Abstract
The respiratory tract is a major mucosal site for microorganism entry into the body, and type I interferon (IFN) and dendritic cells constitute a first line of defense against viral infections. We have analyzed the interaction between a model DNA virus, plasmacytoid dendritic cells, and type I IFN during lung infection of mice. Our data show that murine gammaherpesvirus 68 (γHV68) inhibits type I IFN secretion by dendritic cells and that plasmacytoid dendritic cells are necessary for conventional dendritic cell maturation in response to γHV68. Following γHV68 intranasal inoculation, the local and systemic IFN-α/β response is below detectable levels, and plasmacytoid dendritic cells are activated and recruited into the lung with a tissue distribution that differs from that of conventional dendritic cells. Our results suggest that plasmacytoid dendritic cells and type I IFN have important but independent roles during the early response to a respiratory γHV68 infection. γHV68 infection inhibits type I IFN production by dendritic cells and is a poor inducer of IFN-α/β in vivo, which may serve as an immune evasion strategy.
Respiratory viral infections are the leading cause of acute illnesses worldwide, and several members of the herpesvirus family are responsible for severe pneumonia in neonates and immunocompromised patients (52). Herpes simplex virus, cytomegalovirus, varicella-zoster virus, Epstein-Barr virus, human herpesvirus 6, and Kaposi's sarcoma-associated herpesvirus (KSHV) have all been associated with respiratory diseases (7, 10, 30, 33, 42, 46). Much of our understanding of immune responses to viral infection of the respiratory tract comes from experimental animal models. Murine gammaherpesvirus 68 (γHV68) is structurally and biologically similar to the human gammaherpesviruses Epstein-Barr virus and KSHV (16, 49, 50), and it has become a useful in vivo model of herpesvirus infection. Intranasal infection of mice with γHV68 causes an acute respiratory infection that is rapidly resolved and followed by the establishment of splenic latency mainly in the B-cell compartment (16, 34). Analogous to KSHV (35, 38, 41), γHV68 also infects dendritic cells, a process that may act as a mechanism of immune evasion (18, 20).
Plasmacytoid dendritic cells are professional type I interferon (IFN)-producing cells that quickly respond to most viruses by secreting large amounts of type I IFNs (28). Type I IFN signaling is important for the control of acute γHV68 infection (17, 51). In addition, plasmacytoid dendritic cells secrete cytokines and interact with conventional dendritic cells and T cells (28) and are critical for the defense against parenteral and mucosal infections (1, 13, 25, 29). Although plasmacytoid dendritic cells have been detected in the lungs (12, 14) and have been shown to prevent the development of allergic asthma, we have limited information regarding their role in the antiviral response of the respiratory tract. This is of special importance because the lung is the largest epithelial surface in the body and constitutes a major portal of entry for microorganisms (32). The regulation of immune responses in the respiratory tract must be tightly controlled to elicit an adequate defense against invading agents while maintaining tolerance to innocuous antigens. Dendritic cells have a central role in maintaining homeostasis by discriminating pathogens from harmless antigens and eliciting the right response to induce immunity or tolerance, respectively (44). Not surprisingly, many viruses have developed strategies for disrupting dendritic cell function (36, 37), and the immune system has developed systems such as plasmacytoid dendritic cells to quickly detect and respond to viruses (28).
In this study, we have looked at the interplay between a model double-stranded DNA (dsDNA) virus, plasmacytoid dendritic cells, and type I IFNs during lung infection. The data show that plasmacytoid dendritic cells are necessary for the maturation of conventional dendritic cells and that γHV68 inhibits type I IFN secretion by dendritic cells. Following γHV68 infection, plasmacytoid dendritic cells are activated and recruited into the lung with a tissue distribution that differs from that of conventional dendritic cells. No local or systemic IFN-α/β activity was detected following intranasal γHV68 instillation, and the production of IFN-α mRNA was limited to scattered epithelial cells within the respiratory tract. Our results indicate that plasmacytoid dendritic cells and type I IFN have important but independent roles during the early response to a respiratory DNA virus infection.
MATERIALS AND METHODS
Virus stocks.
γHV68 clone WUMS was propagated and titers were determined on monolayers of NIH 3T3 fibroblasts. Respiratory syncytial virus (RSV) strain A2 was grown in HEp-2 cells. Newcastle disease virus (NDV) was grown in 10-day-old embryonated chicken eggs, and titers were determined by immunofluorescence. Influenza virus A/WSN/33 (H1N1) was grown in Madin-Darby bovine kidney cells, and titers were determined by immunofluorescence.
Animal procedures and virus infection.
C57BL/6J mice were purchased from Taconic Farms or Harlan Sprague Dawley Inc. and housed under specific-pathogen-free conditions in biosafety level 2 containment. IFN-α/β receptor-deficient (IFN-α/βR−/−) and control (129SvEv strain) mice were bred at the Columbus Children's Research Institute (CCRI). The Institutional Animal Care and Use Committee at CCRI approved all studies described here. Mice were anesthetized with 2,2,2-tribromoethanol and inoculated with 103 PFU of γHV68, 102 PFU influenza virus A/WSN/33 (H1N1), or 5 × 105 PFU NDV in Hanks balanced salt solution.
Dendritic cell cultures.
Dendritic cells were generated from bone marrow cultures in complete tissue culture medium supplemented with 20 ng/ml murine recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (Peprotech) or 100 ng/ml human recombinant Flt3-L (Peprotech). On days 6 to 8, the dendritic cells were infected with 1 × 108 to 3.3 × 108 PFU of γHV68, RSV, or NDV and stimulated with 10 μg/ml lipopolysaccharide (LPS) (Sigma) or 2 μg/ml CpG ODN1826 (InvivoGen) if needed.
Fluorescence-activated cell sorter (FACS) analysis.
Single-cell suspensions were obtained from tissues after collagenase D (5 mg/ml; Roche) treatment for 45 min. Cells were next incubated with 5 mM phosphate-buffered saline (PBS)-EDTA for 10 min at room temperature to disrupt multicellular complexes. The cells were Fc blocked and stained with combinations of the following antibodies: CD11c, CD11b, B220, CD8a, CD19, NK1.1, CD3, CD80, CD86, Kb, CD54, I-A, and CD40. Samples were washed and resuspended in 1% paraformaldehyde diluted in PBS before analysis. Flow cytometry data were acquired on a FACSCalibur or LSR (Becton-Dickinson) apparatus and analyzed using FlowJo software (TreeStar, Inc.).
Plaque assay.
To determine the titer of infectious virus, lungs were stored frozen and mechanically homogenized. The lytic virus concentration of the lung homogenates or of dendritic cell culture supernatants was determined in a standard plaque assay on NIH 3T3 fibroblasts.
IFN-α/β bioassay.
