Abstract
The mainstream of recent anti-AIDS vaccines is a prime/boost approach with multiple doses of the target DNA of human immunodeficiency virus type 1 (HIV-1) and recombinant viral vectors. In this study, we have attempted to construct an efficient protein-based vaccine using biodegradable poly(γ-glutamic acid) (γ-PGA) nanoparticles (NPs), which are capable of inducing potent cellular immunity. A significant expansion of CD8+ T cells specific to the major histocompatibility complex class I-restricted gp120 epitope was observed in mice intranasally immunized once with gp120-carrying NPs but not with gp120 alone or gp120 together with the B-subunit of cholera toxin. Both the gp120-encapsulating and -immobilizing forms of NPs could induce antigen-specific spleen CD8+ T cells having a functional profile of cytotoxic T lymphocytes. Long-lived memory CD8+ T cells could also be elicited. Although a substantial decay in the effector memory T cells was observed over time in the immunized mice, the central memory T cells remained relatively constant from day 30 to day 238 after immunization. Furthermore, the memory CD8+ T cells rapidly expanded with boosting with the same immunogen. In addition, γ-PGA NPs were found to be a much stronger inducer of antigen-specific CD8+ T-cell responses than nonbiodegradable polystyrene NPs. Thus, γ-PGA NPs carrying various HIV-1 antigens may have great potential as a novel priming and/or boosting tool in current vaccination regimens for the induction of cellular immune responses.
The development of highly active antiretroviral therapy has achieved a reduced death rate from human immunodeficiency virus type 1 (HIV-1) infection in developed countries. However, considering the high cost and potential toxicity of long-term highly active antiretroviral therapy, it is obvious that the development of vaccines against HIV-1 is the most desirable option for the prevention of viral transmission and disease progression (12, 14). An effective anti-AIDS vaccine will likely need to induce virus-specific neutralizing antibodies and cytotoxic T-lymphocyte (CTL) responses. Although neutralizing antibodies have shown the activity to block HIV-1 and simian immunodeficiency virus (SIV), an immunogen inducing the antibodies that neutralize a diversity of primary HIV-1 isolates has not been obtained. With accumulating evidence for the importance of CTLs in controlling HIV-1 and SIV replication, several vaccine strategies are being pursued for generating HIV-1-specific CTLs (5, 7, 9, 15, 22, 23). Currently, the most promising vaccine strategy for the induction of CTL responses seems to be a heterologous prime/boost regimen employing a plasmid DNA prime dose and a live recombinant-vector boost dose. Since the immunogenicity of plasmid DNA has proved to be modest in human clinical trials, our attempt is to construct a protein-based vaccine capable of inducing potent HIV-1-specific cellular immunity.
Nanoparticles (NPs) are considered to be an efficient antigen carrier and have been widely investigated for their biological potential (20, 21). NPs of an appropriate size are efficiently taken up by dendritic cells (DCs) and can present the carried antigens along with major histocompatibility complex (MHC) class I molecules to CD8+ T cells through the antigen cross-presentation pathway (6, 8, 16). DCs are professional antigen-presenting cells capable of stimulating naïve T cells in the primary immune response and are more-potent antigen-presenting cells than monocyte/macrophages or B cells (4). The superiority of DCs in immunostimulatory activity involves the high-level expression of MHC and costimulatory molecules (CD40, CD80, and CD86), as well as the ability to produce T-helper 1 (Th1) cytokines, such as interleukin-12 (IL-12) and alpha interferon (IFN-α) (4). The ability of DCs to prime naïve T cells with antigens and their presence in various peripheral tissues imply a central role of DCs in mediating immune responses to infectious diseases and cancers.
We have previously reported that antigen-carrying core-corona polystyrene NPs (PSNPs) were efficiently taken up by DCs and did enhance the immunogenicity of antigens (28, 29). Intranasal immunization of mice with heat-inactivated HIV-1-capturing PSNPs demonstrated efficient production of HIV-1-specific neutralizing antibodies in the genital tract and CTL responses in the spleen (2, 10). Furthermore, intranasal immunization with inactivated simian-human immunodeficiency virus (SHIV)-capturing NPs (SHIV-NPs) could induce mucosal immune responses in macaques, and the macaques immunized with SHIV-NPs were partially protected from vaginal and systemic challenge with SHIV (18). However, nonbiodegradable PSNPs may not be applicable in clinical situations as a vaccine material because of their safety issues. To circumvent this problem, we have recently created a novel biodegradable antigen delivery system with self-assembled polymeric NPs using poly(γ-glutamic acid) (γ-PGA) (1). NPs composed of amphiphilic γ-PGA and hydrophobic amino acids can immobilize proteins, peptides, and chemicals onto their surfaces and/or encapsulate these substances into the particles. In addition, γ-PGA NPs were found to be an efficient protein antigen delivery system and adjuvant to DCs in vitro and in vivo (26).
MATERIALS AND METHODS
Preparation of γ-PGA NPs.
