/359, wild type; P < 0.05), day 8 (GMT 1/186, CD40−/−; GMT 1/445, wild type; P < 0.01), and day 10 (GMT 1/62, CD40−/−; GMT 1/278, wild type; P < 0.005).
FIG. 3.
WNV-specific antibody responses. Serum samples from wild-type or CD40−/− mice were collected at the indicated time points. The development of WNV-specific IgM (A) or IgG (B) was determined after incubating serum with purified WNV E protein. (C) Neutralizing activity of serum samples from wild-type or CD40−/− mice at the indicated days was determined by a plaque reduction neutralization titer assay. Asterisks indicate significant differences between wild-type and CD40−/− mice (P < 0.05). Experiments represent 4 to 10 mice per time point, and individual experiments were performed in duplicate.
(ii) IgG.
WNV-specific IgG was first detected 6 days after infection in wild-type mice rising to an average titer of 1/2,500 by day 10 (Fig. 3B). In general, CD40−/− mice exhibited a blunted anti-WNV IgG response. Virus-specific IgG was not detected in CD40-deficient mice until day 8 (GMT 1/100, CD40−/−; GMT 1/700, wild type; P < 0.01) and the levels were significantly lower (GMT 1/500, CD40−/−; GMT 1/2,800, wild type; P < 0.005) than that produced in wild-type mice. Because CD40-CD40L interactions are also required for class-switching (28, 71), we assessed the isotype specificity of the WNV-specific IgG response in CD40−/− mice. Although lower levels of WNV-specific IgG1, IgG2a, IgG2c, and IgG3 were observed, the pattern was not remarkably different compared to wild-type mice (data not shown).
(iii) Neutralizing activity.
To determine how CD40 affected the functional anti-WNV antibody response, we examined neutralizing antibody activity in serum. Low but equivalent levels of neutralizing activity were observed on or before day 6 in wild-type and CD40−/− mice (P ≥ 0.2). However, as expected, at day 10, the increased magnitude of the WNV-specific antibody response led to higher (∼10-fold, P < 0.05) neutralizing activity in serum from wild-type mice (Fig. 3C). To further define the antibody isotype responsible for WNV neutralization, we incubated day 10 serum from CD40−/− and wild-type mice with β-mercaptoethanol to specifically deplete IgM (59). As observed previously (12), this treatment markedly decreased the neutralizing activity of WNV by both wild-type and CD40−/− serum, suggesting that IgM was responsible for much of the WNV neutralizing activity in serum at this time point (data not shown). Collectively, and consistent with its known functions in maturation of antibody responses (29), these experiments demonstrate that a deficiency in CD40 reduced the production of WNV-specific IgM and IgG.
T cell responses in CD40−/− mice.
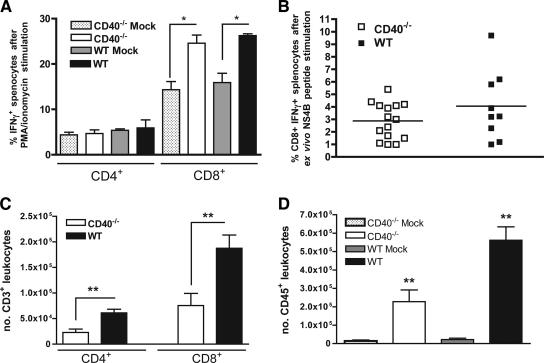
Previous studies have established that CD8+ T cells control and clear WNV from the CNS, whereas CD4+ T cells sustain CD8+ T-cell responses (60, 63, 75). Because we observed uncontrolled replication of WNV in the CNS of CD40−/− mice, we speculated there might be a deficit in WNV-specific T-cell responses in CD40−/− mice. Studies following immunization of mice with ovalbumin, keyhole limpet hemocyanin and human adenovirus type 5 early region 1-expressing mouse embryo cells found that specific T-cell priming was inhibited in the absence of CD40 (4, 22, 58). Initially, we evaluated intracellular IFN-γ expression in splenocytes from mock- and WNV-infected wild-type and CD40−/− mice at day 7 after infection after ex vivo restimulation with the nonspecific agonists PMA and ionomycin (Fig. 4A). We observed a similar 2.5-fold increase in the levels of IFN-γ+ splenic CD8+ T cells in both wild-type and CD40−/− mice (P < 0.01 compared to uninfected mice, P > 0.2 for wild-type compared to CD40−/− mice), suggesting a CD40-independent mechanism for CD8+ T-cell activation. Similar results were obtained when splenocytes were restimulated ex vivo in an antigen-specific manner with a Db-restricted WNV NS4B immunodominant peptide (Fig. 4B). These results showing normal antigen-specific CD8+ T-cell priming in the absence of CD40 are consistent with our virologic results, which demonstrated relatively equivalent clearance kinetics from the spleen: previous studies established a requirement of CD8+ T cells for clearance from this organ (60, 61).
FIG. 4.