An IFN-α/β bioassay was performed as described previously (31). Briefly, supernatants were acid treated to inactivate any input virus as well as other cytokines. Samples were then neutralized, and twofold dilutions of each sample were added to murine fibroblast monolayers. The next day, 1.25 × 105 PFU of vesicular stomatitis virus (VSV) were added to each well. Controls included untreated monolayers plus and minus VSV infection and IFN-α/β standards. After 2 days of incubation, wells were fixed and stained. IFN-α/β concentrations were determined by a comparison of protection from VSV-induced cell killing with that seen with known amounts of IFN-α/β.
Immunohistochemistry.
Frozen tissue sections were fixed in cold acetone for 10 min. Endogenous peroxidase was neutralized using PBS-0.3% H2O2-0.1% sodium azide. The sections were stained with anti-CD11c (eBioscience), anti-mPDCA-1 (Miltenyi), or anti-M3 (23) antiserum followed by peroxidase-conjugated anti-immunoglobulin G antibodies (Jackson ImmunoResearch), and staining was visualized with 3-amino-9-ethylcarbazole. The sections were counterstained with hematoxylin and viewed on an Axioscop 2plus apparatus. Images were captured using a Axiocam HRc digital camera with Axiocam software.
In situ hybridization.
Tissues were harvested on days 2, 3, and 7 after infection with influenza virus or γHV68 or mock infection. To detect type I IFN transcripts in the lung and mediastinal lymph node (MLN) sections, we synthesized digoxigenin-labeled riboprobes of murine IFN-α4 and IFN-β genes using a digoxigenin (DIG) RNA labeling kit (Roche) according to the manufacturer's instructions. After deparaffinization and prehybridization of the tissue sections, DIG-labeled riboprobes at 60 ng/sample were diluted into hybridization buffer and incubated with the sections overnight at 42°C. Next, the sections were stained with anti-DIG alkaline phosphatase (Roche), and the signal was detected with nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate). The sections were washed in water and counterstained with nuclear fast green. Adjacent serial tissue sections were stained with hematoxylin and eosin.
RESULTS
Plasmacytoid dendritic cells are necessary for in vitro activation of conventional dendritic cells in response to γHV68.
Previous studies of the interaction between γHV68 and dendritic cells have shown that γHV68 infection of bone marrow-derived dendritic cells cultured with GM-CSF does not induce dendritic cell maturation, although γHV68 does not prevent the activation of infected dendritic cells by other stimuli (19). Despite the impact of γHV68 on dendritic cell function, infected mice eventually mount an immune response that controls infectious γHV68 but never clears latent infection (16). It is unclear whether plasmacytoid dendritic cells or other dendritic cells mediate the recognition of γHV68 and how the immune response to this virus is initiated at the site of infection. It is thus possible that cells other than conventional dendritic cells first detect the presence of γHV68 infection and initiate the adaptive immune response. Recognition of dsDNA viruses is mediated by Toll-like receptor 9 (TLR-9) and plasmacytoid dendritic cells (25, 26). However, in the mouse, both conventional and plasmacytoid dendritic cells can be activated in vivo by TLR-4, TLR-7, or TLR-9, but they differ in their requirements for type I IFNs for activation and migration (2).
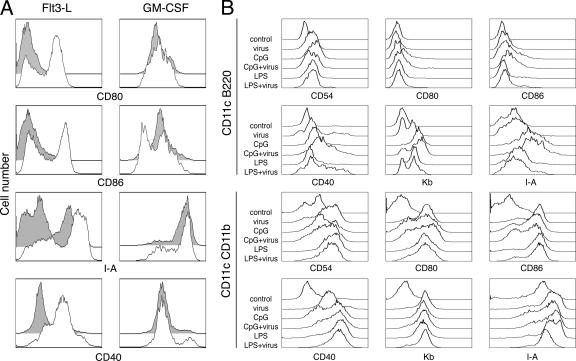
To initially characterize the response of plasmacytoid dendritic cells to γHV68, we generated bone marrow-derived dendritic cells in the presence of GM-CSF or Flt3-L, infected the cultures with γHV68, and monitored cell activation by cell surface analysis of the expression of several costimulatory and major histocompatibility complex molecules. As previously reported (19), γHV68 infection of dendritic cells grown in GM-CSF did not up-regulate the surface expression of CD80, CD86, CD40, or I-A molecules compared with mock-infected controls (Fig. 1A). However, the data show that γHV68 infection of dendritic cells generated in the presence of Flt3-L induced robust cell surface up-regulation of all the activation markers analyzed. Dendritic cells generated in the presence of GM-CSF constitute a homogeneous population with 95% CD11c+ CD11b+ conventional dendritic cells (5; data not shown). Dendritic cells generated with Flt3-L are heterogeneous and contain a mixture of 20 to 30% CD11c+ B220+ plasmacytoid dendritic cells and 70 to 80% CD11c+ CD11b+ conventional dendritic cells (5; data not shown). Next, we questioned whether the activation of Flt3-L-derived dendritic cells by γHV68 was homogeneous or whether conventional and plasmacytoid dendritic cell subpopulations had distinct responses to the virus. We analyzed the activation status of each subpopulation of dendritic cells in Flt3-L cultures under different stimulatory conditions (Fig. 1B). γHV68 induced the up-regulation of surface molecules on plasmacytoid dendritic cells to the same extent as LPS, a TLR-4 agonist. In addition, stimulation with the TLR-9 agonist CpG induced a more robust response by plasmacytoid dendritic cells, and γHV68 infection did not prevent the changes induced by LPS or CpG. Analysis of the conventional dendritic cell subset showed equivalent up-regulation of CD54, CD80, CD86. CD40, Kb, and I-A in response to γHV68, LPS, or CpG. Taken together, our results indicate that plasmacytoid dendritic cells are necessary for conventional dendritic cell activation in response to γHV68 infection.
FIG. 1.
Dendritic cell activation in response to γHV68 infection. (A) Bone marrow-derived dendritic cells grown in the presence of Flt3-L (left column) or GM-CSF (right column) were infected (empty histograms) or not infected (gray histograms) with γHV68 as described in Materials and Methods. Forty hours later, the cells were surface stained with antibodies against CD11c and the indicated activation markers to analyze their fluorescence intensity on a flow cytometer. (B) Bone marrow-derived dendritic cells grown in the presence of Flt3-L were infected or not infected with γHV68 and stimulated with CpG-ODN or LPS as described in Materials and Methods. Forty-eight hours after treatment, the cells were harvested and stained with antibodies against the indicated cell surface markers to analyze their fluorescence intensity. Dendritic cells were previously gated as plasmacytoid dendritic cells (CD11c+ B220+) or conventional dendritic cells (CD11c+ CD11b+). The data are representative of three independent experiments. The data shown in A and B are from two independent experiments.
γHV68 inhibits type I IFN production by dendritic cells.
