Abstract
Although natural killer (NK) cell-mediated control of viral infections is well documented, very little is known about the ability of NK cells to restrain human T-cell leukemia virus type 1 (HTLV-1) infection. In the current study we show that NK cells are unable to kill HTLV-1-infected primary CD4+ T cells. Exposure of NK cells to interleukin-2 (IL-2) resulted in only a marginal increase in their ability to kill HTLV-1-infected primary CD4+ T cells. This inability of NK cells to kill HTLV-1-infected CD4+ T cells occurred despite the down-modulation of major histocompatibility complex (MHC) class I molecules, one of the ligands for the major NK cell inhibitory receptor, by HTLV-1 p12I on CD4+ T cells. One reason for this diminished ability of NK cells to kill HTLV-1-infected cells was the decreased ability of NK cells to adhere to HTLV-1-infected cells because of HTLV-1 p12I-mediated down-modulation of intercellular adhesion molecule 1 (ICAM-1) and ICAM-2. We also found that HTLV-1-infected CD4+ T cells did not express ligands for NK cell activating receptors, NCR and NKG2D, although they did express ligands for NK cell coactivating receptors, NTB-A and 2B4. Thus, despite HTLV-1-mediated down-modulation of MHC-I molecules, HTLV-1-infected primary CD4+ T cells avoids NK cell destruction by modulating ICAM expression and shunning the expression of ligands for activating receptors.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (ATL) (62, 77), an aggressive fatal CD4+ T-cell malignancy, and HTLV-1-associated myelopathy/tropical spastic paraparesis, a neurodegenerative disease of the central nervous system (30, 56). HTLV-1 can infect CD4+ T cells and establish a life-long persistent infection in humans (63). One reason for the persistence of HTLV-1 in the host may be a consequence of the ability of the virus to evade the host immune response. A possible mechanism by which HTLV-1 evades immune responses is by down-modulating the expression of major histocompatibility complex class I (MHC-I) molecules on the surface of infected cells allowing their escape from recognition and destruction by HTLV-1 antigen-specific cytotoxic T lymphocytes (CTLs) (55, 68, 73). The HTLV-1 accessory protein p12I has been previously shown to down-modulate the surface expression of MHC-I on T-cell lines (38). The p12I gene is expressed early after virus entry and is critical for establishing and maintaining viral infection in vivo (1, 21). p12I-mediated suppression of MHC-1 may be a mechanism that allows HTLV-1 to evade early innate immune surveillance while concurrently allowing for the persistent infection of the host.
HTLV-1-mediated down-modulation of MHC-I expression may make HTLV-1-infected cells vulnerable to natural killer (NK) cell-mediated destruction (42). NK cells do not require prior recognition of the pathogen to kill virus-infected cells and are activated by invariant activating ligands present on the cell surface (8, 14, 18). Although uninfected cells may express these activating ligands, NK cells are unable to destroy these cells because MHC-I on the surface of uninfected cells engage specific inhibitory receptors (iNKRs) dampening NK cell cytotoxicity (12, 15, 22, 27). Whether down-modulation of MHC-I leads to NK cell cytotoxicity toward HTLV-1-infected lymphocytes is not yet clearly defined (64, 68, 73).
In addition to loss of inhibitory control through the altered expression of MHC-I, strong adhesion to the target cells, mediated by integrins such as leukocyte function antigen 1 (LFA-1) on NK cells, is critical in triggering NK cell cytotoxicity (4, 33). The engagement of LFA-1 with its natural ligands on target cells is involved in the formation of “NK-target cells immune synapse,” which is important in activation signaling events (33). In addition to immune synapse formation, LFA-1 triggers early signaling events leading to cytotoxic granule polarization, which is important in directing the NK cell killing machinery to the target cells (43). The natural ligands for LFA-1 are intercellular adhesion molecules (ICAMs), which are expressed on a variety of cells, including leukocytes (74, 76). Although NK cells express other integrins, the LFA-1 and ICAM interactions are necessary for efficient adhesion, early signaling, and polarization, resulting in an effective NK cell cytotoxic response (4, 13, 33, 34, 45, 46, 61). Altered ICAM-1 expression has been found on the peripheral blood mononuclear cells (PBMC) of ATL patients (28). However, it remains to be determined whether HTLV-1 can modulate ICAM expression on primary CD4+ T cells, the natural targets of HTLV-1 in vivo (63).
Impaired expression of MHC-I and coengagement of LFA-1 and ICAM may lead to adhesion, synapse formation, and granule polarization, but it is not sufficient to trigger a robust NK cell cytotoxic response toward the target cells (40). The engagement of NK cell activating receptors to their corresponding ligands expressed on the target cells provides the activating signals critical for inducing degranulation, thereby triggering NK cell killing of target cells (49). The major NK cell-specific activating receptors involved in NK cell-mediated destruction of various target cells consist of immunoglobulin-like domain containing natural cytotoxicity receptors (NCRs), NKp30, NKp44, and NKp46 (7, 48, 67), and the C-type lectin NKG2D (49, 59). Although the natural ligands for NCR are still unknown, NKG2D ligands consist of two classes of MHC-I-like molecules, cytomegalovirus (CMV) UL-16 binding proteins and MHC-I-related chain. Although their altered expression might modulate the susceptibility of the infected cells to NK cell cytotoxic response (20, 75), whether ligands for NKG2D and NCR are expressed by HTLV-1-infected primary CD4+ T cells is unknown.
In the present study, we investigated whether HTLV-1 infection leads to down-modulation of MHC-I expression on primary CD4+ T cells and thus makes the HTLV-1-infected primary CD4+ T cells susceptible to autologous NK cell-mediated cytolysis. We sought to determine whether the surface expression of ICAM molecules is affected by infection and whether this modulation alters the ability of the NK cells to adhere to HTLV-1-infected primary CD4+ T cells. We also determined the expression profile of ligands for NK cell activating and coactivating receptors on HTLV-1-infected primary CD4+ T cells. Collectively, the present study investigates whether the ligands critical for initiating the activation signals required for an effective NK cell cytotoxic response are either present or altered after HTLV-1 infection of primary CD4+ T cells.
MATERIALS AND METHODS
Isolation of CD4+ T cells.
Venous blood (60 ml) was obtained from healthy HTLV-1-uninfected donors after informed consent into heparin-treated (143 U) 10-ml Vacutainer tubes (BD Biosciences, Franklin Lakes, NJ) according to the protocols established by the Institutional Review Board for the Protection of Human Subjects of State University of New York, Upstate Medical University (Syracuse, NY). The subsequent steps of isolation and purification of PBMC from the heparin-treated blood by Ficoll-Hypaque gradient (Mediatech, Herndon, VA) centrifugation was performed as previously described (11). Primary CD4+ T cells were isolated from PBMC by using anti-CD4 antibodies coupled to magnetic beads according to the manufacturer's instructions (Invitrogen Life Technologies, Carlsbad, CA), resuspended at 3 × 106 cells/ml in complete medium, consisting of RPMI 1640 medium (Mediatech) with 10% heat-inactivated (56°C for 30 min) fetal bovine serum (FBS; Mediatech), two mM glutamine (Mediatech), 100 μg of streptomycin (Mediatech)/ml, 100 U of penicillin (Mediatech)/ml, and 200 U of recombinant human interleukin-2 (rhIL-2; obtained from the NIH AIDS Research and Reference Reagent Program, Germantown, MD)/ml. The CD4+ T cells were stimulated with 3 μg of phytohemagglutinin (PHA; Sigma)/ml for 3 days.
HTLV-1 infection of primary CD4+ T cells.