T-cell activation and leukocyte trafficking after WNV infection. (A and B) Wild-type or CD40−/− mice were mock treated or infected with WNV. Seven days later, splenocytes were harvested and assayed for activation. The graphs show the percentage of CD4+ IFN-γ+ and CD8+ IFN-γ+ cells after ex vivo restimulation of mock- or WNV-infected splenocytes with phorbol ester and ionomycin (A) or a Db-restricted WNV NS4B peptide (B). The data are an average of at least two independent experiments and reflect 7 to 10 mice per group. Asterisks indicate significant differences (P ≤ 0.05) from mock-treated mice. (C and D) On day 9 after WNV infection of wild-type or CD40−/− mice, brain leukocytes were recovered by Percoll gradient purification and phenotyped with FITC-conjugated anti-CD3 antibody and APC-conjugated anti-CD8 antibody (C) or FITC-conjugated anti-CD45 antibody (D). The data are an average of the total number of CD3+ CD4+, CD3+ CD8+, or CD45+ cells and represent three independent experiments with a total of nine mice. Asterisks indicate significant differences (P ≤ 0.05) between wild-type and CD40−/− mice.
Although no defect in the priming of CD8+ T cells was observed in CD40−/− mice, based on prior studies (60), we suspected that the rise in CNS viral burden in CD40−/− mice was still due to some defect in CD8+ T-cell function or migration. Initially, when we measured the number of WNV-specific CD8+ T cells in the blood at day 7, no difference was observed between wild-type and CD40−/− mice (data not shown). To examine whether a deficiency of CD40 affected T-cell trafficking into the CNS, we measured the levels of leukocytes in the brains of CD40−/− and wild-type mice at day 9 after WNV infection. Using Percoll gradient centrifugation and flow cytometry, we determined the numbers and percentages of CD3+ CD4+, CD3+ CD8+, and CD45+ cells in the brain. Despite higher viral burdens, significantly decreased numbers of infiltrating leukocytes were detected in the brains of CD40−/− mice (Fig. 4C and D). We observed reductions in the number of infiltrating CD3+ CD4+ T cells (wild type, [6.1 ± 2] × 104; CD40−/−, [2.3 ± 1.9] × 104; P < 0.005), CD3+ CD8+ T cells (wild type, [1.9 ± 0.8] × 105; CD40−/−, [0.8 ± 0.7] × 105; P < 0.01), and CD45+ leukocytes (wild type, [5.6 ± 2.1] × 105; CD40, [2.3 ± 1.8] × 105; P < 0.005) in the absence of CD40. These results suggested that CD40-CD40L interactions facilitate T-cell trafficking to the CNS and control of WNV infection.
Chemokine levels in the brain and chemokine receptor expression.
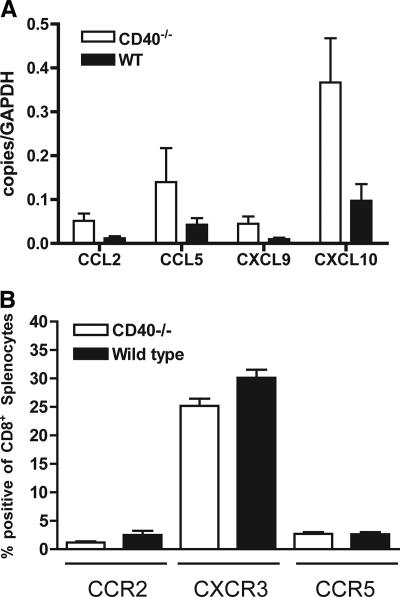
Previous studies demonstrated an essential role for CCR5-CCL5 and CXCR3-CXCL10 chemokine-chemokine receptor interactions in regulating the migration of leukocytes, including CD8+ T cells to the CNS after WNV infection (19, 33). To assess whether a deficiency of CD40 altered the expression of CNS chemokines after WNV infection, we performed quantitative RT-PCR to determine the levels of CCL2, CCL5, CXCL9, and CXCL10. Although these chemokines have documented roles in CNS leukocyte trafficking during viral infections (16, 33, 51), their levels were not decreased in CD40−/− mice (Fig. 5A). Indeed, trends toward higher chemokine levels in the CNS of CD40−/− mice were observed, especially for CXCL10, which likely reflects the elevated viral burden, as seen previously (34). No difference in CCR2, CCR5, and CXCR3 chemokine receptor expression was observed on CD8+ T cells in the spleen (Fig. 5B). Thus, the defect in T-cell trafficking to the CNS observed in CD40−/− mice was not apparently due to altered expression of chemokine or chemokine receptors.
FIG. 5.
Chemokine and chemokine receptor levels. (A) Quantitative RT-PCR analysis of chemokine mRNA levels in the brains of wild-type and CD40−/− mice were measured 9 days after infection with 102 PFU of WNV. Total RNA was analyzed for the expression of CCL2, CCL5, CXCL9, and CXCL10. The data are expressed as copies of chemokine mRNA per copy of glyceraldehyde-3-phosphate dehydrogenase (control) and were obtained from nine mice per group. (B) Chemokine receptor levels on splenic CD8+ T cells. Wild-type or CD40−/− mice were infected with WNV. On day 7, splenocytes were harvested and stained with antibodies against CCR2, CXCR3, and CCR5. Cells were analyzed by flow cytometry. The data are the average of three independent experiments, and none of the differences were statistically significant.
Inflammatory cells localize to blood vessels in the absence of CD40.
