Abstract
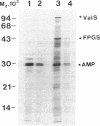
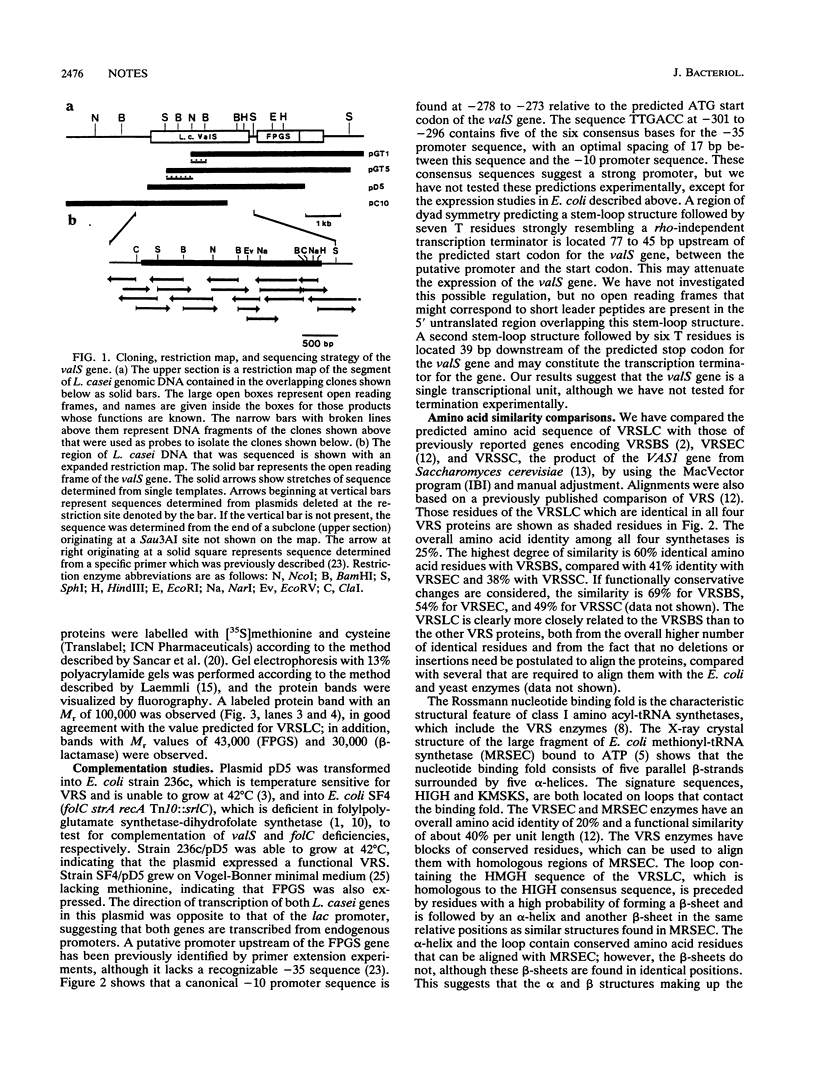
The DNA sequence of the valS gene from Lactobacillus casei and the predicted amino acid sequence of its valyl-tRNA synthetase product have been determined. An open reading frame coding for a protein of 901 amino acids was found. A clone containing the intact L. casei valS gene functionally complemented the temperature-sensitive growth of the valS mutant strain 236c of Escherichia coli. The valS gene and the downstream folylpolyglutamate synthetase gene are transcribed in the same direction but are separated by a putative transcription terminator.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bognar A. L., Osborne C., Shane B., Singer S. C., Ferone R. Folylpoly-gamma-glutamate synthetase-dihydrofolate synthetase. Cloning and high expression of the Escherichia coli folC gene and purification and properties of the gene product. J Biol Chem. 1985 May 10;260(9):5625–5630. [PubMed] [Google Scholar]
- Borgford T. J., Brand N. J., Gray T. E., Fersht A. R. The valyl-tRNA synthetase from Bacillus stearothermophilus has considerable sequence homology with the isoleucyl-tRNA synthetase from Escherichia coli. Biochemistry. 1987 May 5;26(9):2480–2486. doi: 10.1021/bi00383a012. [DOI] [PubMed] [Google Scholar]
- Brand N. J., Fersht A. R. Molecular cloning of the gene encoding the valyl-tRNA synthetase from Bacillus stearothermophilus. Gene. 1986;44(1):139–142. doi: 10.1016/0378-1119(86)90053-3. [DOI] [PubMed] [Google Scholar]
- Brick P., Blow D. M. Crystal structure of a deletion mutant of a tyrosyl-tRNA synthetase complexed with tyrosine. J Mol Biol. 1987 Mar 20;194(2):287–297. doi: 10.1016/0022-2836(87)90376-7. [DOI] [PubMed] [Google Scholar]
- Brunie S., Zelwer C., Risler J. L. Crystallographic study at 2.5 A resolution of the interaction of methionyl-tRNA synthetase from Escherichia coli with ATP. J Mol Biol. 1990 Nov 20;216(2):411–424. doi: 10.1016/S0022-2836(05)80331-6. [DOI] [PubMed] [Google Scholar]
- Cusack S., Berthet-Colominas C., Härtlein M., Nassar N., Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 A. Nature. 1990 Sep 20;347(6290):249–255. doi: 10.1038/347249a0. [DOI] [PubMed] [Google Scholar]
- Cusack S., Härtlein M., Leberman R. Sequence, structural and evolutionary relationships between class 2 aminoacyl-tRNA synthetases. Nucleic Acids Res. 1991 Jul 11;19(13):3489–3498. doi: 10.1093/nar/19.13.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heck J. D., Hatfield G. W. Valyl-tRNA synthetase gene of Escherichia coli K12. Primary structure and homology within a family of aminoacyl-TRNA synthetases. J Biol Chem. 1988 Jan 15;263(2):868–877. [PubMed] [Google Scholar]
- Jordana X., Chatton B., Paz-Weisshaar M., Buhler J. M., Cramer F., Ebel J. P., Fasiolo F. Structure of the yeast valyl-tRNA synthetase gene (VASI) and the homology of its translated amino acid sequence with Escherichia coli isoleucyl-tRNA synthetase. J Biol Chem. 1987 May 25;262(15):7189–7194. [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. Cloning of uvrA, lexC and ssb genes of Escherichia coli. Biochem Biophys Res Commun. 1979 Sep 12;90(1):123–129. doi: 10.1016/0006-291x(79)91598-5. [DOI] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Toy J., Bognar A. L. Cloning and expression of the gene encoding Lactobacillus casei folylpoly-gamma-glutamate synthetase in Escherichia coli and determination of its primary structure. J Biol Chem. 1990 Feb 15;265(5):2492–2499. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]