PHA-stimulated CD4+ T cells were cocultured with a lethally irradiated (103 rads) (137CS source) chronically HTLV-1-infected cell line, SLB-1 (69), at a recipient/donor cell ratio of 1:5 as previously described (41, 71). Mock-infected cells consisted of the PHA-stimulated CD4+ T cells not exposed to SLB-1 cells but were cultured in the same medium used for the infection. After 2 weeks, the cultures were washed with phosphate-buffered saline (PBS), and the media were replaced. Freshly irradiated SLB-1 cells were added again to the primary CD4+ T cells at a recipient/donor cell ratio of 1:5. After 14 days of coculture with SLB-1 cells, CD4+ T cells were washed twice with PBS and then analyzed by flow cytometry or were used as target cells in a 51Cr release assay.
To confirm the lack of viability of lethally irradiated HTLV-1-infected donor cells after 2 weeks in culture, normal and irradiated SLB-1 cells (without primary CD4+ T cells) were analyzed for viability by staining with phycoerythrin (PE)-conjugated annexin V (Biovision, Mountain View, CA) and 7-amino actinomycin D (Calbiochem, La Jolla, CA) at 2 weeks after irradiation (Fig. 1) as described previously (66).
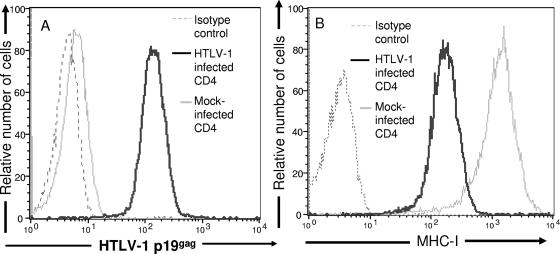
FIG. 1.
Down-modulation of MHC-I expression on primary HTLV-1-infected CD4+ T cells. HTLV-1-infected and mock-infected primary CD4+ T-cell blasts were stained with fluorochrome-conjugated anti-MHC-I antibody and then permeabilized, fixed, and stained with anti-HTLV-1 capsid antibody (p19gag) and analyzed by flow cytometry. (A) Histogram demonstrating HTLV-1 p19gag expression on 104 HTLV-1-infected (black line) or mock-infected (gray line) cells. Thin black lines represent cells stained with secondary antibody in absence of primary antibody. (B) Histogram demonstrating MHC-I expression on 104 HTLV-1-infected (black line) or mock-infected (gray line) cells. Thin black lines represent cells stained with immunoglobulin of similar isotype as MHC-I antibody.
Cytotoxicity assay.
Targets and NK cells for 51Cr release assays were obtained from the same donor. NK cells were isolated from PBMC by immunomagnetic bead isolation with anti-CD56 antibody coupled to magnetic beads (Stem Cell Technologies, Vancouver, British Columbia, Canada). IL-2-treated NK cells were cultured overnight at 3 × 106 cells/ml in 1,000 U of IL-2/ml of culture. Killing of HTLV-1-infected CD4+ T cells or mock-infected CD4+ T cells was determined by using the 51Cr release assay as described previously (9).
Conjugate assay.
The conjugate assay was done as previously described (16). Briefly, NK cells (2 × 106 cells/ml) were labeled with 5 μM concentrations of red fluorescent cell linker dye (PKH26-GL; Sigma), and HTLV-1-infected CD4+ T cells (2 × 106 cells/ml) or mock-infected CD4+ T cells (2 × 106 cells/ml) were labeled with 5 μM green fluorescent cell linker dye (PKH67-GL, Sigma) for 5 min at room temperature. Thereafter, the cells were diluted in 2 volumes of 100% FBS to terminate the staining reaction and washed thrice with two volumes of media. After the final wash the cells were incubated in a 5% CO2 incubator at 37°C for an hour. After the incubation at 37°C the cells were resuspended in cold media with 10% FBS. Effector cells (2 × 105) and target cells (105) were mixed in a final volume of 200 μl of media in a 12-by-75-mm tube (Falcon, Franklin, NJ). The cells were mixed by vortexing the tubes gently and then centrifuged at 4°C for 3 min at 30 × g. The cells were then placed in a 37°C water bath for 0, 10, 20, or 30 min. Thereafter, the cells were mixed by gentle vortexing and fixed by adding 1 ml of cold 0.5% paraformaldehyde in PBS (pH 7.2). The samples were then acquired by using an LSR II flow cytometer (BDIS, San Jose, CA). Acquired samples were then analyzed by using the Cellquestpro 4.0 program (BDIS). The results were expressed as the percentage of target cells that form conjugates with NK cells as calculated by the ratio of cells that are positive for both colors to total cells.
Generation of VSV-G-pseudotyped HTLV-1 p12I expressing lentivirus vectors and transduction of primary CD4+ T cells.
Vesicular stomatitis virus protein G (VSV-G)-pseudotyped lentivirus vector stocks were generated, as previously described (72). Briefly, a three-plasmid transfection system was used consisting of the transfer plasmid (p12I-HA-green fluorescent protein [GFP] or GFP alone [24, 52]), a packaging vector (pCMVΔR8.2ΔVPR), and a vector encoding the VSV-G envelope protein (pHCMV-G). Plasmids were cotransfected into 107 293FT cells (Invitrogen Life Technologies, Carlsbad, CA) by calcium phosphate precipitation (72). Supernatants were harvested at 2 or 4 days posttransfection and filtered through a 0.45-μm-pore-size filter, pooled, and subjected to ultracentrifugation in a Beckman Optima L-90K ultracentrifuge (Beckman-Coulter, Palo Alto, CA) in a SW32 rotor (Beckman-Coulter) at 5 × 104 × g for 2 h at 4°C. The pellets were resuspended overnight at 4°C in a 1/100 initial volume in serum-free Dulbecco modified Eagle medium (DMEM), pooled, and stored at −80°C. Titers of vector stocks were determined by transducing 3 × 105 HeLa cells (American Type Culture Collection, Manassas, VA) with virus vector stocks that were serially diluted (1:10, 1:100, 1:500, and 1:1,000) in DMEM. HeLa cells were analyzed for GFP expression 48 h posttransduction by flow cytometry. Vector titers ranged between 106 to 107 transducing units per ml. Primary CD4+ T cells were transduced with lentivirus vectors (multiplicity of infection = 25) in the presence of Polybrene (8 μg/ml) by spin inoculation in a Beckman GPR centrifuge (3 × 102 × g) in a final volume of 2 ml of serum-free DMEM for 2 h at room temperature (6, 9). Cells were analyzed for GFP expression at 6 days posttransduction by flow cytometry.
Flow cytometry.
HTLV-1-infected or mock-infected primary CD4+ T cells (106) were washed twice with Ca2+/Mg2+-free PBS. After the final wash, 106 of either mock-infected or HTLV-1-infected CD4+ T-cell were resuspended in 100 μl of flow buffer (PBS with 0.1% NaN3) and stained with either 10 μl of soluble fusion proteins of the Fc portion of human immunoglobulin G (IgG) and NKp30, NKp44, NKp46, or NKG2D (R&D Systems, Minneapolis, MN) or 5 μl each of mouse anti-human CD54, CD50, CD48 (Pharmingen, La Jolla, CA), CD102a (Serotec, Raleigh, NC), or NTB-A (R&D Systems, Minneapolis, MN) antibody for 20 min at 4°C. After the samples were washed twice with flow buffer, the cells were stained with PE-conjugated mouse anti-human IgG Fc-specific antibody or PE-conjugated goat anti-mouse IgG Fc-specific antibody (Jackson Immunolabs). Cells were also stained directly with 5 μl of PE-conjugated mouse anti-human pan MHC-I antibody (clone W6/32; Dako, Fort Collins, CO).