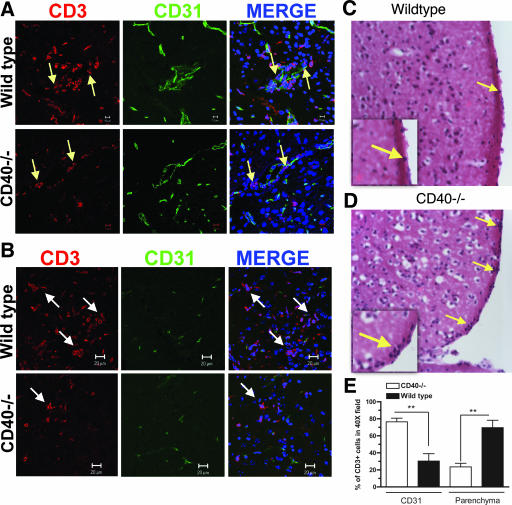
Studies in experimental autoimmune encephalomyelitis have suggested that CD40 is required for CNS inflammation (3, 73, 80). In some of these investigations, the expression of CD40 on endothelial cells increased the adherence of activated CD4+ T cells to the cerebral endothelium, enhancing leukocyte migration (52, 53, 73). We observed a decrease in the overall CNS trafficking of leukocytes in WNV-infected CD40−/− mice. To assess the effect of CD40 expression on trafficking of T cells across the CNS endothelium after WNV infection, histopathological analysis was performed. At day 10 after the infection in wild-type mice, inflammatory cells were scattered throughout the brain parenchyma. In contrast, in CD40−/− mice the inflammatory cells localized primarily near blood vessels or the meninges (Fig. 6C and D and data not shown). To further define this phenotype, brain sections were costained with antibodies to CD3 and CD31 (PECAM), an endothelial cell marker. In agreement with our histopathology findings, in CD40−/− mice CD3+ T cells associated at a greater frequency with CD31-expressing cells, whereas in wild-type mice the majority of CD3+ T cells migrated into the brain parenchyma (Fig. 6A and B). When this difference was quantified, we observed that ∼80% of CD3+ cells localized near CD31+ cells in CD40−/− mice, while in wild-type mice, only ∼30% of CD3+ cells were associated with brain CD31+ cells (P < 0.01) (Fig. 6E). Overall, this analysis suggested that CD40-CD40L interactions were required for efficient T cells egress from the perivascular space into the brain parenchyma during the course of WNV infection.
FIG. 6.
Lymphocyte trafficking patterns in the brain parenchyma after WNV infection. The brains of wild-type and CD40−/− mice were harvested 9 days after infection with WNV, sectioned, and costained for CD31 (green) and CD3 (red); cell nuclei were stained with ToPro3 (blue) (A and B) or stained with hematoxylin and eosin (C and D). Yellow arrows indicate examples of leukocytes associated with meninges or blood vessels, and white arrows indicate CD3+ T cells that have migrated into the parenchyma. Representative images are shown after staining four mice per group in three independent experiments. The insets show a higher-power image and denote the increased density of cells associated with the meninges in CD40−/− mice. (E) Quantitation of colocalization of CD3+ cells with CD31+ cells. Ten different fields were counted at ×400 magnification to determine the percentage of CD3+ cells associated with CD31+ endothelium or in the parenchyma. The data are presented as the percentage of CD31-associated or parenchyma-associated cells in a ×400 field and are derived from experiments from three different mice per group.
Adoptive transfer of CD45.1+ CD8+ T cells into CD45.2+ CD40−/− and wild-type mice.
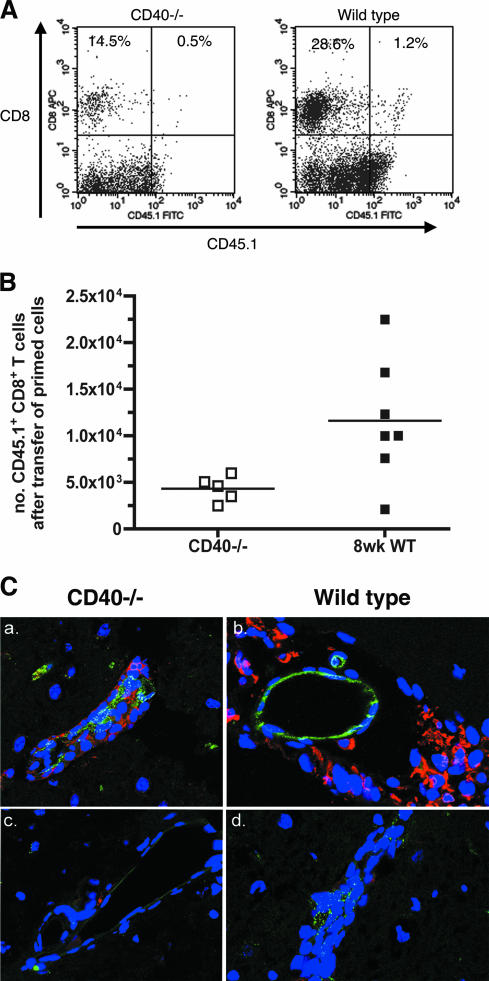
To determine whether CD40 expression in the brain affected the migration of T cells, we adoptively transferred WNV-primed CD45.1+ wild-type CD8+ T cells into CD45.2+ wild-type and CD40−/− mice 4 days after WNV infection. Five days later, the brains were collected, and leukocytes were isolated by Percoll gradient purification, stained for CD8 and CD45.1, and analyzed by flow cytometry (Fig. 7A). Wild-type recipient mice had ∼3-fold-higher numbers of CD45.1+ CD8+ T cells in the brain compared to CD40−/− mice (1.2 × 104 versus 4.3 × 103 cells; P < 0.05) (Fig. 7B). Immunohistochemistry shows that CD45.1 WNV-primed CD8+ T cells migrated into the brain parenchyma of recipient wild-type mice but were retained in the perivascular space of recipient CD40−/− mice (Fig. 7C). Thus, CD40 expression within the brain was crucial for CD8+ T-cell trafficking after WNV infection. Accordingly, although a majority of recipient CD40−/− mice were moribund on day 9, recipient wild-type mice appeared to be completely healthy (data not shown).