γ-PGA (number-average molecular weight, Mn, 380,000) was kindly provided by Meiji Seika Co., Ltd., Tokyo, Japan. The synthesis procedures for γ-PGA NPs, PSNPs, and protein-carrying γ-PGA NPs have been described in our previous report (1, 10). Recombinant HIV-1 (IIIB strain) gp120 protein (Immuno Diagnostics, Woburn, MA) was chosen for the immunization experiments and either immobilized onto or encapsulated into γ-PGA NPs. To prepare the gp120-immobilizing γ-PGA NPs [gp120-NPs (imz)], the carboxyl group of the γ-PGA NPs (10 mg/ml) was activated by water-soluble carbodiimide for 20 min. The NPs, obtained by centrifugation (14,000 × g for 15 min), were mixed with 1 ml of gp120 (0.5 mg/ml) in phosphate-buffered saline (PBS), and the mixture was incubated at 4°C for 24 h. After the reaction, the centrifuged NPs were washed twice with PBS. The gp120-immobilizing PSNPs [gp120-PSNPs (imz)] were prepared by the same method. To prepare the gp120-encapsulating γ-PGA NPs [gp120-NPs (ecp)], γ-PGA-graft-l-phenylalanine ethylester (10 mg/ml) in dimethyl sulfoxide was added to the same volume of gp120 (0.75 mg/ml) in PBS. After the reaction, the centrifuged NPs were washed twice with PBS. The amount of entrapped gp120 protein was evaluated by the Lowry method, as previously described (1). The particle sizes of γ-PGA NPs and protein-carrying γ-PGA NPs in aqueous solution were measured by a dynamic light scattering method. The mean diameters of γ-PGA NPs, PSNPs, gp120-NPs (imz), gp120-NPs (ecp), and gp120-PSNPs (imz) were 210 ± 67 (mean ± standard deviation), 267 ± 76, 301 ± 127, 450 ± 124, and 316 ± 93 nm, respectively. The NPs did not form larger aggregates after they had been mixed with or conjugated to HIV-1 antigens. To determine the distribution of the antigen carried by γ-PGA NPs, fluorescein 5-isothiocyanate-conjugated ovalbumin (FITC-OVA) (Molecular Probes, Inc., Eugene, OR) was selected as a model antigen. FITC-OVA was encapsulated into γ-PGA NPs with the method described above.
Mice and immunization.
Six- to 8-week-old female BALB/c mice were purchased from Charles River Japan (Yokohama, Japan). The experiments were carried out in accordance with the guidelines for animal experimentation in Kagoshima University. The mice were anesthetized by an intraperitoneal injection of sodium pentobarbital and intranasally immunized with various concentrations of antigens in a total volume of 20 μl to one nostril.
Proliferation assay.
Spleen lymphocytes from the immunized mice were isolated by lympholyte-M (Cedarlane, Ontario, Canada) and tested in a standard [3H]thymidine incorporation assay. The cells were cultured in a 96-well flat-bottom plate (2 × 105/well) with gp120 protein or the p18 epitope peptide (RGPGRAFVTI) (25) at various concentrations. They were incubated for 5 days at 37°C and pulsed with 1 μCi of [3H]thymidine per well for 16 h. The cells were harvested on a glass filter paper, and their radioactivity was measured with a liquid scintillation counter. The mean count per min from triplicate wells was used for calculating the stimulation index. The stimulation index is the count per min with antigen stimulation divided by the count per min with stimulation with medium alone.
Pentamer staining and flow cytometric analysis.
Pentameric H-2Dd complexes folded with the p18 epitope peptide (25) were purchased from Proimmune (Oxford, United Kingdom). Spleen lymphocytes from the immunized mice were stained with the p18 pentamer conjugated with allophycocyanin and an FITC-conjugated anti-CD8α monoclonal antibody (MAb) (KT-15; Proimmune) to detect p18-specific CD8+ T cells. The cells were washed in PBS containing 0.1% sodium azide and 0.1% bovine serum albumin and fixed with PBS containing 2.5% formaldehyde (Wako, Tokyo, Japan). For phenotyping of the p18-specific CD8+ T cells, spleen lymphocytes were collected at certain time points after immunization and stained with a peridinin chlorophyll protein-Cy5.5-conjugated anti-CD8α MAb (53-6.7; BD Biosciences, San Jose, CA), a phycoerythrin (PE)-conjugated anti-CD44 MAb (IM7; eBioscience, San Diego, CA), an FITC-conjugated anti-CD62L MAb (MEL-14; eBioscience), a PE-conjugated anti-CD127 MAb (A7R34; eBioscience), and the allophycocyanin-conjugated p18 pentamer. The cells were washed with PBS, and the levels of cell-associated fluorescence were determined by using a multicolor flow cytometer (FACSCalibur; BD Biosciences) and analyzed with CellQuest software (BD Biosciences).
IFN-γ ELISPOT assay.
An enzyme-linked immunospot (ELISPOT) assay was performed to measure IFN-γ production. Spleen lymphocytes from the immunized mice were cultured in a plate with medium alone, the p18 epitope peptide (5 μg/ml), or gp120 protein (5 μg/ml). After a 24-h incubation at 37°C, the plate was washed, and the IFN-γ-producing cells were measured with an ELISPOT assay kit (BD Biosciences), according to the manufacturer's instructions. The data were expressed as the mean number of spot-forming units per million cells ± the standard error of the mean (SEM).
Intracellular cytokine staining and flow cytometric analysis.