After staining with the secondary antibody, cells were washed twice in flow buffer and then permeabilized and fixed by using Cytofix/Cytoperm solution (BDIS) according to the manufacturer's protocol. Cells were then washed twice with permeabilization-wash buffer (BDIS) and resuspended in 100 μl of permeabilization-wash buffer. Cells were then incubated with mouse anti-HTLV-1 Gag (p19) antibody (Zeptometrix, Buffalo, NY) (3 μl/106 cells) for 30 min at 4°C, washed twice with PBS, and then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG monoclonal antibody (1 μl/106 cells; Dak) for 30 min in dark at 4°C. Ten thousand viable and/or HTLV-1 p19+ cells were acquired by using an LSR II flow cytometer. Acquired samples were analyzed with the Cellquestpro 4.0 program.
RESULTS
HTLV-1 modulates the expression of MHC-I molecules on primary CD4+ T cells.
It is unknown whether HTLV-1 infection alters the expression of MHC-I molecules on primary CD4+ T cells, the prime target of HTLV-1 infection in vivo (63). To resolve whether alteration of MHC-I expression occurs after infection on CD4+ T cells with HTLV-1, we infected freshly isolated primary CD4+ T cells with HTLV-1. Greater than 90% of primary CD4+ T cells showed HTLV-1 p19gag expression after 2 weeks of infection (Fig. 1A). The mock-infected and HTLV-1-infected primary CD4+ T cells were then stained with an antibody to a conserved epitope found on all MHC-I molecules (W6/32). The surface expression of MHC-I molecules was drastically reduced on HTLV-1-infected CD4+ T cells compared to MHC-I expression on mock-infected CD4+ T-cell (mean fluorescent intensities [MFIs] of MHC-I expression of 166.7 and 1154, respectively) (Fig. 1B). Thus, HTLV-1 infection leads to down-modulation of MHC-I expression on primary CD4+ T cells.
HTLV-1-infected primary CD4+ T cells are resistant to autologous NK cell-mediated cytotoxicity.
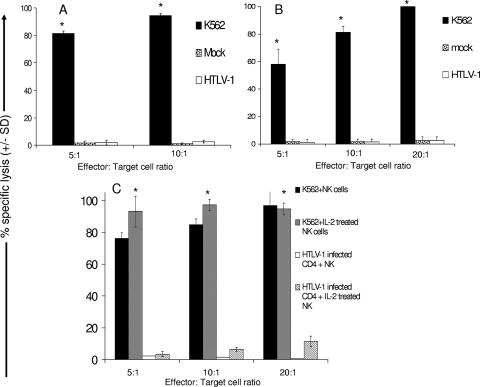
We found that HTLV-1 infection down-modulates MHC-I expression on primary CD4+ T cells, so we wanted to determine whether autologous NK cells were capable of killing HTLV-1-infected primary CD4+ T cells in a cytotoxic assay. We found that NK cell cytotoxicities toward both HTLV-1-infected and mock-infected primary CD4+ T cells (≤3.0% at an effector cell/target cell [E:T] ratio of 20:1) were drastically lower than the NK cell cytotoxicity toward K562 cells (≥95.0% at an E:T ratio of 20:1) (Fig. 2A and B). Notably, the levels of NK cell cytotoxicity were comparable between mock-infected (∼2.5% at an E:T ratio of 20:1) and HTLV-1-infected cells (∼3.0% at E:T ratio of 20:1). This low level of cytotoxicity took place despite the robust down-modulation of MHC-I expression on infected cells compared to mock-infected cells (Fig. 1B). This suggests that NK cells lacked the ability to effectively kill autologous HTLV-1-infected primary CD4+ T cells even though MHC-I expression was substantially reduced on infected cells.
FIG. 2.
HTLV-1-infected primary CD4+ T cells are resistant to lysis by autologous natural killer cells. HTLV-1-infected CD4+ T cells, mock-infected CD4+ T cells, and K562 cells were used as target cells in a 4-h cytotoxic assay with “resting”(A and B) or IL-2-treated (C) (1,000 U of IL-2/ml overnight) autologous NK cells as effector cells. E:T ratios of 5:1, 10:1, and 20:1 were used. All groups were performed in triplicates. Each experiment involved CD4+ T cells and NK cells from two different donors. Statistical analysis was performed by using single-tail analysis of variance and Student t tests (*, P < 0.05).
IL-2 pretreatment marginally increases the NK cells cytotoxic response to autologous HTLV-1-infected primary CD4+ T cells.
IL-2 pretreatment has been shown to evoke NK cell cytotoxicity toward HTLV-1-infected T-cell lines (64). Hence, we wanted to determine whether IL-2 pretreatment could enhance NK cell cytotoxicity toward HTLV-1-infected autologous primary CD4+ T cells since resting NK cells failed to kill HTLV-1-infected targets (Fig. 2A and B). We found that although IL-2 pretreatment increased the ability of NK cells to kill autologous HTLV-1-infected CD4+ T cells in comparison to untreated NK cells (11% versus 3%; Fig. 2C), the level of killing was significantly lower than for NK cell-mediated killing of K562 cells (P < 0.05) (Fig. 2C). Furthermore, although IL-2-pretreated NK cells killed HTLV-1-infected CD4+ T cells, the killing was limited to 11.5% at an E:T ratio of 20:1 and not statistically different from the response at an E:T ratio of 10:1 (P = 0.4). Thus, although IL-2 pretreatment leads to a modest threefold increase in the cytotoxic response of NK cells to HTLV-1-infected CD4+ T cells, it is clearly not up to the maximal potential of the NK cell cytotoxic response demonstrated by the destruction of K562 cells.
HTLV-1 decreased the expression of ICAM-1 and -2 on primary CD4+ T cells.
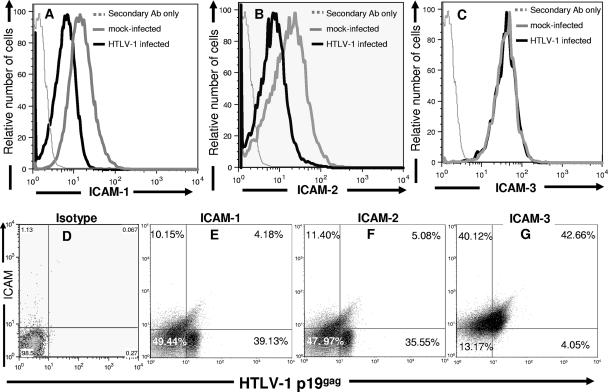
We wanted to investigate whether HTLV-1 infection altered ICAM expression on primary CD4+ T cells because of its importance in engaging LFA-1 on NK cells (4). Interestingly, we found that HTLV-1 infection significantly down-modulates ICAM-1 and -2 expression on the surface of CD4+ T cells compared to mock-infected CD4+ T cells (Fig. 3A and B) (the ICAM-1 MFIs for mock-infected cells and HTLV-1-infected cells were 20.2 and 4.6, respectively, the ICAM-2 MFIs for mock-infected cells and HTLV-1-infected cells were 18.6 and 3.2, respectively). The difference in MFI for ICAM-1 and ICAM-2 between mock-infected CD4+ T cells and HTLV-1-infected CD4+ T cells did not vary significantly between days 7 to 14 postinfection (data not shown). Notably, unlike ICAM-1 and -2, the expression of ICAM-3 was not significantly altered on CD4+ T cells after infection with HTLV-1 (the MFI on mock-infected cells was 19.8, and the MFI on HTLV-1-infected cells was 20.2) (Fig. 3C). Thus, HTLV-1 selectively down-modulates the expression of ICAM-1 and -2, but not ICAM-3, after infection of primary CD4+ T cells.
FIG. 3.