FIG. 7.
Adoptive transfer of CD45.1+ CD8+ T cells into CD45.2+ wild-type and CD40−/− mice. WNV-primed CD45.1+ CD8+ T cells from wild-type mice were adoptively transferred into CD45.2+ wild-type or CD40−/− mice 4 days after WNV infection. Leukocytes were isolated from brains 9 days after infection (5 days after transfer), stained with antibodies to CD45.1 and CD8, and analyzed by flow cytometry. (A) Representative flow cytometry profiles showing CD45.1+ CD8+ T cells in the brain after adoptive transfer into CD40−/− (left) and wild-type (right) mice. (B) Total number of CD45.1+ CD8+ T cells that migrated into the brains of CD40−/− and wild-type mice. The difference in the levels of CD45.1 CD8+ T cells in the brains of wild-type and CD40−/− mice was statistically significant (P < 0.05). (C) The brains of recipient CD40−/− (a and c) and wild-type (b and d) C45.2 mice were harvested 9 days after WNV infection and 5 days after adoptive transfer of WNV-primed CD45.1+CD8+ T cells from wild-type mice (a and c). Brains were sectioned, and costained with anti-CD31 (green) (a, b, and d), anti-CD45.1 (red) (a, b, and d), or isotype control antibodies (c). Cell nuclei were stained with ToPro3 (blue). Panel d is from a WNV-infected wild-type C45.2 control mouse that did not receive CD45.1 cells. Representative images are shown after staining brains from several mice.
DISCUSSION
In this study, we show that CD40-CD40L interactions are necessary to control acute WNV infection. Using genetically deficient mice, we demonstrate that CD40 is required for survival after WNV infection, efficient production of virus-specific antibody, trafficking of CD8+ T cells into the brain parenchyma, and control of WNV in the CNS. Although these experiments agree with previous studies showing that CD40 contributes to controlling viral infections (2, 5, 6), unlike most models, which primarily observed a role in coordinating memory B and T-cell responses, we show an absolute requirement for CD40-CD40L interactions during primary WNV infection and a novel T-cell trafficking phenotype in the CNS.
B cells and antibodies are essential for the control of WNV infection and survival of infected mice (11, 15, 46). WNV-specific IgM and IgG titers were significantly blunted in CD40−/− mice, results that agree with studies that demonstrate a role for CD40 in promoting effective primary antibody responses to viral infection (24, 49, 76). However, the IgM or neutralizing responses prior to day 6 were unaffected, suggesting that CD40-independent pathways contribute to the development of specific antibody soon after WNV infection. Consistent with this, viremia, which is limited by the earliest IgM response against WNV (11, 12), was equivalently controlled in wild-type and CD40−/− mice. These results are consistent with studies that suggested a role for IgM produced in a T-cell-independent manner in the neutralization of WNV (63) or the closely related flavivirus, Japanese encephalitis virus (50).
Although CD40-CD40L interactions are required for optimal maturation of antibody responses, we still observed an increase in WNV-specific IgG antibodies after infection in CD40−/− mice, albeit to a lesser degree than wild type. At day 10 after infection, antibodies to WNV of all IgG subclasses were present. Infections with other viruses have also produced isotype-switched antibodies (18, 36, 39, 66) and suggest that class-switching can occur independent of CD40-CD40L-mediated T-cell help. Nonetheless, WNV-specific IgM production was reduced by day 6 in CD40−/− mice, a result that agrees with previous observations that demonstrated a requirement for CD4+ T cells in sustaining the production of WNV-specific IgM (42, 63).
The role of CD40-CD40L interactions in regulating antiviral CD8+ T-cell responses remains controversial. Although some studies suggest an indirect effect of CD40 interactions on CD8+ T-cell activation through activation of antigen-presenting cells, others observed no effect of CD40 deficiency on primary CD8+ T-cell activation (4, 6, 22, 37, 49). In contrast, CD40-dependent responses were uniformly required for establishing memory CD8+ T cells after viral infection (2, 5, 6, 65). In our studies, after ex vivo antigen-specific restimulation, we observed equivalent IFN-γ production by CD8+ T cells from WNV-infected wild-type and CD40−/− mice. Thus, for WNV infection, CD40-CD40L interactions are not essential for initial priming of CD8+ T cells.