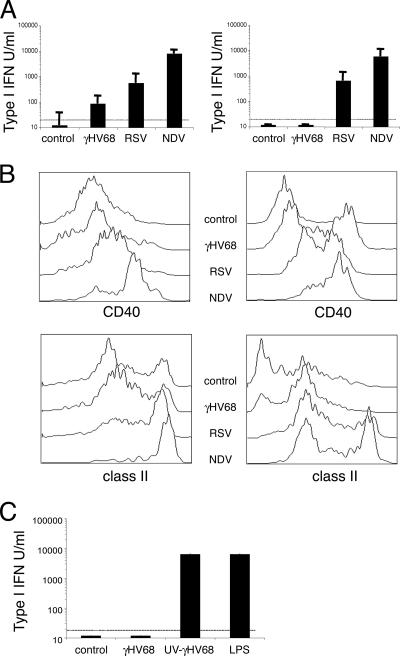
To further investigate the consequences of the interaction of γHV68 with dendritic cells for the induction of immunity, we compared dendritic cell activation and type I IFN production during γHV68 infection with that of two model viruses, RSV and NDV. RSV is a poorly immunogenic virus with reinfections occurring throughout life and a model of inhibition of type I IFNs (39, 43). NDV is a potent inducer of both type I IFN production and dendritic cell maturation (6, 22). First, we analyzed levels of IFN-α/β bioactivity in response to viral stimulation in dendritic cell cultures. The data reported in Fig. 2A show that NDV induced strong type I IFN production with a response 10-fold greater than that of RSV, which correlates with previous observations (31). γHV68 did not induce any significant amounts of type I IFN bioactivity in dendritic cell cultures. A similar pattern of type I IFN bioactivity for all three viruses was obtained using dendritic cells cultured with GM-CSF or with Flt3-L. Second, we analyzed dendritic cell activation after γHV68, RSV, or NDV infection. As shown in Fig. 2B (left column), both RSV and NDV induced robust activation of GM-CSF dendritic cells, as measured by the up-regulation of surface expression of CD40 and I-A molecules. As expected, γHV68 did not induce the up-regulation of the activation markers analyzed. All three viruses induced the activation of Flt3-L-derived dendritic cells although to a different extent (Fig. 2B, right column). Taken together, these results indicate that γHV68 is a poor inducer of type I IFN production by dendritic cells. In addition, the data suggest that the lack of IFN-α/β bioactivity is independent of the maturation state of the dendritic cells. To test whether the lack of IFN-α/β bioactivity in culture supernatants is due to an active viral process that requires γHV68 replication or is due to the poor immunogenicity of γHV68 particles, we used UV-inactivated γHV68 to stimulate dendritic cell cultures. The data show that UV-inactivated γHV68 induced 1,000-fold more IFN-α/β synthesis by dendritic cells than did live γHV68 (Fig. 2C). These data indicate that the inhibition of type I IFN production by dendritic cells is an active process that requires γHV68 replication.
FIG. 2.
γHV68 inhibits type I IFN production by dendritic cells. (A) Type I IFN induction by γHV68, RSV, or NDV was measured in cell culture supernatants 40 h after infection using an IFN-α/β bioassay. The dendritic cells were grown in the presence of GM-CSF (left column) or Flt3-L (right column). (B) The relative abilities of γHV68, RSV, or NDV to induce maturation of dendritic cells were tested using bone marrow-derived dendritic cells grown in the presence of GM-CSF (left column) or Flt3-L (right column). The cell cultures were infected as described in Materials and Methods, and 40 h later, the cells were surface stained with antibodies against CD11c, CD40, and I-A. The histograms shown have been previously gated as CD11c+ cells. (C) Type I IFN induction by UV-inactivated γHV68 in dendritic cell cultures supplemented with GM-CSF. The data presented are the means and standard deviations of triplicate dendritic cell cultures.
Anatomical distribution of dendritic cell subsets within the lung.
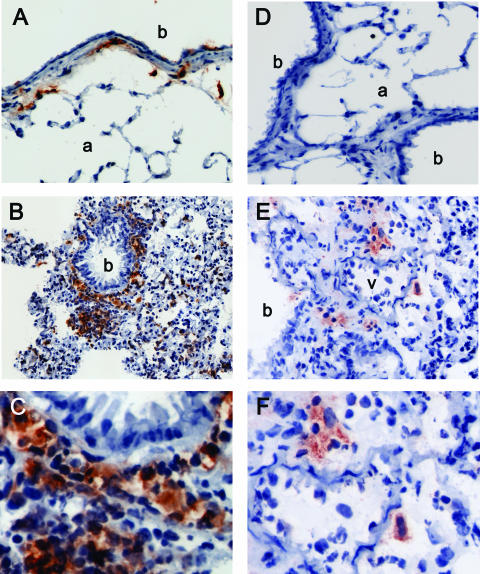
In vivo dendritic cell activation by microbial products and viruses has been shown to induce dendritic cell redistribution in the spleen (2, 9). However, we have limited information regarding the distribution of different subsets of dendritic cells in the respiratory tract. Until recently, the existence of plasmacytoid dendritic cells in the lung and their immunoregulatory role in response to inhaled antigens were unknown (12). We analyzed the distribution of conventional dendritic cells and plasmacytoid dendritic cells within the lung and the spleen at early time points after intranasal infection with γHV68. Because of the low level of expression of CD11c on plasmacytoid dendritic cells in vivo, immunohistochemical staining distinguishes between plasmacytoid dendritic cells and conventional dendritic cells (2). In naïve control mice (Fig. 3A), numerous CD11c+ cells were found interdigitating between respiratory epithelial cells and within the submucosa of conducting airways. In addition, scattered CD11c+ cells were present in the alveolar spaces. After γHV68 infection (Fig. 3B and C), CD11c+ cells were more numerous, forming a continuous layer beneath the bronchiolar epithelium and concentrating in areas of inflammation. Cells positive for mPDCA-1, a marker specific for plasmacytoid dendritic cells, could not be visualized in uninfected control mice (Fig. 3D). However, after γHV68 infection, mPDCA-1+ cells were found scattered or in small clusters in areas of inflammation of the lung parenchyma adjacent to blood vessels (Fig. 3D). These results show that conventional and plasmacytoid dendritic cells are distributed differently within the lung.
FIG. 3.
Lung dendritic cell location after γHV68 infection. Lungs were sampled from naïve mice (A and D) or from γHV68-infected mice at 7 days postinfection (B and E). Serial sections were stained with anti-CD11c (left panels) or anti-mPDCA-1 (right panels) as described in Materials and Methods. Objective magnification, ×20. C and F show a detail of the tissue area adjacent to the bronchi (b) or blood vessel (v) from B and E, respectively. One representative staining out of three mice per group is shown from three independent experiments. a, arteriole.