HTLV-1 infection modulates ICAM-1 and -2 expression on HTLV-1-infected primary CD4+ T cells. Mock-infected and HTLV-1-infected CD4+ T cells were indirectly stained with anti-CD54 (ICAM-1) (A and E), anti-CD102a (ICAM-2) (B and F), or anti-CD50 (ICAM-3) (C and G) antibodies. Cells were then permeabilized, fixed, and stained with anti-HTLV-1 p19 capsid antibody. The extent of ICAM-1 (A), ICAM-2 (B), and ICAM-3 (C) expression on 104 infected (thick black line) and mock-infected CD4+ T cells (thick gray line) was determined. Negative controls (dotted gray line) consisted of secondary antibody in the absence of primary antibody. (D to G) Dot plots of ICAM-1 (E), ICAM-2 (F), and ICAM-3 (G) expression versus HTLV-1 p19 capsid protein expression on 104 HTLV-1-infected CD4+ T cells. Numbers represent percentage of cells in each quadrant.
Ability of NK cells to adhere to autologous HTLV-1-infected CD4+ T cells.
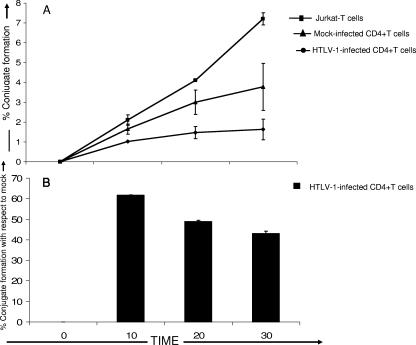
HTLV-1 down-modulation of ICAM-1 and -2 expression may affect the ability of NK cells to adhere to the infected cells (Fig. 4). To test this, a two-colored flow cytometry assay was used to determine the percentage of cells that formed NK cell and target cell heteroconjugates (16). IL-2 treatment has been shown to enhance LFA-1-mediated adhesion of NK cells to ICAMs on target cells, so we pretreated the NK cells with IL-2 (5). The percentage of heteroconjugates formed between HTLV-1-infected CD4+ T cells and autologous IL-2 pretreated NK cells was significantly lower (Fig. 4A) (mean = 1.5% after 30 min) than the percentage of heteroconjugates formed between mock-infected CD4+ T cells and autologous NK cells (Fig. 4A) (mean = 3.5% after 30 min). In contrast, CD4+ Jurkat T cells, which have normal levels of ICAM expression, formed ca. 8% heteroconjugates with NK cells after 30 min of incubation at 37°C (Fig. 4A). Notably, the percentage of heteroconjugate formation between HTLV-1-infected CD4+ T cells and autologous NK cells was significantly higher after 10 min of incubation compared to 20 and 30 min of incubation (P < 0.05), suggesting that cellular adhesions were relatively rapid and did not require longer incubation times (Fig. 4B). Thus, the ability of the NK cells to physically adhere to CD4+ T cells is significantly reduced after infection with HTLV-1.
FIG. 4.
HTLV-1-infected CD4+ T cells have a decreased ability to adhere to autologous natural killer cells. (A) Heteroconjugate formation between Jurkat T cells, HTLV-1-infected CD4+ T cells, or uninfected CD4+ T cells and autologous IL-2-treated NK cells was measured at 0, 10, 20, and 30 min. Experiments were done using three separate healthy donors. (B) Percent heteroconjugate formation by HTLV-1-infected CD4+ T cells over time with respect to 100% heteroconjugate formation by mock-infected cells. All groups were evaluated in triplicate with cells from three different donors. Statistical analysis was performed by using single-tail analysis of variance and Student t tests (*, P < 0.05).
HTLV-1 p12I is sufficient for down-modulation of MHC-I, ICAM-1, and ICAM-2 on primary CD4+ T cells.
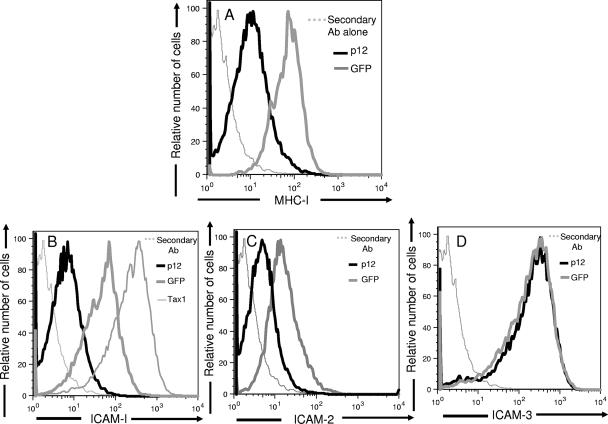
To determine whether HTLV-1 p12I decreases the expression of MHC-I on primary CD4+ T cells, we transduced primary CD4+ T cells with a bicistronic lentivirus vector encoding HTLV-1 p12I and GFP. This vector has been shown, by reverse transcription-PCR and Western blot analysis, to coexpress GFP and p12I-HA protein, as described previously (24, 52). As a control, the primary CD4+ T cells from the same donor were transduced with a lentivirus vector that expressed GFP only. HTLV-1 p12I transduction in CD4+ T cells resulted in significant down-modulation of MHC-I expression compared to MHC-I expression on CD4+ T cells transduced with control GFP vector alone (Fig. 5A) (the MFIs of MHC-1 were 710.3 and 79.3 for mock-treated and p12I-transduced cells, respectively).
FIG. 5.
HTLV-1 p12I down-modulates MHC-I, ICAM-1, and ICAM-2 expressions on primary CD4+ T cells. Primary CD4+ T cells were transduced with lentivirus vectors encoding both GFP and HTLV-1 p12I (black line). As a control CD4+ cells were transduced with a vector expressing GFP only (gray line). Transduced CD4+ T cells were stained with PE-conjugated anti-MHC-I antibody (W6/32) (A), anti-CD54 (ICAM-1) (B), anti-CD102a (ICAM-2) (C), or anti-CD50 (ICAM-3) (D) antibody. The histogram represents gated 104 GFP-positive cells. Negative controls (gray dotted line) consisted of cells stained with secondary antibody in the absence of primary antibody.
To evaluate whether p12I could affect the expression of ICAMs on CD4+ T cells, primary CD4+ T cells were transduced with lentivirus vectors encoding p12I and GFP. As a control, the primary CD4+ T cells from the same donor were transduced with a lentivirus vector that expressed GFP only (52). As illustrated in Fig. 5B and C, p12I-transduced CD4+ T cells showed significantly decreased levels of ICAM-1 and -2 expression compared to GFP only-transduced CD4+ T cells (the MFIs of ICAM-1 and ICAM-2 for GFP-transduced CD4+T cells were 141.14 and 83.2, respectively; those for p12I-transduced CD4+ T cells were 92.44 and 25.2, respectively). Similar to the results observed with HTLV-1-infected CD4+ T cells, we did not find any significant difference in the surface expression of ICAM-3 between p12I-transduced and GFP only-transduced CD4+ T cells (Fig. 5D) (the MFIs of ICAM-3 were 18.4 and 16.2 for GFP-transduced CD4+ T cells p12I-transduced CD4+ T cells, respectively). Notably, we found that Tax1 transduction leads to the up-regulation of ICAM-1 expression on CD4+ T cells, an observation in accordance with previously published data (57). Thus, HTLV-1 p12I selectively down-modulates the expression of ICAM-1 and -2 on primary CD4+ T cells, leaving the expression of ICAM-3 unaltered on the surface.
HTLV-1-infected CD4+ T cells do not express ligands for NK cell activating receptors NCRs and NKG2D but do express ligands for NK cell coactivating receptors 2B4 and NTB-A.