Spleen lymphocytes from the immunized mice were stimulated with the p18 epitope peptide (10 μg/ml) or medium alone for 6 h. The protein transport inhibitor BD GolgiPlug (1:1,000; BD Biosciences) was added to accumulate intracellular cytokines. The cells were washed, incubated for 10 min at 4°C with a purified anti-mouse CD16/32 to block Fc receptors, and stained with a PE-conjugated anti-CD8α MAb (53-6.7; BD Biosciences) for 30 min at 4°C. The cells were permeabilized (BD Cytofix/Cytoperm Plus; BD Biosciences) and stained with an FITC-conjugated anti-IFN-γ MAb (XMG 1.2; BD Biosciences), an FITC-conjugated anti-IL-2 MAb (JES6-5H4; BD Biosciences), or an FITC-conjugated anti-tumor necrosis factor alpha (TNF-α) MAb (MP6-XT22; BD Biosciences) for 30 min at 4°C. The cells were washed with PBS, and the levels of cell-associated fluorescence were determined by using a FACSCalibur and analyzing with CellQuest.
CTL assay.
Spleen lymphocytes from the immunized mice were suspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 mM 2-mercaptoethanol, and 20 U/ml recombinant IL-2 (Sigma, St. Louis, MO). The cells (4 × 106 cells/ml) were cultured with the p18 epitope peptide (10 μg/ml) in a 24-well plate for 4 days. MHC-matched P815 cells (H-2d) were used as the target cells. The target cells (1 × 105 cells/ml) were preincubated with the p18 epitope peptide (10 μg/ml) for 16 h. The cytolytic activity of the effector cells was evaluated with a lactate dehydrogenase (LDH) cytotoxicity detection assay kit (Takara, Tokyo, Japan), as previously described (11). Prior to the assay, dead cells were removed from the effector cells by density gradient centrifugation with lympholyte-M. The effector and peptide-pulsed target cells were cocultured in a 96-well round-bottom plate at various effector/target ratios. After being incubated for 4 h, the culture supernatants were harvested and examined for their LDH levels. The LDH level of cell-free medium (background) was subtracted from the LDH levels of the samples. The percent specific lysis was calculated as follows: (sample LDH − effector spontaneous LDH − target spontaneous LDH)/(target maximum LDH − target spontaneous LDH) × 100. The effector and target spontaneous LDH levels were determined by culturing the cells with medium alone, and the target maximum LDH level was determined by adding 1% Triton X-100 to the target cells. To clarify the roles of CD4+ and CD8+ T cells in gp120-NP-induced cytolytic activity, an anti-CD4 MAb (GK1.5; eBioscience) and an anti-CD8 MAb (53-6.7; eBioscience) were used for depletion of CD4+ and CD8+ T cells, respectively. The MAbs were mixed with the effector cells before being added to the target cells at a final concentration of 10 μg/ml and incubated for 45 min at 4°C.
Statistical analysis.
Statistical tests were performed by using Student's t test. A P value of less than 0.05 was considered significant.
RESULTS
Induction of antigen-specific CD8+ T cells by gp120-NPs.
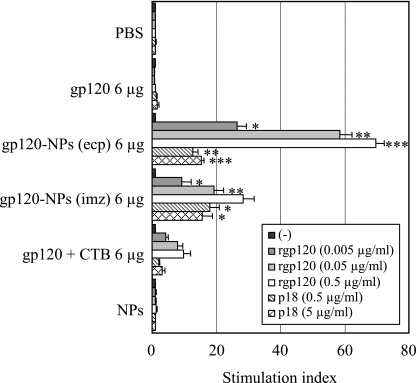
Since γ-PGA NPs act as an efficient protein antigen delivery system and adjuvant to DCs in vitro and in vivo (26), the ability of γ-PGA NPs to elicit HIV-1-specific CD8+ T-cell responses was examined in mice. Recombinant HIV-1 gp120 protein was selected as an immunogen, and gp120-NPs (ecp) and gp120-NPs (imz) were created. The mice were intranasally immunized once with gp120-NPs, and their cellular immune responses were measured with a lymphocyte proliferation assay. As shown in Fig. 1, antigen-specific lymphocyte proliferation was not observed in the spleen lymphocytes obtained from the mice immunized with PBS, gp120 alone, or NPs alone. In contrast, the spleen cells obtained from the mice immunized with gp120-NPs (ecp) and gp120-NPs (imz) showed significant antigen-specific proliferation in a dose-dependent fashion compared to the proliferation in the spleen cells obtained from mice immunized with a mixture of gp120 and the B-subunit of cholera toxin (gp120 + CTB) (Fig. 1). Higher lymphocyte proliferation was observed in the mice immunized with gp120-NPs (ecp) than in those immunized with gp120-NPs (imz). Furthermore, both gp120-NPs (ecp) and gp120-NPs (imz) elicited potent p18-specific lymphocyte proliferation. These results suggest that both gp120-NPs (ecp) and gp120-NPs (imz) have great potential to induce antigen-specific cellular immune responses, especially, CD8+ T-cells.
FIG. 1.