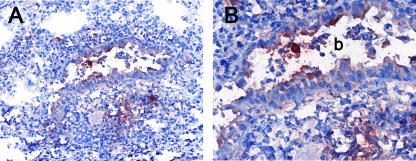
To determine whether plasmacytoid dendritic cells were recruited toward infection sites or to the lung in general, we analyzed the distribution of the γHV68 antigen M3 in the lung of infected mice. As shown in Fig. 4, M3 expression is detected on cells of the airway epithelium, mononuclear cells in the airways, and individual cells in areas of inflammation of the lung parenchyma. Altogether, these data suggest that mPDCA-1+ plasmacytoid dendritic cells and γHV68 antigens localize in inflamed areas of the lung parenchyma of infected mice.
FIG. 4.
Location of viral antigens in the lung of γHV68-infected mice. Lungs were sampled from γHV68-infected mice at 7 days postinfection, and sections were stained with anti-γHV68 M3 antiserum. (A) Area of inflammation in the lung parenchyma. Magnification, ×20. (B) Detail of the bronchi (b) from A. Magnification, ×40.
Plasmacytoid dendritic cell recruitment to the lung following γHV68 infection.
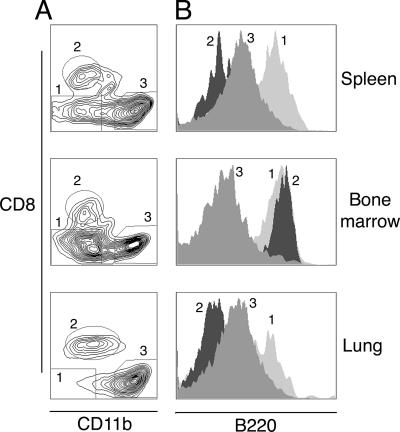
We have used enzymatic tissue digestion and flow cytometry to analyze the migration of dendritic cells in the respiratory tract in response to γHV68 infection. We have compared this information with that for the spleen, where dendritic cell subsets are well described, and bone marrow, where dendritic cell precursors are generated. During the study, lineage-positive cells (CD19+, CD3+, and NK1.1+) and macrophages (CD11b+ CD11cdim and/or large forward-scatter/side-scatter autofluorescent cells) were excluded from the analysis. Dendritic cells were gated using forward scatter/side scatter and low autofluorescence as plasmacytoid dendritic cells (CD11cdim B220+) and conventional dendritic cells (CD11chigh CD8α+ or CD11chigh CD11b+). Figure 5 shows a representative plot of the CD11c+ population in lung, bone marrow, and spleen 5 days after infection and the B220 expression profile of three different subsets of dendritic cells. The different subsets of respiratory tract dendritic cells presented the same B220 staining profile as their splenic counterparts.
FIG. 5.
Identification of dendritic cell subsets in respiratory tract mPDCA-1+ cells. (A) Expression of CD8α and CD11b in previously gated CD11c+ cells defines different dendritic cell subsets in the lung, bone marrow, and spleen of mice 5 days after γHV68 infection: 1, plasmacytoid dendritic cells; 2, CD8 conventional dendritic cells; 3, conventional CD11b dendritic cells. (B) B220 cell surface expression on mouse dendritic cell subsets as previously gated in A. Data are representative of three independent experiments.
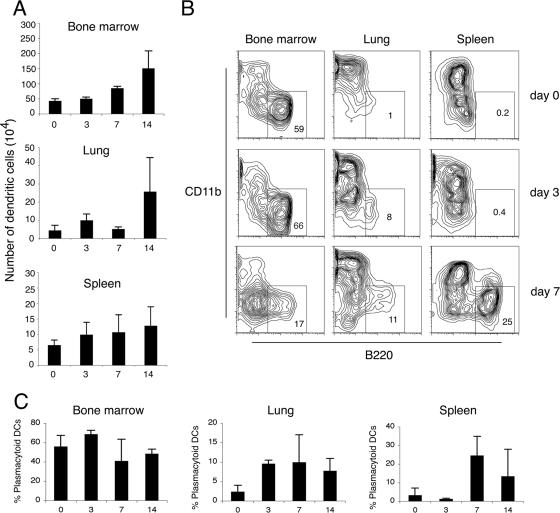
We next did a temporal kinetic analysis of the numbers of dendritic cells in various tissues following γHV68 infection. The data in Fig. 6A show an increase in the absolute numbers of dendritic cells after γHV68 infection in all the tissues analyzed. This increase starts at day 3 after infection in lung and spleen, although at that time, viral replication is restricted to the respiratory tract (8, 45). By the time that lytic virus is cleared from the respiratory tract and viral latency peaks in the spleen at day 14 (8), the dendritic cell numbers have increased two- to threefold in lung, spleen, and bone marrow. To analyze the composition of the different subsets of dendritic cells and the frequency of plasmacytoid dendritic cells present in the lung at different times after γHV68 infection, we digested whole lungs and compared them with spleen and bone marrow. As observed in the representative plots shown in Fig. 6B, plasmacytoid dendritic cells migrate into the lung after intranasal instillation with γHV68, and by days 3 to 7, a distinct population can be observed in the FACS contour plots. Simultaneously, the frequency of plasmacytoid dendritic cells in the spleen also increased, with a distinct population becoming evident by day 7. An analysis of the frequency of plasmacytoid dendritic cells in lung showed a twofold increase in the percentage of plasmacytoid dendritic cells from days 3 to 14 after infection and up to a sixfold increase in the spleen at day 7 after infection (Fig. 6C). Altogether, these data indicate that (i) plasmacytoid dendritic cells are differentially recruited into the lung after gammaherpesvirus infection and (ii) plasmacytoid dendritic cells are also being recruited into the spleen, although viral replication is restricted to the lung.
FIG. 6.
Dendritic cell migration into the lung and spleen in response to γHV68 infection. (A) Time course analysis of the absolute dendritic cell numbers in bone marrow, lung, and spleen after γHV68 infection. The data presented are the means and standard deviations of three independent experiments, each containing three mice. (B) Plasmacytoid dendritic cells migrate into the lung and spleen after γHV68 infection. Numbers indicate the percentages of cells inside the gate. One representative of three experiments is shown. (C) Time course analysis of the frequency of plasmacytoid dendritic cells (DC) in bone marrow, lung, and spleen after γHV68 infection. The data presented are the means and standard deviations of three independent experiments, each containing three mice. In all the panels shown, dendritic cells were analyzed as CD11c+ and lineage-negative (CD3, CD19, and NK1.1) nonautofluorescent cells.
Kinetics of dendritic cell activation and type I IFN secretion in response to γHV68 infection.