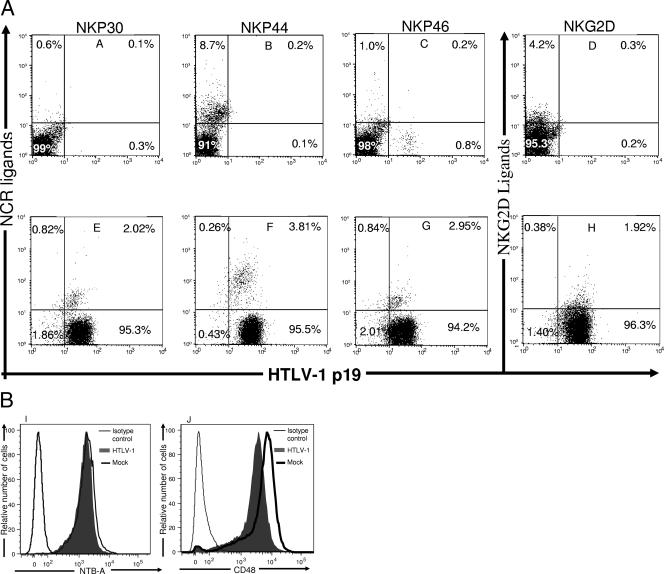
Reduced expression of MHC-I on the target cells or a robust adhesion of the NK cells to the target cells is insufficient to trigger NK cell cytotoxicity (40) and requires activating signals provided by the engagement of NK cell activating receptors such as NCRs and/or NKG2D to their corresponding ligands on target cells (49). We found that ≥95% HTLV-1-infected primary CD4+ T cells did not express NCR and NKG2D ligands on their surface (Fig. 6E to H). Notably, only 2.02, 2.95, and 1.92% of HTLV-1-infected primary CD4+ T cells express ligands for NKp30, NKp46, and NKG2D levels, respectively, which were comparable to the expression levels on mock-infected CD4+ T cells (0.6, 1.0, and 4.2%, respectively) (Fig. 6). Also, only 3.8% of HTLV-1-infected CD4+ T cells express ligands for NKp44 compared to 8.7% of mock-infected cells (Fig. 6B and F). This indicates that the lack of expression of NCR and NKG2D ligands on HTLV-1-infected CD4+ T cells might affect the ability of NK cells to trigger an effective cytotoxic response toward the HTLV-1-infected targets.
FIG. 6.
Expression of ligands for NCR, NKG2D, 2B4, and NTB-A on HTLV-1-infected primary CD4+ T cells. Mock-infected (A to D) and HTLV-1-infected (E to H) CD4+ T cells were stained with recombinant human NKp30 and IgG Fc fusion proteins (A and E), human NKp44 and IgG Fc fusion proteins (B and F), human NKp56 and IgG Fc fusion proteins (C and G), and human NKG2D and IgG Fc fusion proteins (D and H). Cells were then permeabilized, fixed, and stained with anti-HTLV-1 p19 capsid antibody. Numbers represent the percentage of cells in each quadrant. Mock-infected and HTLV-1-infected CD4+ T cells were stained with mouse anti-human NTB-A (I) and CD48 (J) antibody. Cells were then permeabilized, fixed, and stained with anti-HTLV-1 p19 capsid antibody. The extent of CD48 and NTB-A expressed on 104 infected cells (filled gray line) and 104 mock-infected CD4+ T cells (thick black line) were determined. Negative controls (thin black line) consisted of secondary antibody in the absence of primary antibody.
Alteration in the expression of NK cell coactivating receptor ligands may also affect the ability of NK cells to kill target cells (60), so we wanted to determine whether HTLV-1 infection alters the expression of coactivating receptor ligands on primary CD4+ T cells. After screening primary CD4+ T cells for coactivating receptor ligands, we found that they express CD48 and NTB-A, ligands for NK cell coactivating receptors 2B4 and NTB-A (Fig. 6I and J). Also, HTLV-1 infection did not alter the expression of CD48 and NTB-A on primary CD4+ T cells (the MFIs of CD48 were 76.8 and 95.5 for mock-infected and HTLV-1-infected cells, respectively, and the MFIs of NTB-A were 8.5 and 9.8 for mock-infected and HTLV-1-infected cells, respectively) (Fig. 6I and J). Hence, both mock-infected and HTLV-1-infected primary CD4+ T cells express ligands for NK cell coactivating receptors, and HTLV-1 infection does not modulate the expression of these ligands on CD4+ T cells.
DISCUSSION
The NK cell-mediated cytotoxic response may be pivotal in controlling viremia during the early stages of primary HTLV-1 infection prior to the development of adaptive immunity because NK cells do not require prior recognition of HTLV-1 to kill the virus-infected cells (3, 58). Notably, transient suppression of NK activity was previously shown to be necessary for the establishment and growth of tumors derived from HTLV-1 transformed cell lines in SCID mice, indicating that NK cells play a prominent role in controlling the establishment and growth of HTLV-1-derived tumors in vivo (26, 69). Moreover, NK cells may help in eliminating infected cells when an HTLV-1-specific CTL response is impaired because of the down-modulation of MHC-I molecules (68). Earlier studies have reported that NK cells are cytotoxic for target cells with abnormally low levels of MHC-I expression (55, 68). In the present study, we found that the down-modulation of MHC-I on HTLV-1-infected primary CD4+ T cells did not enhance their killing by autologous NK cells (Fig. 2).
Apart from the inhibitory control via the coengagement of iNKRs with MHC-I on target cells, NK cell cytotoxicity also involves adhesion, synapse formation, polarization, and degranulation. These processes involve the coengagement of a variety of distinct receptors with their corresponding ligands on target cells. Coengagement of LFA-1 with ICAMs on target cells is critical for inducing adhesion, synapse formation, and early activation signals, leading to cytotoxic granule polarization in NK cells (4, 13, 33). We found that HTLV-1 down-regulates ICAM-1 and -2 expression on primary CD4+ T cells (Fig. 3) and, furthermore, the ability of primary CD4+ T cells to adhere to autologous NK cells was significantly reduced after infection with HTLV-1 (Fig. 4). On the contrary, mock-infected primary CD4+ T cells and Jurkat T cells that maintained normal surface expression levels of ICAMs had significantly greater ability to adhere to autologous NK cells (Fig. 4). IL-2 pretreatment did not enhance the adhesion of NK cells toward HTLV-1-infected targets, although IL-2 treatment has previously been shown to enhance LFA-1-mediated adhesion of NK cells to ICAM on target cells (5). Thus, the down-modulation of ICAM-1 and -2 on HTLV-1-infected target cells may contribute to the reduced ability of NK cells to adhere to these infected target cells. This may severely hamper early steps in NK cell cytotoxic response, including synapse formation and granular polarization, leading to the inability of NK cells to kill HTLV-1-infected CD4+ T cells. Indeed, the down-modulation of ICAM-1 expression on cell lines derived from ATL patients indicate that altered ICAM expression may help in disease progression under selective pressure from NK cell immune surveillance (28). Notably, other transforming viruses, such as the Epstein-Barr virus, has been previously reported to down-regulate the expression of ICAMs to escape NK cell cytotoxic responses (31).
It is notable that p12 is expressed early after HTLV-1 infection and is involved in T-cell activation, as well as in the enhancement of viral infection (21, 23, 24). We show here, for the first time, that HTLV-1 p12I down-modulates the expression of ICAM-1 and -2 on primary CD4+ T cells (Fig. 5B-D). This down-modulation was selective because p12I did not alter the expression of ICAM-3 on CD4+ T cells. It has been previously reported that p12I enhances LFA-1 clustering, which leads to increased T-cell adhesion without the need to up-regulate LFA-1 expression (39). Although LFA-1-dependent T-cell to T cells adhesion is a complex process involving cooperative signaling through TCR, chemokine and other coreceptors (17, 74), adhesion of NK cells to T cells is more dependent on LFA-1 expression on NK cells and its interaction with ICAMs on T cells and does not require signaling through other receptors (5). Therefore, down-modulation of ICAM-1 and -2 on HTLV-1-infected T cells may contribute to reduced LFA-1 mediated adhesion of the infected cells with NK cells.