Induction of antigen-specific lymphocyte proliferation by gp120-NPs. Mice were intranasally immunized once with the indicated antigens. Spleen lymphocytes were isolated on day 10 after immunization and stimulated in vitro with the indicated concentrations of the recombinant gp120 (rgp120) or p18 peptide for 5 days. The cells were exposed to [3H]thymidine for the final 16 h, and the levels of incorporation of radioactivity were determined. All data represent the means ± SEMs of the results for four mice per group. Statistical analysis was carried out in comparison with the results for the gp120 + CTB group. −, no stimulus; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
When the CD8+ T-cell responses to the H-2d-restricted p18 epitope were examined with the pentamer-staining assay, immunization with gp120-NPs (ecp) and gp120-NPs (imz) had strongly induced p18-specific CD8+ T-cell responses in a dose-dependent fashion (Fig. 2A). Higher responses were observed even in the mice immunized with gp120-NPs at a dose of 0.24 μg than in those immunized with gp120 alone or gp120 + CTB at a dose of 6 μg. Although the immune responses induced by gp120-NPs (ecp) and gp120-NPs (imz) were comparable at a dose of 6 μg, stronger CD8+ T-cell responses were induced by gp120-NPs (ecp) than by gp120-NPs (imz) at a dose of 1.2 μg. Surprisingly, a simple mixture of gp120 and NPs (gp120 + NPs) could also induce potent p18-specific CD8+ T-cell responses in mice (Fig. 2A).
FIG. 2.
Induction of gp120-specific CD8+ T-cell responses by gp120-NPs. Mice were intranasally immunized once with the indicated antigens. Spleen lymphocytes were isolated on day 10 after immunization. (A) Antigen-specific CD8+ T cells were detected by H-2Dd/p18 pentamer staining. Data are expressed as the percentages of the gated CD8+ T cells that bound to the pentamer, as measured by flow cytometry. All data represent the mean ± SEM of the results for four mice per group. Statistical analysis was carried out in comparison with the results for the gp120 + CTB group. *; P < 0.05; **; P < 0.001. (B) Spleen lymphocytes were evaluated by ELISPOT for IFN-γ production after stimulation with no peptide (−), the p18 peptide (5 μg/ml), or recombinant gp120 (rgp120; 5 μg/ml). Data are expressed as the numbers of antigen-specific spots per million cells. All data represent the mean ± SEM of the results for four mice per group. Statistical analysis was carried out in comparison with the results for the gp120 + CTB group. *, P < 0.05; **, P < 0.001. (C) CD8+ T cells were evaluated by intracellular cytokine staining for the production of IL-2, IFN-γ, and TNF-α after stimulation with the p18 peptide. Data are expressed as the percentages of cytokine-positive CD8+ cells. (D) Spleen lymphocytes were cultured for 4 days in the presence of the p18 peptide (10 μg/ml). The cells were harvested and used as effector cells to assess P815 target cell lysis by measuring LDH release after overnight incubation with medium alone or the p18 peptide. Data are expressed as the levels of peptide-specific lysis, calculated by subtracting the percent specific lysis of the control target cells from the percent specific lysis of the peptide-pulsed target cells at the indicated effector-to-target (E:T) cell ratio.
The functional capacity of gp120-NP-induced specific CD8+ T cells was assessed with an IFN-γ ELISPOT assay following stimulation with the p18 peptide and gp120. As shown in Fig. 2B, a number of p18- and gp120-specific IFN-γ-producing cells were identified in the mice immunized with gp120-NPs, and the IFN-γ-producing cells were more abundant than those in the mice receiving gp120 + CTB (Fig. 2B). No antigen-specific IFN-γ-producing cells were detected in the mice immunized with PBS or gp120 alone. Similar to the results of pentamer staining (Fig. 2A), gp120-NPs (ecp) were a more potent inducer of the IFN-γ-producing cells than gp120-NPs (imz) at a dose of 1.2 μg of gp120. Again, gp120 + NPs also potently induced the IFN-γ-producing cells in mice. When the production of IL-2 and TNF-α from the antigen-specific CD8+ T cells was evaluated by intracellular cytokine staining, both cytokines could be detected for the cells obtained from the gp120-NP-immunized mice (Fig. 2C). Similar to the ELISPOT results, the production of these cytokines was also higher in the mice immunized with gp120-NPs (ecp) than in those immunized with gp120-NPs (imz).
To assess the cytolytic activities of gp120-NP-induced CD8+ T cells, spleen lymphocytes from the immunized mice were cultured with the p18 peptide for 4 days and used as the effector cells. As shown in Fig. 2D, p18-specific cytotoxic activity was not observed for the spleen lymphocytes of the mice immunized with gp120 alone or gp120 + CTB. In contrast, the spleen lymphocytes of the mice immunized with gp120-NPs (ecp) or gp120-NPs (imz) displayed significant cytolytic activity for the target cells. Modest cytolytic activity was observed for the spleen lymphocytes of the mice immunized with gp120 + NPs. These results indicate that the antigen-specific CD8+ T cells induced by gp120-NPs are capable of secreting IFN-γ, IL-2, and TNF-α and functioning as CTLs.
Comparison of γ-PGA NPs with PSNPs for the induction of antigen-specific CD8+ T cells.