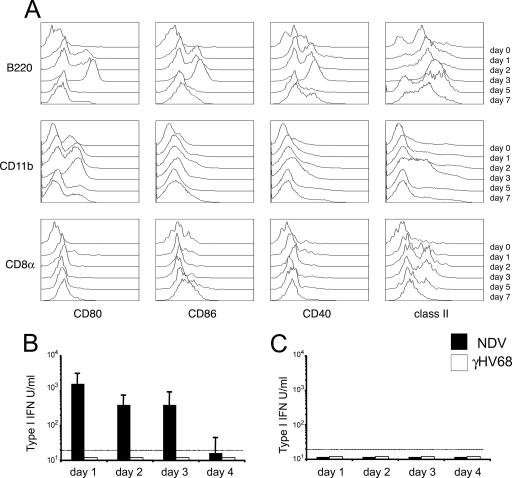
Dendritic cells play a central role in the induction of adaptive and innate immune responses to respiratory tract infections. Although “myeloid” dendritic cells constitute the predominant population of pulmonary dendritic cells in humans (47) and mice (11), other subsets also play essential roles in the response to inhaled antigens (12, 48). Our previous in vitro results suggest that plasmacytoid dendritic cells are essential for host detection of γHV68 infection. Thus, we questioned which subset(s) of respiratory dendritic cells was activated in response to infection in vivo. To investigate the kinetics of plasmacytoid and conventional dendritic cell activation in the lung, we monitored the surface expression of several costimulatory (CD80, CD86, and CD40) and major histocompatibility complex (I-A) molecules on B220+ plasmacytoid dendritic cells, conventional CD11b+ dendritic cells, and conventional CD8α+ dendritic cells. Plasmacytoid dendritic cells up-regulated all activation markers analyzed by 24 h after infection, and this state of activation was maintained until day 5 after infection (Fig. 7A). Conventional dendritic cells did not show any phenotypic changes until days 2 to 3 and then only partially up-regulated some of the markers analyzed: CD80 (CD11b+ dendritic cells) and class II (CD11b+ and CD8α+ dendritic cells). Thus, plasmacytoid dendritic cells are the first dendritic cell subpopulation at the site of infection that up-regulates activation markers in response to γHV68. In addition, plasmacytoid dendritic cells display a more robust state of activation than their conventional dendritic cell counterparts in the lung. These data suggest that plasmacytoid dendritic cells are the first lung dendritic cell population to detect γHV68 infection after intranasal inoculation.
FIG. 7.
Dendritic cell maturation and IFN-α/β production in γHV68-infected mice. (A) Dendritic cell subsets undergo differential maturation in the lung in response to γHV68 infection. Lung dendritic cells were analyzed at different time points after γHV68 infection (days 0 to 7) for the level of cell surface expression of several activation markers (CD80, CD86, CD40, and I-A). Histograms are previously gated as CD11c+ B220+ (plasmacytoid dendritic cells) (first row), CD11c+ CD11b+ (conventional dendritic cells) (second row), or CD11c+ CD8a+ (conventional dendritic cells) (third row). (B) Type I IFN bioactivity in BAL fluid of γHV68- and NDV-infected mice at different time points after infection. (C) Type I IFN bioactivity in serum of γHV68- and NDV-infected mice at different time points after infection. The data presented are the means and standard deviations for three to four mice.
The recognition of dsDNA viruses by plasmacytoid dendritic cells triggers IFN-α secretion (28), and the activation and migration of plasmacytoid dendritic cells is thought to be dependent on type I IFN (2). To determine the role of IFN-α/β during γHV68 respiratory infection, we measured the amount of type I IFN present in bronchoalveolar lavage (BAL) samples and sera of γHV68-infected mice. Mice intranasally infected with NDV were used as positive controls. The data in Fig. 7B show that no IFN-α/β activity could be detected in BAL samples or sera of γHV68-infected mice. In addition, although intranasal infection with NDV induces potent IFN-α/β bioactivity in BAL samples, the amount of systemic type I IFNs in serum was below the level of detection of our bioassay.
Type I IFN production and dendritic cell activation during γHV68 infection of the lung.
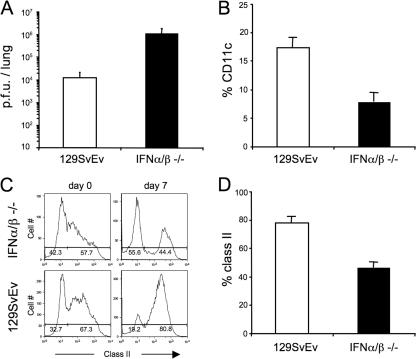
Type I IFNs are critical in the defense against viral infections by direct inhibition of viral replication in infected cells as well as through immunoregulatory effects (4). IFN-α/β has been shown to be important for plasmacytoid dendritic cell activation and for conventional dendritic cell activation and migration (2, 22). Our previous results suggested that plasmacytoid dendritic cell activation may be critical for the induction of a conventional dendritic cell response to γHV68 in mice and demonstrated that γHV68 does not induce a detectable type I IFN response on dendritic cell cultures or in the lung. Next, we analyzed lung virus titers and dendritic cell activation and recruitment into the lung of γHV68-infected mice using IFN-α/βR−/− and wild-type mice (Fig. 8). As expected, IFN-α/βR−/− mice showed a 100-fold increase in infectious virus compared with normal mice. These data corroborate the important role of type I IFN signaling in the control of γHV68 lytic infection (17, 51). In addition, and despite the increased virus production, IFN-α/βR−/− mice showed a two- to threefold decrease in the recruitment of dendritic cells into the lung after γHV68 infection (Fig. 8B). The frequency of activated dendritic cells as measured by class II expression was also lower in IFN-α/βR−/− mice (40%) than in wild-type mice (80%) after γHV68 infection (Fig. 8C and D). These data suggest that type I IFN signaling enhances, but is not an absolute requirement, for dendritic cell recruitment into and activation in the lung after γHV68 infection.
FIG. 8.
Role of type I IFN signaling in virus control and dendritic cell recruitment and activation in the lung. (A) Infectious virus titers in the lung of 129SvEv or IFN-α/βR−/− mice were determined by plaque assay on day 5 after virus inoculation. (B) Frequency of CD11c+ dendritic cells in the lung of γHV68-infected mice. (C) Representative histograms of the level of expression of class II molecules on CD11c+ cells in the lung. Numbers indicate the percentage of cells inside the gate. (D) Frequency of class II expression on CD11c+ cells in the lung. FACS analysis was performed on naïve and γHV68-infected mice on day 7. The data presented are the means and standard deviations for three individual mice.