CD40−/− mice had significantly reduced leukocyte accumulation in the brain. Several independent and interdependent mechanisms promote leukocyte recruitment into the CNS, including the production of inflammatory cytokines and chemokines and the upregulation and activation of adhesion molecules (14, 47, 70, 79). Although chemokine interactions direct inflammatory responses to the CNS after WNV infection (19, 33), CD40-CD40L interactions had little effect on chemokine receptor expression on activated leukocytes. Unexpectedly, we observed that an absence of CD40 attenuated T-cell migration from the perivascular space into the brain parenchyma. This occurred even though increased levels of CXCL10 were measured in the brain tissues of CD40−/− mice. Thus, enhanced production of CXCL10 in the CNS, by itself, was not sufficient to facilitate T-cell recruitment across the blood-brain barrier. These results agree with recent studies that found no significant increase in T-cell infiltration in transgenic mice in which CXCL10 was constitutively expressed by astrocytes (8).
Our results agree with prior studies that suggest an important effect of CD40 on leukocyte migration across endothelial cells and into tumors (55, 69). The defect in T-cell migration to the CNS in CD40−/− mice is also consistent with studies on CD4+ T-cell trafficking during experimental autoimmune encephalomyelitis (1, 3, 26, 31, 68) and suggest a critical role for CD40-CD40L interactions in the regulation of lymphocyte movement into the CNS. The adoptive transfer experiments showed that CD40 expression in CNS tissues facilitates CD8+ T cells migration into the brain parenchyma. Because CD40 is expressed on microglia and some endothelial cells in the CNS (30, 48, 67), lymphocytes expressing CD40L may interact with CD40 to upregulate or activate adhesion molecules that allow migration across the blood-brain barrier. CD40-CD40L interactions also could induce changes in CNS endothelial cells. Indeed, CXCL12 distribution on blood-brain barrier endothelial cells modulates leukocyte migration across the perivascular space (40), and CD40 engagement can modulate CXCL12 expression (44). Finally, CD40 interactions could also have independent effects on T-cell expansion in the CNS since CD40 engagement on microglial cells enhances antigen presentation (53) and CD40-CD40L interactions between CD4+ and CD8+ T cells may be required to license memory CD8+ T cells (7). In support of this, mice deficient in CD4+ T cells failed to sustain WNV-specific CD8+ T-cell responses in the CNS (63).
Studies with immunodeficient mice continue to define mechanisms of pathogenesis and protection against WNV infection. CD40-CD40L interactions are required for efficient production of antibody and trafficking of effector CD8+ T cells into the CNS. In theory, targeted pharmacological blockade of CD40-CD40L interactions could diminish CNS inflammation and injury after viral infections. Although this may not be beneficial for a highly virulent and cytolytic virus such as WNV, it could be useful for CNS pathogens that cause disease primarily through immunopathological mechanisms.
Acknowledgments
The study was supported by the NIH (grants AI061373 to M.S.D., NS052632 to R.S.K. and M.S.D., NS045607 to R.S.K., and U54 AI057160 and U54 AI05716005 [Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research]) and by the Washington University Markey Scholar Program (E.S.).
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Abromson-Leeman, S., E. Maverakis, R. Bronson, and M. E. Dorf. 2001. CD40-mediated activation of T cells accelerates, but is not required for, encephalitogenic potential of myelin basic protein-recognizing T cells in a model of progressive experimental autoimmune encephalomyelitis. Eur. J. Immunol. 31:527-538. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, M. F., L. Hunziker, R. M. Zinkernagel, T. Storni, and M. Kopf. 2004. Maintenance of memory CTL responses by T helper cells and CD40-CD40 ligand: antibodies provide the key. Eur. J. Immunol. 34:317-326. [DOI] [PubMed] [Google Scholar]
- 3.Becher, B., B. G. Durell, A. V. Miga, W. F. Hickey, and R. J. Noelle. 2001. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J. Exp. Med. 193:967-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett, S. R., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478-480. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, P., A. Tishon, S. Lee, J. Xu, I. S. Grewal, M. B. Oldstone, and R. A. Flavell. 1996. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J. Exp. Med. 183:2129-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow, P., D. F. Tough, D. Eto, A. Tishon, I. S. Grewal, J. Sprent, R. A. Flavell, and M. B. Oldstone. 1998. CD40 ligand-mediated interactions are involved in the generation of memory CD8+ cytotoxic T lymphocytes (CTL) but are not required for the maintenance of CTL memory following virus infection. J. Virol. 72:7440-7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourgeois, C., B. Rocha, and C. Tanchot. 2002. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T-cell memory. Science 297:2060-2063. [DOI] [PubMed] [Google Scholar]