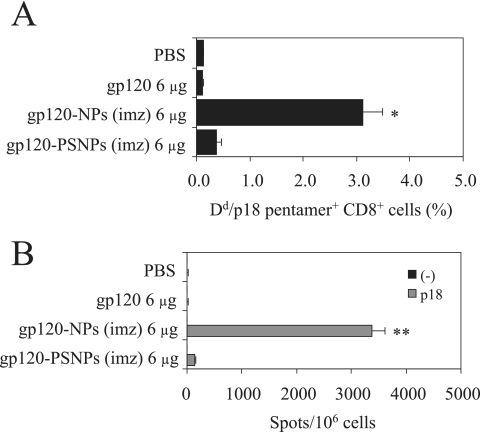
To compare the efficacy of γ-PGA NPs and PSNPs in inducing antigen-specific CD8+ T-cell responses, the binding of H-2Dd/p18 pentamers to CD8+ T cells was evaluated on day 10 after immunization with gp120-NPs (imz) or gp120-PSNPs (imz). As shown in Fig. 3A, gp120-NPs (imz) could induce the p18-specific CD8+ T-cell responses more strongly than gp120-PSNPs (imz) and gp120 alone. Furthermore, the p18-specific IFN-γ-producing cells were identified as being more abundant in the mice immunized with gp120-NPs (imz) than in the mice receiving gp120-PSNPs (imz) (Fig. 3B). These results indicate that γ-PGA NPs are superior to PSNPs as an inducer of antigen-specific CD8+ T cells.
FIG. 3.
Induction of gp120-specific CD8+ T-cell responses by gp120-NPs (imz) and gp120-PSNPs (imz). Mice were intranasally immunized once with the indicated antigens. Spleen lymphocytes were isolated on day 10 after immunization. (A) Antigen-specific CD8+ T cells were detected by H-2Dd/p18 pentamer staining. Data are expressed as the percentages of the gated CD8+ T cells that bound to the pentamer, as measured by flow cytometry. All data represent the means ± SEMs of the results for three mice per group. Statistical analysis was carried out in comparison with the results for the gp120-PSNPs group. *, P < 0.05. (B) Spleen lymphocytes were evaluated by ELISPOT for their IFN-γ production after stimulation with no peptide (−) or the p18 peptide (5 μg/ml). Data are expressed as the numbers of antigen-specific spots per million cells. All data represent the means ± SEMs of the results for three mice per group. Statistical analysis was carried out in comparison with the results for the gp120-PSNPs group. **, P < 0.001.
Induction of long-lived memory CD8+ T cells.
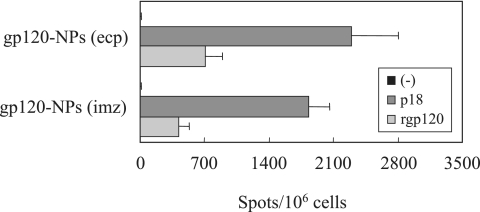
To determine whether intranasal immunization with gp120-NPs could generate memory CD8+ T cells, the spleen lymphocytes were harvested after 4 weeks of immunization and subjected to the IFN-γ ELISPOT and CTL assays. As shown in Fig. 4, considerable numbers of p18- and gp120-specific IFN-γ-producing cells were identified in the mice immunized with gp120-NPs. Again, gp120-NPs (ecp) were a more potent inducer of the IFN-γ-producing cells than gp120-NPs (imz). Furthermore, the cells could efficiently kill the target cells pulsed with the p18 peptide (data not shown). These results suggest that a pool of the antigen-specific memory CD8+ T cells was generated after 4 weeks.
FIG. 4.
Functional analysis of the memory CD8+ T cells. Mice were intranasally immunized once with 6 μg of gp120-NPs. Spleen lymphocytes were collected on day 30 after immunization. Spleen lymphocytes were evaluated by ELISPOT for their IFN-γ production after stimulation with no peptide (−), the p18 peptide (5 μg/ml), or recombinant gp120 (rgp120; 5 μg/ml). Data are expressed as the numbers of antigen-specific spots per million cells. All data represent the means ± SEMs of the results for four mice per group.
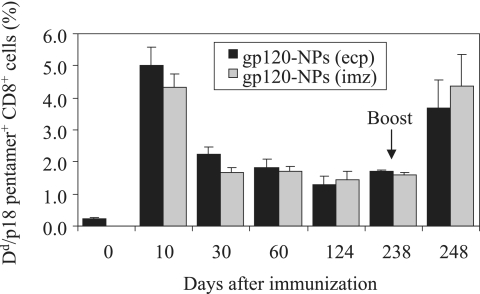
To determine whether the antigen-specific memory CD8+ T cells were long-lived or not, the binding of the H-2Dd/p18 pentamer to CD8+ T cells was evaluated at the indicated times after the immunization with gp120-NPs. As shown in Fig. 5, both gp120-NPs (ecp) and gp120-NPs (imz) displayed similar kinetics of p18-specific CD8+ T-cell responses. After a peak in the number of pentamer-positive cells on day 10, the positive cell number decreased on day 30 and remained stable from day 30 to day 238. However, the pentamer-positive cell number rapidly increased again immediately after boosting with gp120-NPs. To further characterize the antigen-specific memory CD8+ T cells, their maturation stages were determined by assessing the surface expression of CD44, CD127, and CD62L on day 30 after immunization by using flow cytometry. All of the pentamer-positive cells expressed CD44, indicating that they were activated (Fig. 6A). Most of the cells had the profiles of effector memory cells (pentamer positive, CD127+, and CD62Llow), and substantial decay of these cells was observed over time (Fig. 6A and 6B). On the other hand, the number of central memory cells (pentamer positive, CD127+, and CD62Lhigh) was lower than the number of effector memory cells. The number of central memory cells remained relatively constant and tended to increase between days 30 and 238 after immunization. There was no significant difference between gp120-NP (ecp) and gp120-NP (imz) immunization in the resulting levels and subsets of memory cells. These results indicate that gp120-NPs can generate both effector and long-lived central memory HIV-1-specific CD8+ T cells.