There is a discrepancy between the important role of IFN-α/β in controlling γHV68 respiratory infection and our inability to detect IFN-α/β bioactivity in BAL and serum samples of infected mice. To resolve this apparent contradiction, we used in situ hybridization to analyze the production of type I IFN in lung and draining lymph nodes and to determine which cells are responsible for its production in vivo. We analyzed infected tissues on days 2, 3, and 7 after intranasal viral inoculation using probes for IFN-α4 and for IFN-β. Mice infected with 102 PFU of influenza virus were used as positive controls. The data show that in influenza virus-infected mice, a strong IFN-α/β signal is detected in the epithelium of the bronchi and in a few scattered cells in the airways (Fig. 9A to C). On the contrary, γHV68-infected mice showed a very weak IFN-α/β-positive signal in lung sections exclusively in scattered bronchiolar epithelial cells (Fig. 9D to F). The analysis of tissue sections from MLN of influenza virus-infected mice revealed IFN-α/β-positive cells distributed throughout the lymph node (Fig. 9G and H). Mice infected with γHV68 showed no IFN-α/β signal in the draining lymph node (Fig. 9I and J). Similar results were obtained using the α and β probes and at all different time points analyzed. Altogether, the data indicate that intranasal γHV68 infection does not induce a potent type I IFN response in either the lung or its draining lymph nodes.
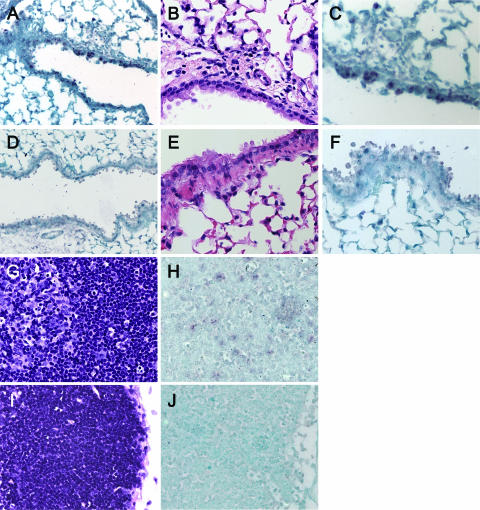
FIG. 9.
Absence of type I IFN-producing cells in lung and draining lymph nodes of γHV68-infected mice. Lung (A to F) and MLN (G to J) tissue sections from influenza virus-infected (A to C, G, and H) or γHV68-infected (D to F, I, and J) mice (days 2, 3, and 7) or control mice were labeled with riboprobes of murine IFN-α4 and IFN-β genes. (A) Influenza virus-infected lung, day 2, with an IFN-β probe. Magnification, ×20. A detail is shown is shown in B and C. (B) Influenza virus-infected lung, day 2, with staining with hematoxylin and eosin. Magnification, ×40. (C) Influenza virus-infected lung, day 2, with an IFN-β probe. Magnification, ×40. (D) γHV68-infected lung, day 2, with an IFN-β probe. Magnification, ×20. A detail is shown in E and F. (E) γHV68-infected lung, day 2, with staining with hematoxylin and eosin. Magnification, ×40. (F) γHV68-infected lung, day 2, with IFN-β probe. Magnification, ×40. (G) Influenza virus-infected MLN, day 3, with staining with hematoxylin and eosin. Magnification, ×40. (H) Influenza virus-infected MLN, day 3, with IFN-α4 probe. Magnification, ×40. (I) γHV68-infected MLN, day 3, with staining with hematoxylin and eosin. Magnification, ×40. (J) γHV68-infected MLN, day 3, with IFN-α4 probe. Magnification, ×40.
DISCUSSION
In this paper, we show that γHV68 infection inhibits type I IFN production in dendritic cells and is a poor inducer of IFN-α/β in vivo. In addition, our data show that dendritic cell activation and recruitment to the lung after γHV68 respiratory infection occur in spite of an IFN-α/β response that is below the limit of detection or in mice that lack IFN-α/β signaling. Our data also indicate that plasmacytoid dendritic cells play an important role in the response to gammaherpesviruses by detecting γHV68 and promoting the activation of conventional dendritic cells regardless of the weak IFN-α/β response to infection.
Type I IFNs are essential for the defense against viral infections (4), and γHV68 is not an exception. IFN-α/β is important for the control of acute γHV68 infection (17, 51) and also for the control of latency (3). Thus, it is not surprising that γHV68 has evolved strategies to subvert type I IFN responses. Our data showing a lack of type I IFN production in response to γHV68 infection by cultured dendritic cells generated in the presence of GM-CSF or Flt3-L suggest the existence of specific IFN-inhibitory mechanisms. The activation of Flt3-L-derived dendritic cells by infectious γHV68 in the absence of type I IFN production also supports this conclusion. In addition, the ability of UV-inactivated, but not live, γHV68 to induce IFN-α/β production in dendritic cell cultures gives strong support to the hypothesis that γHV68 inhibition of type I IFN production is an active process. Several mechanisms common to gammaherpesviruses may account for the following findings: (i) M2 gene expression inhibits IFN-mediated transcriptional activation by down-regulating STAT1 and STAT2 (27), and (ii) ORF45, a gene conserved among the gammaherpesviruses that is essential for γHV68 replication (24), blocks interferon regulatory factor 7 (IRF-7) phosphorylation and nuclear accumulation (54). Regardless of the mechanism inhibiting IFN-α/β production, the data show that γHV68 inhibits type I IFN synthesis by cultured dendritic cells, a process that may help the virus to evade immune control in vivo. This idea is also supported by (i) in vivo data showing the lack of detectable IFN-α/β bioactivity in BAL and serum samples of γHV68-infected mice, (ii) in situ hybridization data showing weak IFN-α and IFN-β signals only from respiratory epithelial cells, and (ii) previous studies showing that γHV68 infects dendritic cells (18-20).
Our results indicate that plasmacytoid dendritic cells are the first dendritic cell population in the lung to show signs of activation in response to γHV68 intranasal inoculation and that γHV68 induces the activation of conventional dendritic cells in vitro only when external “help” in the form of plasmacytoid dendritic cells is present. These data correlate with a requirement for plasmacytoid dendritic cell “help” in conventional dendritic cell function during cutaneous herpes simplex virus infection (53). However, in a model of genital herpes simplex virus type 2 infection, plasmacytoid dendritic cells were not required to mediate Th1 immunity (29). Thus, it seems that the type of herpesvirus and/or the route of infection is likely to contribute to fundamental differences in the response. In addition, our data demonstrate that in response to a gammaherpesvirus, (i) plasmacytoid dendritic cell activation in culture is independent of IFN-α/β, (ii) plasmacytoid dendritic cell recruitment and activation in the lung occur in the presence of a local and systemic IFN-α/β response that is below the limits of detection by bioassay, and (iii) dendritic cell activation and recruitment into the lung, albeit reduced, still occur in IFN-α/βR−/− mice. Altogether, our findings suggest that type I IFN signaling is important but not an absolute requirement for dendritic cells to respond to γHV68 infection. These findings contrast with the previously observed requirement for IFN-α/β signaling during the activation of conventional dendritic cells in culture (22) as well as the need for type I IFN signaling for plasmacytoid dendritic cell migration and activation in response to TLR-7 and TLR-9 ligands (2). It is possible that low levels of IFN-α/β below the limit of detection of our assays may contribute to the recruitment and activation of dendritic cells and that dendritic cell activation can also be induced by alternative mechanisms such as a CD40/CD40L interaction or membrane-bound interleukin-15. In addition, it is likely that the response to a DNA virus infection in vivo is more complex and may account for the differences observed between experimental systems.