- 8.Boztug, K., M. J. Carson, N. Pham-Mitchell, V. C. Asensio, J. DeMartino, and I. L. Campbell. 2002. Leukocyte infiltration, but not neurodegeneration, in the CNS of transgenic mice with astrocyte production of the CXC chemokine ligand 10. J. Immunol. 169:1505-1515. [DOI] [PubMed] [Google Scholar]
- 9.Campbell, G. L., A. A. Marfin, R. S. Lanciotti, and D. J. Gubler. 2002. West Nile virus. Lancet Infect. Dis. 2:519-529. [DOI] [PubMed] [Google Scholar]
- 10.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T-cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond, M. S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond, M. S., E. M. Sitati, L. D. Friend, S. Higgs, B. Shrestha, and M. Engle. 2003. A critical role for induced IgM in the protection against West Nile virus infection. J. Exp. Med. 198:1853-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebel, G. D., A. P. Dupuis, 2nd, K. Ngo, D. Nicholas, E. Kauffman, S. A. Jones, D. Young, J. Maffei, P. Y. Shi, K. Bernard, and L. D. Kramer. 2001. Partial genetic characterization of West Nile virus strains, New York State, 2000. Emerg. Infect. Dis. 7:650-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelhardt, B., and R. M. Ransohoff. 2005. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 26:485-495. [DOI] [PubMed] [Google Scholar]
- 15.Engle, M. J., and M. S. Diamond. 2003. Antibody prophylaxis and therapy against West Nile virus infection in wild-type and immunodeficient mice. J. Virol. 77:12941-12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eugenin, E. A., K. Osiecki, L. Lopez, H. Goldstein, T. M. Calderon, and J. W. Berman. 2006. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 26:1098-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontana, S., D. Moratto, M. Surinder, M. De Francesco, W. Vermi, S. Ferrari, F. Facchetti, N. Kutukculer, C. Fiorini, M. Duse, P. K. Das, L. D. Notarangelo, A. Plebani, and F. Badolato. 2003. Functional defects of dendritic cells in patients with CD40 deficiency. Blood 102:4099-4106. [DOI] [PubMed] [Google Scholar]
- 18.Franco, M. A., and H. B. Greenberg. 1997. Immunity to rotavirus in T-cell deficient mice. Virology 238:169-179. [DOI] [PubMed] [Google Scholar]
- 19.Glass, W. G., D. H. McDermott, J. K. Lim, S. Lekhong, S. F. Yu, W. A. Frank, J. Pape, R. C. Cheshier, and P. M. Murphy. 2006. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J. Exp. Med. 203:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granwehr, B. P., K. M. Lillibridge, S. Higgs, P. W. Mason, J. F. Aronson, G. A. Campbell, and A. D. Barrett. 2004. West Nile virus: where are we now? Lancet Infect. Dis. 4:547-556. [DOI] [PubMed] [Google Scholar]
- 21.Grewal, I. S., H. G. Foellmer, K. D. Grewal, J. Xu, F. Hardardottir, J. L. Baron, C. A. Janeway, Jr., and R. A. Flavell. 1996. Requirement for CD40 ligand in costimulation induction, T-cell activation, and experimental allergic encephalomyelitis. Science 273:1864-1867. [DOI] [PubMed] [Google Scholar]
- 22.Grewal, I. S., J. Xu, and R. A. Flavell. 1995. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature 378:617-620. [DOI] [PubMed] [Google Scholar]
- 23.Harnett, M. M. 2004. CD40: a growing cytoplasmic tale. Sci. STKE 2004:pe25. [DOI] [PubMed]
- 24.Hebeis, B. J., K. Klenovsek, P. Rohwer, U. Ritter, A. Schneider, M. Mach, and T. H. Winkler. 2004. Activation of virus-specific memory B cells in the absence of T-cell help. J. Exp. Med. 199:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollenbaugh, D., H. D. Ochs, R. J. Noelle, J. A. Ledbetter, and A. Aruffo. 1994. The role of CD40 and its ligand in the regulation of the immune response. Immunol. Rev. 138:23-37. [DOI] [PubMed] [Google Scholar]
- 26.Howard, L. M., A. J. Miga, C. L. Vanderlugt, M. C. Dal Canto, J. D. Laman, R. J. Noelle, and S. D. Miller. 1999. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J. Clin. Investig. 103:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain, A., T. P. Atkinson, P. E. Lipsky, J. E. Slater, D. L. Nelson, and W. Strober. 1999. Defects of T-cell effector function and post-thymic maturation in X-linked hyper-IgM syndrome. J. Clin. Investig. 103:1151-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janeway, C. A., P. Travers, M. Walport, and M. Shlomick. 2001. Immunobiology: the immune system in health and disease, 5th ed. Garland Publishing, New York, NY.