FIG. 5.
Long-lived p18-specific memory CD8+ T cells induced by gp120-NPs. Mice were intranasally immunized once with 6 μg of gp120-NPs. On day 238, mice were boosted with the same gp120-NPs as those used for the first immunization. Spleen lymphocytes were collected from the immunized mice at the indicated times, and antigen-specific CD8+ T cells were detected by H-2Dd/p18 pentamer staining. Data are expressed as the percentages of the gated CD8+ T cells that bound to the pentamer, as measured by flow cytometry. All data represent the means ± SEMs or range of the results for two to four mice per group.
FIG. 6.
Analysis of antigen-specific CD8+ central and effector memory T cells induced by gp120-NPs. Mice were intranasally immunized once with 6 μg of gp120-NPs. (A) Spleen lymphocytes were collected from the immunized mice on day 30 and examined by flow cytometry for the expression of CD44, CD127, and CD62L in the H-2Dd/p18 pentamer-positive CD8+ T cells. The numbers in the upper left and right corners of each panel indicate the percentages of H-2Dd/p18 pentamer-positive and indicated marker (CD44, CD127, or CD62L) low and high CD8+ T cells, respectively. (B) Spleen lymphocytes were collected from the immunized mice at the indicated times and examined for the expression of CD127 and CD62L on the H-2Dd/p18 pentamer-positive CD8+ T cells by flow cytometry. Data are expressed as the percentages of CD127+ CD8+ T cells. All data represent the means ± SEMs or range of the results for two to four mice per group.
DISCUSSION
The results presented here demonstrate that novel biodegradable γ-PGA NPs hold great promise as an antigen delivery system and effective adjuvant for inducing HIV-1-specific cellular immune responses. Protein-based vaccines generally induce strong MHC class II-mediated humoral immune responses but weak MHC class I-mediated cellular immune responses. It was reported that the encapsulation of protein antigens into NPs could modulate certain immune responses, including the enhancement of CTL against the particle-associated antigen (17, 19). DCs are considered to be an initiator and modulator of immune responses and capable of presenting antigens through both MHC class I and II pathways. The cells efficiently take up poly(d,l-lactate-co-glycolide) NPs of an appropriate size and present particle-associated antigens along with MHC class I molecules to CD8+ T cells through the antigen cross-presentation pathway (6, 8, 16). γ-PGA NPs were found to deliver protein antigens to DCs in vitro and in vivo (26). Furthermore, our preliminary data suggest that γ-PGA NPs could also efficiently deliver the encapsulated antigen into CD11c+ and CD86+ cells with low granularity in the lung, which were considered to be pulmonary DCs, after intranasal administration (data not shown). Thus, targeting of protein antigen to DCs appears to be an important mechanism of γ-PGA NPs in inducing HIV-1-specific cellular immune responses.
Cellular immunity plays a critical role in controlling HIV-1 replication in the acute phase of infection and maintaining a low viral load in the chronic phase. Therefore, an effective anti-AIDS vaccine should induce potent CTLs against HIV-1 antigen-expressing cells. In fact, the CD8+ T cells induced by gp120-NPs exhibited effector functions, such as cytolytic activity and the production of various cytokines (Fig. 2). Such CD8+ T cells seem to play a critical role in protecting against disease progression in SIV-infected monkeys (13, 24). In comparison with gp120-NPs (imz), gp120-NPs (ecp) showed a slightly better ability to induce functional antigen-specific CD8+ T cells (Fig. 2B, 2C, and 2D). To our surprise, either gp120-NPs (ecp) or gp120-NPs (imz) induced low, if any, levels of gp120-specific antibodies in sera and vaginal fluids of the mice even after four intranasal immunizations (data not shown), whereas the mice immunized with gp120 + CTB had high levels of gp120-specific antibodies in both sera and vaginal fluids. Thus, γ-PGA NPs are different from CTB and have the ability to potently induce cellular immunity rather than humoral immunity through intranasal immunization. Since an efficacious vaccine against HIV-1 should elicit both humoral and cellular responses, the modification of γ-PGA NPs for eliciting both humoral and cellular responses, especially for the production of immunoglobulin A antibodies on the mucous membrane, is now under investigation. Another interesting observation is that even gp120 + NPs could induce considerable cellular immunity (Fig. 2A, 2B, and 2D), probably because negatively charged γ-PGA NPs adsorbed positively charged gp120 on their surface and thereby behaved like gp120-NPs (imz). These results suggest that, although it may not be applicable to all antigens, a simple mixture of some antigens and γ-PGA NPs is more practical as a vaccine formulation in developing protein-based vaccines than antigen-encapsulating or -immobilizing γ-PGA NPs.