Tax1 has previously been reported to induce MHC-I expression in glial cells (65). Interestingly, p12I down modulated the surface expression of MHC-I molecules on primary CD4+ T cells, apparently overriding the Tax1 activation function (Fig. 5A). This is in accordance with previously published reports that HTLV-1 p12I down-modulates MHC-I and IL-2-receptor expression on T-cell lines (38, 51). HTLV-1 p12I seems to mimic the function of KSHV K5 protein in down-modulating the expression of both MHC-I and ICAMs on infected primary CD4+ T cells, thereby successfully evading both a CTL and an NK cell cytotoxic response (36, 37). Notably, we found that lentivirus vector-mediated expression of Tax1 up-regulates ICAM-1 expression on primary CD4+ T cells, indicating that this may contribute to the heightened expression of ICAM-1 on HTLV-1 transformed T-cell lines where Tax1 might be overexpressed (28). This indicates that HTLV-1 p12I and Tax1 may have divergent and counteractive functionality in modulating adhesion molecule expression on infected cells. However, in the context of HTLV-1 infection of primary CD4+ T cells in our system, it appears that the p12I-mediated down-modulation of ICAM-1 overcomes the up-regulation of ICAM-1 by Tax1.
We are currently in the process of delineating the molecular mechanism of ICAM down-regulation by HTLV-1 p12I. This may involve the translocation of ICAMs from the endoplasmic reticulum to the proteasome as opposed to its normal pathway of migrating from the endoplasmic reticulum to the Golgi and finally to the surface, similar to p12I-mediated down-modulation of MHC-I (54). Alternatively, HTLV-1 p12I may inhibit the transcription of ICAM by modulating the interaction of various transcriptional regulatory factors with the ICAM promoter. It has been recently shown that p12I modulates the expression of various calcium-regulated genes involved in cell adhesion, presumably by modulating the expression of transcriptional regulators such as p300 (52, 53). The ICAM-1 promoter contains potential binding sites for a variety of transcriptional regulatory factors, including the CRE motif that can bind to p300/CBP complex (19, 70). Interestingly, the active promoter sequence of both ICAM-1 and -2 are homologous but differ in comparison to that of the ICAM-3 promoter (32), and this may explain the selective down-modulation ICAM-1 and -2 by p12I.
Adhesion through LFA-1 and ICAM is required for effective NK cell synapse formation and polarization, but cooperative signaling through engagement of NK cell activating receptors (NCR and NKG2D) with their ligands on target cells is necessary for degranulation and effective destruction of the target cells by NK cells (49, 50). We found that <4% of HTLV-1-infected primary CD4+ T cells express ligands for NCR (Fig. 6). At this time the only known ligands for NCR are viral in origin (2). It has been hypothesized that expression of these ligands on infected cells and their subsequent recognition by the NCRs might be a putative mechanism by which NK cells recognize and kill virally infected target cells. Our data suggest that there was very little expression of NCR ligands on CD4+ T cells after infection with HTLV-1. We also found that HTLV-1-infected CD4+ T cells showed very little surface expression of ligands for another NK cell activating receptor, NKG2D. In contrast to NCR ligands, the NKG2D ligands MHC-I-related chain and UL-16 binding protein are well-characterized cellular proteins. These proteins are induced after DNA damage and may be abundantly expressed on transformed cells (29). Moreover, HTLV-1 has been shown to induce DNA damage and transform T lymphocytes (29, 44). The poor expression of NCR and NKG2D ligands on infected cells was not due to the inability of the soluble receptors to detect the ligands on the cell surface because the ligands were detected on the surfaces of COS7, 293T, and HeLa cells at the concentrations of soluble receptors used in our assays (data not shown). Collectively, our data suggest that HTLV-1-infected primary CD4+ T cells have a very low expression profile of NCR and NKG2D ligands, which might interfere with proper degranulation and the cytotoxic response of NK cells to HTLV-1-infected targets.
Normally, NK-cell “activating coreceptors” are important in the signaling events through which NK cell activating receptors (NCR and NKG2D) are able to trigger NK cell-mediated cytotoxicity (8, 25, 35, 47, 50). We found both NTB-A and CD48 on the HTLV-1-infected cells, and the level of expression was unaltered in comparison to uninfected cells. CD155 and CD112, the ligands for another important NK cell coactivating receptor, DNAM-1 (10), were not expressed on primary CD4+ T cells (data not shown) and thus may not have an apparent role in triggering NK cell cytotoxicity toward HTLV-1-infected targets.
We found that the expression of ICAM-3, CD48, and NTB-A was not significantly altered on primary CD4+ T cells after infection with HTLV-1 (Fig. 3C and 6I and J). It still remains to be determined whether the presence of the coactivating receptors triggers NK cell-mediated killing of HTLV-1-infected cells, especially in the presence of IL-2. Indeed, we found that IL-2-treated NK cells have a threefold-enhanced cytotoxic response to HTLV-1-infected CD4+ T cells, although this was still significantly lower than the NK cell response against K562 cells (Fig. 2). This indicates that IL-2 treatment and cooperative activation signals from the simultaneous interaction of LFA-1 and ICAM-3, as well as 2B4 and CD48 and/or NTB-A and NTB-A, may not be sufficient to profoundly increase the cytotoxic response of NK cells to HTLV-1-infected targets.
Combined with the modulation of ICAM-1 and -2 and the lack of expression of NCR and NKG2D ligands, the present study indicates that the ligands on target cells that trigger the critical steps of NK cell cytotoxic response to target cells are either robustly down-regulated or not present on the HTLV-1-infected CD4+ T cells. Furthermore, even when the coactivating receptor ligands are present they are not able to induce a strong NK cytotoxic response, indicating the limited role of these coactivating receptor ligands in triggering NK cell cytotoxicity in the absence of activation through NCR and NKG2Ds.
Acknowledgments
We thank Michael Lairmore from Ohio State University, Columbus, OH, for providing us with the p121-GFP lentivirus vector and Jeffrey Ward for technical assistance.
Footnotes
Published ahead of print on 3 July 2007.