- 29.Kawabe, T., T. Naka, K. Yoshida, T. Tanaka, H. Fujiwara, S. Suematsu, N. Yoshida, T. Kishimoto, and H. Kikutani. 1994. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1:167-178. [DOI] [PubMed] [Google Scholar]
- 30.Ke, Z. J., N. Y. Calingasan, L. A. DeGiorgio, B. T. Volpe, and G. E. Gibson. 2005. CD40-CD40L interactions promote neuronal death in a model of neurodegeneration due to mild impairment of oxidative metabolism. Neurochem. Int. 47:204-215. [DOI] [PubMed] [Google Scholar]
- 31.Ke, Z. J., N. Y. Calingasan, S. S. Karuppagounder, L. A. DeGiorgio, B. T. Volpe, and G. E. Gibson. 2005. CD40L deletion delays neuronal death in a model of neurodegeneration due to mild impairment of oxidative metabolism. J. Neuroimmunol. 164:85-92. [DOI] [PubMed] [Google Scholar]
- 32.Kemball, C. C., E. D. Lee, E. Szomolanyi-Tsuda, T. C. Pearson, C. P. Larsen, and A. E. Lukacher. 2006. Costimulation requirements for antiviral CD8+ T cells differ for acute and persistent phases of polyomavirus infection. J. Immunol. 176:1814-1824. [DOI] [PubMed] [Google Scholar]
- 33.Klein, R. S., E. Lin, B. Zhang, A. D. Luster, J. Tollett, M. A. Samuel, M. Engle, and M. S. Diamond. 2005. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 79:11457-11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanciotti, R. S., A. J. Kerst, R. S. Nasci, M. S. Godsey, C. J. Mitchell, H. M. Savage, N. Komar, N. A. Panella, B. C. Allen, K. E. Volpe, B. S. Davis, and J. T. Roehrig. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, B. O., L. Hartson, and T. D. Randall. 2003. CD40-deficient, influenza-specific CD8 memory T cells develop and function normally in a CD40-sufficient environment. J. Exp. Med. 198:1759-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, B. O., J. Rangel-Moreno, J. E. Moyron-Quiroz, L. Hartson, M. Makris, F. Sprague, F. E. Lund, and T. D. Randall. 2005. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J. Immunol. 175:5827-5838. [DOI] [PubMed] [Google Scholar]
- 37.Lu, Z., L. Yuan, X. Zhou, E. Sotomayor, H. I. Levitsky, and D. M. Pardoll. 2000. CD40-independent pathways of T-cell help for priming of CD8+ cytotoxic T lymphocytes. J. Exp. Med. 191:541-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mach, F., U. Schonbeck, and P. Libby. 1998. CD40 signaling in vascular cells: a key role in atherosclerosis? Atherosclerosis 137(Suppl.):S89-S95. [DOI] [PubMed] [Google Scholar]
- 39.Maloy, K. J., B. Odermatt, H. Hengartner, and R. M. Zinkernagel. 1998. Interferon gamma-producing γδ T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc. Natl. Acad. Sci. USA 95:1160-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCandless, E. E., Q. Wang, B. M. Woerner, J. M. Harper, and R. S. Klein. 2006. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J. Immunol. 177:8053-8064. [DOI] [PubMed] [Google Scholar]
- 41.Mehlhop, E., and M. S. Diamond. 2006. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J. Exp. Med. 203:1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehlhop, E., K. Whitby, T. Oliphant, A. Marri, M. Engle, and M. S. Diamond. 2005. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J. Virol. 79:7466-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullbacher, A., M. Lobigs, and E. Lee. 2003. Immunobiology of mosquito-borne encephalitic flaviviruses. Adv. Virus Res. 60:87-120. [DOI] [PubMed] [Google Scholar]
- 44.Nanki, T., K. Hayashida, H. S. El-Gabalawy, S. Suson, K. Shi, H. J. Girschick, S. Yavuz, and P. E. Lipsky. 2000. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J. Immunol. 165:6590-6598. [DOI] [PubMed] [Google Scholar]
- 45.O'Keefe, G. M., V. T. Nguyen, and E. N. Benveniste. 2002. Regulation and function of class II major histocompatibility complex, CD40, and B7 expression in macrophages and microglia: implications in neurological diseases. J. Neurovirol. 8:496-512. [DOI] [PubMed] [Google Scholar]
- 46.Oliphant, T., M. Engle, G. E. Nybakken, C. Doane, S. Johnson, L. Huang, S. Gorlatov, E. Mehlhop, A. Marri, K. M. Chung, G. D. Ebel, L. D. Kramer, D. H. Fremont, and M. S. Diamond. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 11:522-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Omari, K. M., R. Chui, and K. Dorovini-Zis. 2004. Induction of beta-chemokine secretion by human brain microvessel endothelial cells via CD40/CD40L interactions. J. Neuroimmunol. 146:203-208. [DOI] [PubMed] [Google Scholar]
- 48.Omari, K. M., and K. Dorovini-Zis. 2003. CD40 expressed by human brain endothelial cells regulates CD4+ T-cell adhesion to endothelium. J. Neuroimmunol. 134:166-178. [DOI] [PubMed] [Google Scholar]
- 49.Oxenius, A., K. A. Campbell, C. R. Maliszewski, T. Kishimoto, H. Kikutani, H. Hengartner, R. M. Zinkernagel, and M. F. Bachmann. 1996. CD40-CD40 ligand interactions are critical in T-B cooperation but not for other antiviral CD4+ T cell functions. J. Exp. Med. 183:2209-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan, C. H., H. W. Chen, H. W. Huang, and M. H. Tao. 2001. Protective mechanisms induced by a Japanese encephalitis virus DNA vaccine: requirement for antibody but not CD8+ cytotoxic T-cell responses. J. Virol. 75:11457-11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patterson, C. E., J. K. Daley, L. A. Echols, T. E. Lane, and G. F. Rall. 2003. Measles virus infection induces chemokine synthesis by neurons. J. Immunol. 171:3102-3109. [DOI] [PubMed] [Google Scholar]
- 52.Pluvinet, R., J. Petriz, J. Torras, I. Herrero-Fresneda, J. M. Cruzado, J. M. Grinyo, and J. M. Aran. 2004. RNAi-mediated silencing of CD40 prevents leukocyte adhesion on CD154-activated endothelial cells. Blood 104:3642-3646. [DOI] [PubMed] [Google Scholar]