The ability of plasmacytoid dendritic cells to recognize and respond to viruses is critical for providing a first line of defense at mucosal surfaces. The importance of plasmacytoid dendritic cells during the immune response to γHV68 is supported by our analysis of infected mice. The immunohistochemistry and flow cytometry data show that plasmacytoid dendritic cells are rapidly recruited to the lung after intranasal instillation of γHV68. This increase in the number of respiratory plasmacytoid dendritic cells is accompanied by the early induction of an activation phenotype. The tissue distribution and migration of plasmacytoid dendritic cells into the respiratory tract are less well defined in comparison to those of conventional dendritic cells. Our data are consistent with human and mouse data indicating that conventional dendritic cells in the lung are mainly CD11b+ or “myeloid” dendritic cells and that they form a contiguous subepithelial network (40, 47). Lung plasmacytoid dendritic cells have recently been described as BDCA2+/CD123+ cells in humans (14) and as Gr-1+/B220+ cells scattered throughout the lung interstitium in mice (12). Our analysis indicates that plasmacytoid dendritic cells constitute a small fraction (2%) of pulmonary dendritic cells and that they were not visualized during steady-state conditions by immunohistochemistry using mPDCA-1. However, after respiratory γHV68 inoculation, plasmacytoid dendritic cells (CD11c+ B220+) are recruited into the lung, constitute 10% of lung dendritic cells, and can be visualized as mPDCA-1+ cells in areas of inflammation. Thus, the distribution of plasmacytoid dendritic cells in the lung is different from that of conventional CD11c+ dendritic cells. While conventional dendritic cells mostly form a dense network underneath the epithelium and in areas of inflammation, plasmacytoid dendritic cells appear to migrate into the lung in response to an inflammatory stimulus.
It is becoming increasingly evident that plasmacytoid dendritic cells play an essential dual role in the initiation of antiviral responses: as professional type I IFN producer cells and in regulating the function of conventional dendritic cells by IFN-independent pathways (9, 28). The data presented here support this hypothesis using the γHV68 model of infection. Due to the key role of type I IFNs in antiviral defense, it is not surprising that many viruses have developed immune evasion mechanisms to block their production (21). Plasmacytoid dendritic cells are resistant to many of these strategies either because they cannot be infected by the virus or because the virus targets IRF-3-dependent pathways, which are not essential for type I IFN induction by TLR-7 and TLR-9 (15). The exceptions to this rule include some highly successful pathogens including measles virus, RSV, KSHV (21, 54), and γHV68.
Acknowledgments
We thank the Morphology Core at CCRI for technical help.
This work was supported by NIH grant AI-59603 and by CCRI.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144-1150. [DOI] [PubMed] [Google Scholar]
- 2.Asselin-Paturel, C., G. Brizard, K. Chemin, A. Boonstra, A. O'Garra, A. Vicari, and G. Trinchieri. 2005. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J. Exp. Med. 201:1157-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton, E. S., M. L. Lutzke, R. Rochford, and H. W. Virgin IV. 2005. Alpha/beta interferons regulate murine gammaherpesvirus latent gene expression and reactivation from latency. J. Virol. 79:14149-14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biron, C. A. 2001. Interferons alpha and beta as immune regulators—a new look. Immunity 14:661-664. [DOI] [PubMed] [Google Scholar]
- 5.Brawand, P., D. R. Fitzpatrick, B. W. Greenfield, K. Brasel, C. R. Maliszewski, and T. De Smedt. 2002. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand supplemented bone marrow cultures are immature APCs. J. Immunol. 169:6711-6719. [DOI] [PubMed] [Google Scholar]
- 6.Brehm, G., and H. Kirchner. 1986. Analysis of the interferons induced in mice in vivo and in macrophages in vitro by Newcastle disease virus and by polyinosinic-polycytidylic acid. J. Interf. Res. 6:21-28. [DOI] [PubMed] [Google Scholar]
- 7.Bruynseels, P., P. G. Jorens, H. E. Demey, H. Goossens, S. R. Pattyn, M. M. Elseviers, J. Weyler, L. L. Bossaert, Y. Mentens, and M. Ieven. 2003. Herpes simplex virus in the respiratory tract of critical care patients: a prospective study. Lancet 362:1536-1541. [DOI] [PubMed] [Google Scholar]
- 8.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colonna, M., G. Trinchieri, and Y. J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219-1226. [DOI] [PubMed] [Google Scholar]
- 10.Cool, C. D., P. R. Rai, M. E. Yeager, D. Hernandez-Saavedra, A. E. Serls, T. M. Bull, M. W. Geraci, K. K. Brown, J. M. Routes, R. M. Tuder, and N. F. Voelkel. 2003. Expression of human herpesvirus 8 in primary pulmonary hypertension. N. Engl. J. Med. 349:1113-1122. [DOI] [PubMed] [Google Scholar]
- 11.de Heer, H. J., H. Hammad, M. Kool, and B. N. Lambrecht. 2005. Dendritic cell subsets and immune regulation in the lung. Semin. Immunol. 17:295-303. [DOI] [PubMed] [Google Scholar]
- 12.de Heer, H. J., H. Hammad, T. Soullie, D. Hijdra, N. Vos, M. A. Willart, H. C. Hoogsteden, and B. N. Lambrecht. 2004. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200:89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delale, T., A. Paquin, C. Asselin-Paturel, M. Dalod, G. Brizard, E. E. Bates, P. Kastner, S. Chan, S. Akira, A. Vicari, C. A. Biron, G. Trinchieri, and F. Briere. 2005. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J. Immunol. 175:6723-6732. [DOI] [PubMed] [Google Scholar]
- 14.Demedts, I. K., G. G. Brusselle, K. Y. Vermaelen, and R. A. Pauwels. 2005. Identification and characterization of human pulmonary dendritic cells. Am. J. Respir. Cell Mol. Biol. 32:177-184. [DOI] [PubMed] [Google Scholar]
- 15.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 16.Doherty, P. C., J. P. Christensen, G. T. Belz, P. G. Stevenson, and M. Y. Sangster. 2001. Dissecting the host response to a gamma-herpesvirus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:581-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutia, B. M., D. J. Allen, H. Dyson, and A. A. Nash. 1999. Type I interferons and IRF-1 play a critical role in the control of a gammaherpesvirus infection. Virology 261:173-179. [DOI] [PubMed] [Google Scholar]