REFERENCES
- 1.Albrecht, B., N. D. Collins, M. T. Burniston, J. W. Nisbet, L. Ratner, P. L. Green, and M. D. Lairmore. 2000. Human T-lymphotropic virus type 1 open reading frame I p12I is required for efficient viral infectivity in primary lymphocytes. J. Virol. 74:9828-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnon, T. I., M. Lev, G. Katz, Y. Chernobrov, A. Porgador, and O. Mandelboim. 2001. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur. J. Immunol. 31:2680-2689. [DOI] [PubMed] [Google Scholar]
- 3.Bangham, C. R., and M. Osame. 2005. Cellular immune response to HTLV-1. Oncogene 24:6035-6046. [DOI] [PubMed] [Google Scholar]
- 4.Barber, D. F., M. Faure, and E. O. Long. 2004. LFA-1 contributes an early signal for NK cell cytotoxicity. J. Immunol. 173:3653-3659. [DOI] [PubMed] [Google Scholar]
- 5.Barber, D. F., and E. O. Long. 2003. Coexpression of CD58 or CD48 with intercellular adhesion molecule 1 on target cells enhances adhesion of resting NK cells. J. Immunol. 170:294-299. [DOI] [PubMed] [Google Scholar]
- 6.Biagi, E., F. Bambacioni, G. Gaipa, C. Casati, J. Golay, A. Biondi, and M. Introna. 2001. Efficient lentiviral transduction of primary human acute myelogenous and lymphoblastic leukemia cells. Hematologica 86:13-16. [PubMed] [Google Scholar]
- 7.Biassoni, R., C. Cantoni, M. Falco, D. Pende, R. Millo, L. Moretta, C. Bottino, and A. Moretta. 2000. Human natural killer cell activating receptors. Mol. Immunol. 37:1015-1024. [DOI] [PubMed] [Google Scholar]
- 8.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 9.Bonaparte, M. I., and E. Barker. 2004. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood 104:2087-2094. [DOI] [PubMed] [Google Scholar]
- 10.Bottino, C., R. Castriconi, D. Pende, P. Rivera, M. Nanni, B. Carnemolla, C. Cantoni, J. Grassi, S. Marcenaro, N. Reymond, M. Vitale, L. Moretta, M. Lopez, and A. Moretta. 2003. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 198:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyum, A. 1968. Separation of leukocytes from blood and bone marrow: introduction. Scand. J. Clin. Lab. Investig. Suppl. 97:7. [PubMed] [Google Scholar]
- 12.Brutkiewicz, R. R., and R. M. Welsh. 1995. Major histocompatibility complex class I antigens and the control of viral infections by natural killer cells. J. Virol. 69:3967-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryceson, Y. T., M. E. March, D. F. Barber, H. G. Ljunggren, and E. O. Long. 2005. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 202:1001-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bukowski, J. F., J. F. Warner, G. Dennert, and R. M. Welsh. 1985. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J. Exp. Med. 161:40-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burshtyn, D. N., A. S. Lam, M. Weston, N. Gupta, P. A. Warmerdam, and E. O. Long. 1999. Conserved residues amino-terminal of cytoplasmic tyrosines contribute to the SHP-1-mediated inhibitory function of killer cell Ig-like receptors. J. Immunol. 162:897-902. [PubMed] [Google Scholar]
- 16.Burshtyn, D. N., J. Shin, C. Stebbins, and E. O. Long. 2000. Adhesion to target cells is disrupted by the killer cell inhibitory receptor. Curr. Biol. 10:777-780. [DOI] [PubMed] [Google Scholar]
- 17.Carman, C. V., and T. A. Springer. 2003. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr. Opin. Cell Biol. 15:547-556. [DOI] [PubMed] [Google Scholar]
- 18.Cerwenka, A., and L. L. Lanier. 2001. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 1:41-49. [DOI] [PubMed] [Google Scholar]
- 19.Chan, H. M., and N. B. La Thangue. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114:2363-2373. [DOI] [PubMed] [Google Scholar]
- 20.Chisholm, S. E., and H. T. Reyburn. 2006. Recognition of vaccinia virus-infected cells by human natural killer cells depends on natural cytotoxicity receptors. J. Virol. 80:2225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins, N. D., G. C. Newbound, B. Albrecht, J. L. Beard, L. Ratner, and M. D. Lairmore. 1998. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood 91:4701-4707. [PubMed] [Google Scholar]
- 22.Colonna, M., F. Navarro, T. Bellon, M. Llano, P. Garcia, J. Samaridis, L. Angman, M. Cella, and M. Lopez-Botet. 1997. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 186:1809-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding, W., B. Albrecht, R. E. Kelley, N. Muthusamy, S. J. Kim, R. A. Altschuld, and M. D. Lairmore. 2002. Human T-cell lymphotropic virus type 1 p12I expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J. Virol. 76:10374-10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding, W., S. J. Kim, A. M. Nair, B. Michael, K. Boris-Lawrie, A. Tripp, G. Feuer, and M. D. Lairmore. 2003. Human T-cell lymphotropic virus type 1 p12I enhances interleukin-2 production during T-cell activation. J. Virol. 77:11027-11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falco, M., E. Marcenaro, E. Romeo, F. Bellora, D. Marras, F. Vely, G. Ferracci, L. Moretta, A. Moretta, and C. Bottino. 2004. Homophilic interaction of NTBA, a member of the CD2 molecular family: induction of cytotoxicity and cytokine release in human NK cells. Eur. J. Immunol. 34:1663-1672. [DOI] [PubMed] [Google Scholar]
- 26.Feuer, G., S. A. Stewart, S. M. Baird, F. Lee, R. Feuer, and I. S. Chen. 1995. Potential role of natural killer cells in controlling tumorigenesis by human T-cell leukemia viruses. J. Virol. 69:1328-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fruh, K., A. Gruhler, R. M. Krishna, and G. J. Schoenhals. 1999. A comparison of viral immune escape strategies targeting the MHC class I assembly pathway. Immunol. Rev. 168:157-166. [DOI] [PubMed] [Google Scholar]
- 28.Fukudome, K., M. Furuse, N. Fukuhara, S. Orita, T. Imai, S. Takagi, M. Nagira, Y. Hinuma, and O. Yoshie. 1992. Strong induction of ICAM-1 in human T cells transformed by human T-cell-leukemia virus type 1 and depression of ICAM-1 or LFA-1 in adult T-cell-leukemia-derived cell lines. Int. J. Cancer 52:418-427. [DOI] [PubMed] [Google Scholar]
- 29.Gasser, S., S. Orsulic, E. J. Brown, and D. H. Raulet. 2005. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436:1186-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 31.Gregory, C. D., R. J. Murray, C. F. Edwards, and A. B. Rickinson. 1988. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T-cell surveillance. J. Exp. Med. 167:1811-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayflick, J. S., J. Stine, R. Fox, D. Hoekstra, and W. M. Gallatin. 1997. Functional mapping of the cytoplasmic region of intercellular adhesion molecule-3 reveals important roles for serine residues. J. Biol. Chem. 272:22207-22214. [DOI] [PubMed] [Google Scholar]
- 33.Helander, T. S., and T. Timonen. 1998. Adhesion in NK cell function. Curr. Top. Microbiol. Immunol. 230:89-99. [DOI] [PubMed] [Google Scholar]
- 34.Hildreth, J. E., F. M. Gotch, P. D. Hildreth, and A. J. McMichael. 1983. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur. J. Immunol. 13:202-208. [DOI] [PubMed] [Google Scholar]
- 35.Hodge, D. L., W. B. Schill, J. M. Wang, I. Blanca, D. A. Reynolds, J. R. Ortaldo, and H. A. Young. 2002. IL-2 and IL-12 alter NK cell responsiveness to IFN-gamma-inducible protein 10 by down-regulating CXCR3 expression. J. Immunol. 168:6090-6098. [DOI] [PubMed] [Google Scholar]
- 36.Ishido, S., J. K. Choi, B. S. Lee, C. Wang, M. DeMaria, R. P. Johnson, G. B. Cohen, and J. U. Jung. 2000. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity 13:365-374. [DOI] [PubMed] [Google Scholar]
- 37.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson, J. M., C. Nicot, J. Fullen, V. Ciminale, L. Casareto, J. C. Mulloy, S. Jacobson, and G. Franchini. 2001. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12I protein. J. Virol. 75:6086-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim, S. J., A. M. Nair, S. Fernandez, L. Mathes, and M. D. Lairmore. 2006. Enhancement of LFA-1-mediated T-cell adhesion by human T lymphotropic virus type 1 p12I1. J. Immunol. 176:5463-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanier, L. L. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225-274. [DOI] [PubMed] [Google Scholar]