An ideal priming vector for inducing cellular immune responses should elicit a large population of CD8+ T cells that differentiate rapidly into memory cells, which will generate vigorous secondary immune responses immediately after boosting. In our study, the antigen-specific CD8+ T cells induced by gp120-NPs rapidly differentiated to the memory cells on day 30 and retained a stable level from day 30 to day 238 (Fig. 5). These memory CD8+ T cells rapidly exhibited effector functions, such as antigen-specific cytotoxicity and IFN-γ production after in vitro stimulation (Fig. 4 and data not shown). Furthermore, the memory CD8+ T cells rapidly expanded in vivo after being boosted with the same antigen (Fig. 5). The characterization of the memory CD8+ T cells revealed that the majority of the cells were effector memory cells on day 30, yet central memory cells also existed and stayed stable for more than 200 days after immunization (Fig. 6). It is noteworthy that intranasal immunization of mice only once with gp120-NPs could induce a stable population of antigen-specific central memory CD8+ T cells. The ability of gp120-NPs to generate the long-lived central memory cells is particularly important, since these cells expand and mediate protective immunity after a challenge with pathogens (24, 30). Further studies are needed to determine whether antigen-carrying γ-PGA NPs have such activity in other species.
The factors that regulate the differentiation of CD8+ T cells to memory cells have not been fully understood yet. Several events, such as inflammation, DC maturation, and antigen persistence, affect the differentiation process. It was recently shown that the vaccination of mice with peptide-pulsed mature DCs resulted in accelerated differentiation of memory CD8+ T cells (3). Indeed, γ-PGA NPs upregulated the expression of MHC and costimulatory molecules of DCs in vitro and in vivo (26). Furthermore, γ-PGA NPs could also induce the production of inflammatory cytokines from DCs. These finding indicate that γ-PGA NPs are capable of inducing the maturation of DCs, which may rapidly generate memory CD8+ T cells.
In conclusion, the present work demonstrates the ability of gp120-carrying γ-PGA NPs to induce robust cellular immune responses in mice. Thus, γ-PGA NPs may have great potential as an antigen carrier and novel protein-based vaccine against HIV-1 infection.
Acknowledgments
This work was supported by Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Agency (JST).
Footnotes
Published ahead of print on 3 July 2007.
REFERENCES
- 1.Akagi, T., T. Kaneko, T. Kida, and M. Akashi. 2005. Preparation and characterization of biodegradable nanoparticles based on poly(γ-glutamic acid) with l-phenylalanine as a protein carrier. J. Control. Release 108:226-236. [DOI] [PubMed] [Google Scholar]
- 2.Akagi, T., M. Kawamura, M. Ueno, K. Hiraishi, M. Adachi, T. Serizawa, M. Akashi, and M. Baba. 2003. Mucosal immunization with inactivated HIV-1-capturing nanospheres induces a significant HIV-1-specific vaginal antibody response in mice. J. Med. Virol. 69:163-172. [DOI] [PubMed] [Google Scholar]
- 3.Badovinac, V. P., K. A. Messingham, A. Jabbari, J. S. Haring, and J. T. Harty. 2005. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat. Med. 11:748-756. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 6.Fonteneau, J. F., D. G. Kavanagh, M. Lirvall, C. Sanders, T. L. Cover, N. Bhardwaj, and M. Larsson. 2003. Characterization of the MHC class I cross-presentation pathway for cell-associated antigens by human dendritic cells. Blood 102:4448-4455. [DOI] [PubMed] [Google Scholar]
- 7.Goonetilleke, N., S. Moore, L. Dally, N. Winstone, I. Cebere, A. Mahmoud, S. Pinheiro, G. Gillespie, D. Brown, V. Loach, J. Roberts, A. Guimaraes-Walker, P. Hayes, K. Loughran, C. Smith, J. De Bont, C. Verlinde, D. Vooijs, C. Schmidt, M. Boaz, J. Gilmour, P. Fast, L. Dorrell, T. Hanke, and A. J. McMichael. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 80:4717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guermonprez, P., L. Saveanu, M. Kleijmeer, J. Davoust, P. Van Endert, and S. Amigorena. 2003. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425:397-402. [DOI] [PubMed] [Google Scholar]
- 9.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawamura, M., T. Naito, M. Ueno, T. Akagi, K. Hiraishi, I. Takai, M. Makino, T. Serizawa, K. Sugimura, M. Akashi, and M. Baba. 2002. Induction of mucosal IgA following intravaginal administration of inactivated HIV-1-capturing nanospheres in mice. J. Med. Virol. 66:291-298. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura, M., X. Wang, T. Uto, K. Sato, M. Ueno, T. Akagi, K. Hiraishi, T. Matsuyama, M. Akashi, and M. Baba. 2005. Induction of dendritic cell-mediated immune responses against HIV-1 by antigen-capturing nanospheres in mice. J. Med. Virol. 76:7-15. [DOI] [PubMed] [Google Scholar]