- 18.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 19.Flano, E., B. Kayhan, D. L. Woodland, and M. A. Blackman. 2005. Infection of dendritic cells by a gamma2-herpesvirus induces functional modulation. J. Immunol. 175:3225-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flano, E., I. J. Kim, J. Moore, D. L. Woodland, and M. A. Blackman. 2003. Differential gamma-herpesvirus distribution in distinct anatomical locations and cell subsets during persistent infection in mice. J. Immunol. 170:3828-3834. [DOI] [PubMed] [Google Scholar]
- 21.Hengel, H., U. H. Koszinowski, and K. K. Conzelmann. 2005. Viruses know it all: new insights into IFN networks. Trends Immunol. 26:396-401. [DOI] [PubMed] [Google Scholar]
- 22.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen, K. K., S. C. Chen, R. W. Hipkin, M. T. Wiekowski, M. A. Schwarz, C. C. Chou, J. P. Simas, A. Alcami, and S. A. Lira. 2003. Disruption of CCL21-induced chemotaxis in vitro and in vivo by M3, a chemokine-binding protein encoded by murine gammaherpesvirus 68. J. Virol. 77:624-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia, Q., V. Chernishof, E. Bortz, I. McHardy, T. T. Wu, H. I. Liao, and R. Sun. 2005. Murine gammaherpesvirus 68 open reading frame 45 plays an essential role during the immediate-early phase of viral replication. J. Virol. 79:5129-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krug, A., A. R. French, W. Barchet, J. A. Fischer, A. Dzionek, J. T. Pingel, M. M. Orihuela, S. Akira, W. M. Yokoyama, and M. Colonna. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21:107-119. [DOI] [PubMed] [Google Scholar]
- 26.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 27.Liang, X., Y. C. Shin, R. E. Means, and J. U. Jung. 2004. Inhibition of interferon-mediated antiviral activity by murine gammaherpesvirus 68 latency-associated M2 protein. J. Virol. 78:12416-12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, Y. J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275-306. [DOI] [PubMed] [Google Scholar]
- 29.Lund, J. M., M. M. Linehan, N. Iijima, and A. Iwasaki. 2006. Cutting edge: plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J. Immunol. 177:7510-7514. [DOI] [PubMed] [Google Scholar]
- 30.Mackie, P. L. 2003. The classification of viruses infecting the respiratory tract. Paediatr. Respir. Rev. 4:84-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Sobrido, L., N. Gitiban, A. Fernandez-Sesma, J. Cros, S. E. Mertz, N. A. Jewell, S. Hammond, E. Flano, R. K. Durbin, A. Garcia-Sastre, and J. E. Durbin. 2006. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J. Virol. 80:1130-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masten, B. J. 2004. Initiation of lung immunity: the afferent limb and the role of dendritic cells. Semin. Respir. Crit. Care Med. 25:11-20. [DOI] [PubMed] [Google Scholar]
- 33.Merk, J., F. X. Schmid, M. Fleck, S. Schwarz, C. Lehane, S. Boehm, B. Salzberger, and D. E. Birnbaum. 2005. Fatal pulmonary failure attributable to viral pneumonia with human herpes virus 6 (HHV6) in a young immunocompetent woman. J. Intensive Care Med. 20:302-306. [DOI] [PubMed] [Google Scholar]
- 34.Nash, A. A., B. M. Dutia, J. P. Stewart, and A. J. Davison. 2001. Natural history of murine gammaherpesvirus infection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Offermann, M. K. 1999. Consideration of host-viral interactions in the pathogenesis of Kaposi's sarcoma. J. Acquir. Immune Defic. Syndr. 21(Suppl. 1):S58-S65. [PubMed] [Google Scholar]
- 36.Palucka, K., and J. Banchereau. 2002. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 14:420-431. [DOI] [PubMed] [Google Scholar]
- 37.Rescigno, M., and P. Borrow. 2001. The host-pathogen interaction: new themes from dendritic cell biology. Cell 106:267-270. [DOI] [PubMed] [Google Scholar]
- 38.Rettig, M. B., H. J. Ma, R. A. Vescio, M. Pold, G. Schiller, D. Belson, A. Savage, C. Nishikubo, C. Wu, J. Fraser, J. W. Said, and J. R. Berenson. 1997. Kaposi's sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science 276:1851-1854. [DOI] [PubMed] [Google Scholar]
- 39.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K. K. Conzelmann. 2005. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schon-Hegrad, M. A., J. Oliver, P. G. McMenamin, and P. G. Holt. 1991. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J. Exp. Med. 173:1345-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulz, T. F. 1998. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8). J. Gen. Virol. 79:1573-1591. [DOI] [PubMed] [Google Scholar]
- 42.Simoons-Smit, A. M., E. M. Kraan, A. Beishuizen, R. J. S. van Schijndel, and C. M. Vandenbroucke-Grauls. 2006. Herpes simplex virus type 1 and respiratory disease in critically-ill patients: real pathogen or innocent bystander? Clin. Microbiol. Infect. 12:1050-1059. [DOI] [PubMed] [Google Scholar]
- 43.Spann, K. M., K. C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78:4363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinman, R. M., D. Hawiger, and M. C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685-711. [DOI] [PubMed] [Google Scholar]
- 45.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 46.Verboon-Maciolek, M. A., T. G. Krediet, L. J. Gerards, A. Fleer, and T. M. van Loon. 2005. Clinical and epidemiologic characteristics of viral infections in a neonatal intensive care unit during a 12-year period. Pediatr. Infect. Dis. J. 24:901-904. [DOI] [PubMed] [Google Scholar]
- 47.Vermaelen, K., and R. Pauwels. 2005. Pulmonary dendritic cells. Am. J. Respir. Crit. Care Med. 172:530-551. [DOI] [PubMed] [Google Scholar]
- 48.Vermaelen, K. Y., I. Carro-Muino, B. N. Lambrecht, and R. A. Pauwels. 2001. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med. 193:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virgin, H. W., and S. H. Speck. 1999. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr. Opin. Immunol. 11:371-379. [DOI] [PubMed] [Google Scholar]
- 50.Virgin, H. W., IV, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weck, K. E., A. J. Dal Canto, J. D. Gould, A. K. O'Guin, K. A. Roth, J. E. Saffitz, S. H. Speck, and H. W. Virgin. 1997. Murine gamma-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus-induced vascular disease. Nat. Med. 3:1346-1353. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. 2004. Changing history. The World Health Report. World Health Organization, Geneva, Switzerland.
- 53.Yoneyama, H., K. Matsuno, E. Toda, T. Nishiwaki, N. Matsuo, A. Nakano, S. Narumi, B. Lu, C. Gerard, S. Ishikawa, and K. Matsushima. 2005. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202:425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu, F. X., S. M. King, E. J. Smith, D. E. Levy, and Y. Yuan. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA 99:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]