- 53.Ponomarev, E. D., L. P. Shriver, and B. N. Dittel. 2006. CD40 expression by microglial cells is required for their completion of a two-step activation process during central nervous system autoimmune inflammation. J. Immunol. 176:1402-1410. [DOI] [PubMed] [Google Scholar]
- 54.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 55.Ryschich, E., A. Marten, E. Schmidt, M. Linnebacher, N. Wentzensen, S. Eisold, E. Klar, M. W. Buchler, and J. Schmidt. 2006. Activating anti-CD40 antibodies induce tumour invasion by cytotoxic T-lymphocytes and inhibition of tumour growth in experimental liver cancer. Eur. J. Cancer 42:981-987. [DOI] [PubMed] [Google Scholar]
- 56.Samuel, M. A., and M. S. Diamond. 2005. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 79:13350-13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samuel, M. A., and M. S. Diamond. 2006. Pathogenesis of West Nile virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 80:9349-9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoenberger, S. P., R. E. Toes, E. I. van der Voort, R. Offringa, and C. J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483. [DOI] [PubMed] [Google Scholar]
- 59.Scott, D., and R. K. Gershon. 1970. Determination of total and mercaptoethanol-resistant antibody in the same serum sample. Clin. Exp. Immunol. 6:313-316. [PMC free article] [PubMed] [Google Scholar]
- 60.Shrestha, B., and M. S. Diamond. 2004. Role of CD8+ T cells in control of West Nile virus infection. J. Virol. 78:8312-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shrestha, B., M. A. Samuel, and M. S. Diamond. 2006. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J. Virol. 80:119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shrestha, B., T. Wang, M. A. Samuel, K. Whitby, J. Craft, E. Fikrig, and M. S. Diamond. 2006. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J. Virol. 80:5338-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sitati, E. M., and M. S. Diamond. 2006. CD4+ T cell responses are required for clearance of West Nile virus from the central nervous system. J. Virol. 80:12060-12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun, J. C., and M. J. Bevan. 2004. Cutting edge: long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J. Immunol. 172:3385-3389. [DOI] [PubMed] [Google Scholar]
- 65.Sun, J. C., M. A. Williams, and M. J. Bevan. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szomolanyi-Tsuda, E., and R. M. Welsh. 1998. T-cell-independent antiviral antibody responses. Curr. Opin. Immunol. 10:431-435. [DOI] [PubMed] [Google Scholar]
- 67.Tan, J., T. Town, T. Mori, D. Obregon, Y. Wu, A. DelleDonne, A. Rojiani, F. Crawford, R. A. Flavell, and M. Mullan. 2002. CD40 is expressed and functional on neuronal cells. EMBO J. 21:643-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan, J., T. Town, D. Paris, A. Placzek, T. Parker, F. Crawford, H. Yu, J. Humphrey, and M. Mullan. 1999. Activation of microglial cells by the CD40 pathway: relevance to multiple sclerosis. J. Neuroimmunol. 97:77-85. [DOI] [PubMed] [Google Scholar]
- 69.Thienel, U., J. Loike, and M. J. Yellin. 1999. CD154 (CD40L) induces human endothelial cell chemokine production and migration of leukocyte subsets. Cell. Immunol. 198:87-95. [DOI] [PubMed] [Google Scholar]
- 70.Ubogu, E. E., M. B. Cossoy, and R. M. Ransohoff. 2006. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol. Sci. 27:48-55. [DOI] [PubMed] [Google Scholar]
- 71.van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 67:2-17. [DOI] [PubMed] [Google Scholar]
- 72.Vishnevetsky, D., V. A. Kiyanista, and P. J. Gandhi. 2004. CD40 ligand: a novel target in the fight against cardiovascular disease. Ann. Pharmacother. 38:1500-1508. [DOI] [PubMed] [Google Scholar]
- 73.Wagner, A. H., B. Guldenzoph, B. Lienenluke, and M. Hecker. 2004. CD154/CD40-mediated expression of CD154 in endothelial cells: consequences for endothelial cell-monocyte interaction. Arterioscler. Thromb. Vasc. Biol. 24:715-720. [DOI] [PubMed] [Google Scholar]
- 74.Wang, T., E. Scully, Z. Yin, J. H. Kim, S. Wang, J. Yan, M. Mamula, J. F. Anderson, J. Craft, and E. Fikrig. 2003. IFN-gamma-producing gamma delta T cells help control murine West Nile virus infection. J. Immunol. 171:2524-2531. [DOI] [PubMed] [Google Scholar]
- 75.Wang, Y., M. Lobigs, E. Lee, and A. Mullbacher. 2003. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J. Virol. 77:13323-13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitmire, J. K., M. K. Slifka, I. S. Grewal, R. A. Flavell, and R. Ahmed. 1996. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J. Virol. 70:8375-8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Willer, D. O., and S. H. Speck. 2005. Establishment and maintenance of long-term murine gammaherpesvirus 68 latency in B cells in the absence of CD40. J. Virol. 79:2891-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winkelstein, J. A., M. C. Marino, H. Ochs, R. Fuleihan, P. R. Scholl, R. Geha, E. R. Stiehm, and M. E. Conley. 2003. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine 82:373-384. [DOI] [PubMed] [Google Scholar]
- 79.Yednock, T. A., C. Cannon, L. C. Fritz, F. Sanchez-Madrid, L. Steinman, and N. Karin. 1992. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 356:63-66. [DOI] [PubMed] [Google Scholar]
- 80.Yellin, M. J., J. Brett, D. Baum, A. Matsushima, M. Szabolcs, D. Stern, and L. Chess. 1995. Functional interactions of T cells with endothelial cells: the role of CD40L-CD40-mediated signals. J. Exp. Med. 182:1857-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]