- 12.Klein, M. 2003. Prospects and challenges for prophylactic and therapeutic HIV vaccines. Vaccine 21:616-619. [DOI] [PubMed] [Google Scholar]
- 13.Letvin, N. 2006. Virus-specific cellular immune correlates of survival in vaccinated monkeys after SIV challenge. Retrovirology 3(Suppl. 1):S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letvin, N. L. 2002. Strategies for an HIV vaccine. J. Clin. Investig. 110:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letvin, N. L., Y. Huang, B. K. Chakrabarti, L. Xu, M. S. Seaman, K. Beaudry, B. Korioth-Schmitz, F. Yu, D. Rohne, K. L. Martin, A. Miura, W. P. Kong, Z. Y. Yang, R. S. Gelman, O. G. Golubeva, D. C. Montefiori, J. R. Mascola, and G. J. Nabel. 2004. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J. Virol. 78:7490-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutsiak, M. E., D. R. Robinson, C. Coester, G. S. Kwon, and J. Samuel. 2002. Analysis of poly(d,l-lactic-co-glycolic acid) nanosphere uptake by human dendritic cells and macrophages in vitro. Pharm. Res. 19:1480-1487. [DOI] [PubMed] [Google Scholar]
- 17.Maloy, K. J., A. M. Donachie, D. T. O'Hagan, and A. M. Mowat. 1994. Induction of mucosal and systemic immune responses by immunization with ovalbumin entrapped in poly(lactide-co-glycolide) microparticles. Immunology 81:661-667. [PMC free article] [PubMed] [Google Scholar]
- 18.Miyake, A., T. Akagi, Y. Enose, M. Ueno, M. Kawamura, R. Horiuchi, K. Hiraishi, M. Adachi, T. Serizawa, O. Narayan, M. Akashi, M. Baba, and M. Hayami. 2004. Induction of HIV-specific antibody response and protection against vaginal SHIV transmission by intranasal immunization with inactivated SHIV-capturing nanospheres in macaques. J. Med. Virol. 73:368-377. [DOI] [PubMed] [Google Scholar]
- 19.Moore, A., P. McGuirk, S. Adams, W. C. Jones, J. P. McGee, D. T. O'Hagan, and K. H. Mills. 1995. Immunization with a soluble recombinant HIV protein entrapped in biodegradable microparticles induces HIV-specific CD8+ cytotoxic T lymphocytes and CD4+ Th1 cells. Vaccine 13:1741-1749. [DOI] [PubMed] [Google Scholar]
- 20.Otten, G., M. Schaefer, C. Greer, M. Calderon-Cacia, D. Coit, J. Kazzaz, A. Medina-Selby, M. Selby, M. Singh, M. Ugozzoli, J. zur Megede, S. W. Barnett, D. O'Hagan, J. Donnelly, and J. Ulmer. 2003. Induction of broad and potent anti-human immunodeficiency virus immune responses in rhesus macaques by priming with a DNA vaccine and boosting with protein-adsorbed polylactide coglycolide microparticles. J. Virol. 77:6087-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otten, G. R., M. Schaefer, B. Doe, H. Liu, I. Srivastava, J. Megede, J. Kazzaz, Y. Lian, M. Singh, M. Ugozzoli, D. Montefiori, M. Lewis, D. A. Driver, T. Dubensky, J. M. Polo, J. Donnelly, D. T. O'Hagan, S. Barnett, and J. B. Ulmer. 2005. Enhanced potency of plasmid DNA microparticle human immunodeficiency virus vaccines in rhesus macaques by using a priming-boosting regimen with recombinant proteins. J. Virol. 79:8189-8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 23.Seaman, M. S., L. Xu, K. Beaudry, K. L. Martin, M. H. Beddall, A. Miura, A. Sambor, B. K. Chakrabarti, Y. Huang, R. Bailer, R. A. Koup, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2005. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J. Virol. 79:2956-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun, Y., J. E. Schmitz, A. P. Buzby, B. R. Barker, S. S. Rao, L. Xu, Z. Y. Yang, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2006. Virus-specific cellular immune correlates of survival in vaccinated monkeys after simian immunodeficiency virus challenge. J. Virol. 80:10950-10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi, H., Y. Nakagawa, C. D. Pendleton, R. A. Houghten, K. Yokomuro, R. N. Germain, and J. A. Berzofsky. 1992. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science 255:333-336. [DOI] [PubMed] [Google Scholar]
- 26.Uto, T., X. Wang, K. Sato, M. Haraguchi, T. Akagi, M. Akashi, and M. Baba. 2007. Targeting of antigen to dendritic cells with poly(γ-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J. Immunol. 178:2979-2986. [DOI] [PubMed] [Google Scholar]
- 27.Reference deleted.
- 28.Wang, X., T. Akagi, M. Akashi, and M. Baba. 2007. Development of core-corona type polymeric nanoparticles as an anti-HIV-1 vaccine. Mini-Rev. Org. Chem. 4:51-59. [Google Scholar]
- 29.Wang, X., T. Uto, K. Sato, K. Ide, T. Akagi, M. Okamoto, T. Kaneko, M. Akashi, and M. Baba. 2005. Potent activation of antigen-specific T cells by antigen-loaded nanospheres. Immunol. Lett. 98:123-130. [DOI] [PubMed] [Google Scholar]
- 30.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]