- 41.Lehky, T. J., E. P. Cowan, L. A. Lampson, and S. Jacobson. 1994. Induction of HLA class I and class II expression in human T-lymphotropic virus type I-infected neuroblastoma cells. J. Virol. 68:1854-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ljunggren, H. G., and K. Karre. 1985. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J. Exp. Med. 162:1745-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacFarlane, A. W. T., and K. S. Campbell. 2006. Signal transduction in natural killer cells. Curr. Top. Microbiol. Immunol. 298:23-57. [DOI] [PubMed] [Google Scholar]
- 44.Marriott, S. J., F. J. Lemoine, and K. T. Jeang. 2002. Damaged DNA and miscounted chromosomes: human T-cell leukemia virus type I tax oncoprotein and genetic lesions in transformed cells. J. Biomed. Sci. 9:292-298. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto, G., M. P. Nghiem, N. Nozaki, R. Schmits, and J. M. Penninger. 1998. Cooperation between CD44 and LFA-1/CD11a adhesion receptors in lymphokine-activated killer cell cytotoxicity. J. Immunol. 160:5781-5789. [PubMed] [Google Scholar]
- 46.Matsumoto, G., Y. Omi, U. Lee, T. Nishimura, J. Shindo, and J. M. Penninger. 2000. Adhesion mediated by LFA-1 is required for efficient IL-12-induced NK and NKT cell cytotoxicity. Eur. J. Immunol. 30:3723-3731. [DOI] [PubMed] [Google Scholar]
- 47.Messmer, B., P. Eissmann, S. Stark, and C. Watzl. 2006. CD48 stimulation by 2B4 (CD244)-expressing targets activates human NK cells. J. Immunol. 176:4646-4650. [DOI] [PubMed] [Google Scholar]
- 48.Moretta, A., R. Biassoni, C. Bottino, M. C. Mingari, and L. Moretta. 2000. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol. Today 21:228-234. [DOI] [PubMed] [Google Scholar]
- 49.Moretta, A., C. Bottino, M. Vitale, D. Pende, C. Cantoni, M. C. Mingari, R. Biassoni, and L. Moretta. 2001. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19:197-223. [DOI] [PubMed] [Google Scholar]
- 50.Moretta, L., and A. Moretta. 2004. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 23:255-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulloy, J. C., R. W. Crownley, J. Fullen, W. J. Leonard, and G. Franchini. 1996. The human T-cell leukemia/lymphotropic virus type 1 p12I proteins bind the interleukin-2 receptor beta and gamma chains and affects their expression on the cell surface. J. Virol. 70:3599-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nair, A., B. Michael, H. Hiraragi, S. Fernandez, G. Feuer, K. Boris-Lawrie, and M. Lairmore. 2005. Human T lymphotropic virus type 1 accessory protein p12I modulates calcium-mediated cellular gene expression and enhances p300 expression in T lymphocytes. AIDS Res. Hum. Retrovir. 21:273-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair, A. M., B. Michael, A. Datta, S. Fernandez, and M. D. Lairmore. 2006. Calcium-dependent enhancement of transcription of p300 by human T-lymphotropic type 1 p12I. Virology 353:247-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicot, C., R. L. Harrod, V. Ciminale, and G. Franchini. 2005. Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene 24:6026-6034. [DOI] [PubMed] [Google Scholar]
- 55.Ohashi, T., S. Hanabuchi, R. Suzuki, H. Kato, T. Masuda, and M. Kannagi. 2002. Correlation of major histocompatibility complex class I downregulation with resistance of human T-cell leukemia virus type 1-infected T cells to cytotoxic T-lymphocyte killing in a rat model. J. Virol. 76:7010-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-1 associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 57.Owen, S. M., D. L. Rudolph, C. S. Dezzutti, N. Shibata, S. Naik, S. W. Caughman, and R. B. Lal. 1997. Transcriptional activation of the intercellular adhesion molecule 1 (CD54) gene by human T lymphotropic virus types I and II Tax is mediated through a palindromic response element. AIDS Res. Hum. Retrovir. 13:1429-1437. [DOI] [PubMed] [Google Scholar]
- 58.Parker, C. E., S. Daenke, S. Nightingale, and C. R. Bangham. 1992. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology 188:628-636. [DOI] [PubMed] [Google Scholar]
- 59.Pende, D., C. Cantoni, P. Rivera, M. Vitale, R. Castriconi, S. Marcenaro, M. Nanni, R. Biassoni, C. Bottino, A. Moretta, and L. Moretta. 2001. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur. J. Immunol. 31:1076-1086. [DOI] [PubMed] [Google Scholar]
- 60.Pende, D., G. M. Spaggiari, S. Marcenaro, S. Martini, P. Rivera, A. Capobianco, M. Falco, E. Lanino, I. Pierri, R. Zambello, A. Bacigalupo, M. C. Mingari, A. Moretta, and L. Moretta. 2005. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood 105:2066-2073. [DOI] [PubMed] [Google Scholar]
- 61.Perez, O. D., D. Mitchell, G. C. Jager, and G. P. Nolan. 2004. LFA-1 signaling through p44/42 is coupled to perforin degranulation in CD56+ CD8+ natural killer cells. Blood 104:1083-1093. [DOI] [PubMed] [Google Scholar]
- 62.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richardson, J. H., A. J. Edwards, J. K. Cruickshank, P. Rudge, and A. G. Dalgleish. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64:5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruscetti, F. W., J. A. Mikovits, V. S. Kalyanaraman, R. Overton, H. Stevenson, K. Stromberg, R. B. Herberman, W. L. Farrar, and J. R. Ortaldo. 1986. Analysis of effector mechanisms against HTLV-I- and HTLV-III/LAV-infected lymphoid cells. J. Immunol. 136:3619-3624. [PubMed] [Google Scholar]
- 65.Sawada, M., A. Suzumura, M. Yoshida, and T. Marunouchi. 1990. Human T-cell leukemia virus type I trans activator induces class I major histocompatibility complex antigen expression in glial cells. J. Virol. 64:4002-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sieburg, M., A. Tripp, J. W. Ma, and G. Feuer. 2004. Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 tax oncoproteins modulate cell cycle progression and apoptosis. J. Virol. 78:10399-10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sivori, S., D. Pende, C. Bottino, E. Marcenaro, A. Pessino, R. Biassoni, L. Moretta, and A. Moretta. 1999. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur. J. Immunol. 29:1656-1666. [DOI] [PubMed] [Google Scholar]
- 68.Sonoda, S., S. Yashiki, K. Takahashi, N. Arima, Y. Daitoku, M. Matsumoto, T. Matsumoto, M. Tara, K. Shinmyozu, K. Sato, et al. 1987. Altered HLA antigens expressed on T and B lymphocytes of adult T-cell leukemia/lymphoma patients and their relatives. Int. J. Cancer 40:629-634. [DOI] [PubMed] [Google Scholar]
- 69.Stewart, S. A., G. Feuer, A. Jewett, F. V. Lee, B. Bonavida, and I. S. Chen. 1996. HTLV-1 gene expression in adult T-cell leukemia cells elicits an NK cell response in vitro and correlates with cell rejection in SCID mice. Virology 226:167-175. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka, Y., M. Hayashi, S. Takagi, and O. Yoshie. 1996. Differential transactivation of the intercellular adhesion molecule 1 gene promoter by Tax1 and Tax2 of human T-cell leukemia viruses. J. Virol. 70:8508-8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tripp, A., P. Banerjee, M. Sieburg, V. Planelles, F. Li, and G. Feuer. 2005. Induction of cell cycle arrest by human T-cell lymphotropic virus type 1 Tax in hematopoietic progenitor (CD34+) cells: modulation of p21cip1/waf1 and p27kip1 expression. J. Virol. 79:14069-14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tripp, A., Y. Liu, M. Sieburg, J. Montalbano, S. Wrzesinski, and G. Feuer. 2003. Human T-cell leukemia virus type 1 tax oncoprotein suppression of multilineage hematopoiesis of CD34+ cells in vitro. J. Virol. 77:12152-12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uno, H., H. Matsuoka, M. Suzuki, K. Tsuda, and H. Tsubouchi. 1995. Altered expression of class I HLA antigen on peripheral mononuclear cells in patients with adult T-cell leukemia: inverse relationship with natural killer susceptibility. Cancer Epidemiol. Biomarkers Prev. 4:367-372. [PubMed] [Google Scholar]
- 74.van Kooyk, Y., and C. G. Figdor. 2000. Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr. Opin. Cell Biol. 12:542-547. [DOI] [PubMed] [Google Scholar]
- 75.Vieillard, V., J. L. Strominger, and P. Debre. 2005. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc. Natl. Acad. Sci. USA 102:10981-10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang, J., and T. A. Springer. 1998. Structural specializations of immunoglobulin superfamily members for adhesion to integrins and viruses. Immunol. Rev. 163:197-215. [DOI] [PubMed] [Google Scholar]
- 77.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]